Abstract
In April 2009, following the first school closure due to 2009 pandemic influenza A (H1N1) (pH1N1) in Chicago, Illinois, area hospitals were inundated with patients presenting with influenza-like illness (ILI). The extent of disease spread into the surrounding community was unclear. We performed a household survey to estimate the ILI attack rate among community residents and compared reported ILI with confirmed pH1N1 cases and ILI surveillance data (ie, hospital ILI visits, influenza testing, and school absenteeism). The estimated ILI attack rate was 4.6% (95% confidence interval, 2.8%-7.4%), with cases distributed throughout the 5-week study period. In contrast, 36 (84%) of 43 confirmed pH1N1 cases were identified the week of the school closure. Trends in surveillance data peaked during the same week and rapidly decreased to near baseline. Public awareness and health care practices impact standard ILI surveillance data. Community-based surveys are a valuable tool to help assess the burden of ILI in a community.
On 26 April 2009, the Chicago Department of Public Health (CDPH) held its first press conference regarding 2009 pandemic influenza A (H1N1) (pH1N1) virus. At that time, little was known about the severity and potential impact of pH1N1 in the city. However, by 28 April, several probable pH1N1 cases had been reported to CDPH, including a case in an ill elementary school student from a northeastern Chicago community (community A) [1]. The probable pH1N1 case in the student, along with additional suspect cases in the same school (school X), led to the school closure on 29 April. That same day, CDPH received reports that emergency departments (EDs) serving community A were inundated with patients seeking care for influenza-like illness (ILI).
On 30 April, because of the large volume of mildly ill patients seeking medical care and an excess of specimens being submitted to the state laboratory for confirmatory testing, CDPH recommended that testing for pH1N1 be performed only on hospitalized patients. At that time, community A had the highest incidence of pH1N1 in Chicago, with the majority of confirmed cases occurring among school X students and their household contacts, suggesting an outbreak at the school [1]. However, it was unknown how much pH1N1 had spread into the surrounding community and if the available surveillance data could accurately depict the burden of ILI in the community. To investigate these issues, we performed a household survey to estimate the ILI attack rate among community residents, and compared reported ILI to confirmed pH1N1 cases, hospital surveillance data (ILI visits and influenza testing), and school absenteeism.
METHODS
Community ILI Survey
Sample size calculations were performed using a typical seasonal ILI rate (± 2.5%; 95% confidence interval [CI]) with a design effect of 2 to account for household clustering. On the basis of a previous community survey, we estimated 25% participation of households and, therefore, oversampled by 75% [2]. We used a stratified, two-stage cluster design to sample six community A Census tracts serving as the catchment area for school X [3]. Using 2000 Census data, the 115 Census blocks in the selected area were divided into 2 strata based on the number of occupied households to account for population density. The higher density stratum contained 30 blocks forming the upper quartile of the number of households, and the lower density stratum contained the remaining 85 blocks. Thirteen higher-density blocks and 32 lower-density blocks were selected proportional to the occupied number of households, without replacement. In selected blocks, 354 households were identified for inclusion in the study. With use of proportional allocation and simple random sampling, 10 households were selected in higher stratum blocks and 7 households in lower stratum blocks.
The door-to-door survey was performed during 11–17 May 2009. Buildings were excluded if they were vacant; contained a business, dormitory, or nursing home; or had no way to access an individual unit (eg, locked entry ways or broken buzzers). Households visited at least 2 times with no response were considered to be nonresponders. If the household was classified as a nonresponder, an adjacent household was randomly selected for enrollment. Households that were visited only once but without response were not eligible for inclusion. All enrolled households were classified as a single family dwelling (1 housing unit per building), multifamily dwelling (2–10 housing units per building), or apartment dwelling (>10 housing units per building).
All members of an enrolled household were invited to participate. A household member was defined as an individual who spent a mean of ≥2 nights per week in the home. Participants or their parent or guardian provided informed verbal consent. Information was obtained directly from participants or an adult member of the household. This survey was part of the emergency public health practice response to the pandemic and was reviewed by a human subjects coordinator at CDPH and the Centers for Disease Control (CDC) and deemed not to be research in accordance with the federal human subjects' protection regulations at 45 Code of Federal Regulations 46.101c and 46.102d and CDC's Guidelines for Defining Public Health Research and Public Health Non-Research.
A standardized questionnaire was administered to enrolled participants regarding household characteristics, demographic characteristics, symptoms consistent with ILI, existing medical conditions, illness outcome, and exposures, such as travel and employment and/or attendance in a school or health care setting. A case of ILI was defined in survey participants who reported fever with cough and/or sore throat from 13 April through the day of the interview.
Estimates of population parameters, standard errors (SEs), CIs, risk factor modeling estimates, and hypothesis tests were calculated accounting for the sampling design and incorporating sampling weights and finite population correction with the statistical software R (www.r-project.org). The age, sex, and race distribution of participants were compared with those of the community population with use of a χ2 goodness-of-fit test [4]. Standard calibration estimators were used to adjust for nonresponse by calibrating the sampled data to the 2000 Census block population totals by race (white/nonwhite), sex, and age group, simultaneously [5]. Categorical variables and ILI symptoms were assessed using the Rao-Scott correction to the χ2 test. CIs were computed using Wald-type intervals. Binomial regression was used to provide adjusted relative risk estimates and CIs, and model effects were evaluated for statistical significance with use of Wald tests. We report both crude values describing the survey participants and population estimates.
Estimated proportions and 95% CIs established by the community survey were used to calculate the total number of ILI cases and the number patients with ILI who sought medical care or were hospitalized in the community over the 5-week period. Incidence rates were calculated using the 2000 Census data for blocks in the surveyed area.
Diagnostic Sampling and Laboratory Testing
Patients with ILI with onset of fever within 7 days prior to completing the survey were asked to provide a nasopharyngeal swab specimen. Specimens were collected using sterile swabs with a synthetic tip and plastic shaft. The swabs were placed in viral transport media and kept at 4°C for <6 h and then stored at –70°C. Real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) for 2009 H1N1 influenza viral RNA was performed at the Illinois Department of Public Health Division of Laboratories Chicago, IL [6].
Description of Confirmed Cases
A standardized case report form was used to collect demographic and exposure information on confirmed cases reported to CDPH by Chicago area hospitals and health care providers. A confirmed case was defined in a patient with ILI onset during the period from 13 April through 17 May and laboratory evidence of 2009 pH1N1 virus infection by rRT-PCR or viral culture. ILI was defined as a fever (temperature, ≥37.8°C) with cough and/or sore throat. Data obtained from patients with confirmed H1N1 who resided in community A were compared with data from participants with ILI who were identified through the household survey.
Hospital ILI and School Absenteeism Data
We obtained information on outpatient visits for ILI and influenza testing from 3 hospitals serving community A. Residents from community A were differentiated by zip code. ILI information was extracted based on the International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis codes for fever with cough and/or sore throat or influenza diagnoses (ie, ICD-9 codes 780.6, 786.2, 462, 784.1, and 487).
Chicago Public Schools provided school attendance data for the 5 public elementary schools in community A, including school X. The proportion of absent students (absenteeism) was calculated from the number of students absent on a given day and the total number of students reported to be enrolled.
Evaluating Trends Over Time
For the purpose of this analysis, events were grouped into 3 periods: (1) baseline period from 13 through 26 April; (2) school outbreak period from 27 April through 3 May, which includes the identification of the first case and school closure in community A; and (3) post–school outbreak period from 4 through 17 May, which includes the week of the school reopening through the last day of the survey.
RESULTS
Community ILI Survey
Of the 711 eligible households approached for enrollment in the survey, 316 (44%) were nonresponders, 155 (22%) declined, and 240 (34%) agreed to participate. Enrolled households had a total of 644 residents and a median of 3 residents per household (range, 1–8). When compared with census data for the 38,351 residents of the survey area, enrolled participants were similar in age and sex distribution but were more likely to be White and nonHispanic (Table 1).
Table 1.
Demographic Characteristics of Survey Participants, Compared with 2000 Census Tract Data for Community A
| Survey participants (n = 644) |
Community A 2000 Censusa (n = 38,351) |
|||
| No. | (%) | No. | (%) | |
| Male | 324 | (50) | 20,020 | (52) |
| Age group | ||||
| <5 years | 55 | (9) | 3124 | (8) |
| 5–14 years | 62 | (10) | 4571 | (12) |
| 15–24 years | 124 | (19) | 7339 | (19) |
| 25–49 years | 272 | (42) | 17,287 | (45) |
| 50–64 years | 90 | (14) | 3654 | (10) |
| ≥65 years | 41 | (6) | 2376 | (6) |
| White raceb | 465 | (72) | 18,902 | (49) |
| Hispanic ethnicityb | 131 | (20) | 11,890 | (31) |
Excludes individuals living on census tracts or blocks that were excluded from the study
Goodness of fit - P< .01
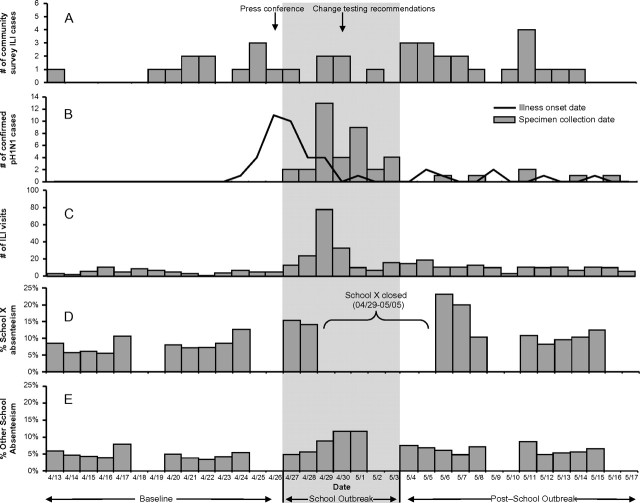
Of the 644 enrolled participants, 37 met the ILI case definition for an estimated population ILI attack rate of 4.6% (95% CI, 2.8%–7.4%). Illness onset for these ILI cases occurred from 13 April through 14 May 2009 and was evenly distributed, with a median of 1 case reported per day (range, 0–4) (Figure 1A). An estimated 44% of ILI occurred in male individuals, and the mean age was 25 years (95% CI, 17–33 years) (Table 2). The oldest survey participants with ILI among survey participants was 59 years of age. The majority of patients were white and nonHispanic. An estimated 62% (95% CI, 43%–82%) of survey participants with ILI sought medical care for their illness, and 3.0% (95% CI, 0.0%–8.8%) were hospitalized. The median duration of illness for survey participants with ILI was 5.0 days (95% CI, 2.3–6.0 days), and 74% (95% CI, 58%–90%) missed work or school because of their illness rather than school/work closures or taking care of ill family members. Almost all survey participants with ILI (96%; 95% CI, 84%–99%) lived in a multifamily or apartment dwelling (higher-density housing), 17% (95% CI, 4.5%–29%) had an underlying condition that predisposed them to more severe disease (Table 2), and 37% (95% CI, 18%–60%) attended or worked at a primary school (ie, daycare, preschool, or elementary school; 11 students and 1 staff). None of the ILI survey participants with ILI attended, worked at, or had household contacts affiliated with school X.
Figure 1.
Correlation of epidemiologic indicators of 2009 pandemic influenza A (H1N1) (pH1N1) virus activity in community A, from 13 April through 17 May 2009. A, Influenza-like illness (ILI) cases identified through the community survey by illness onset date. B, Confirmed cases of pH1N1 in community A, by specimen collection date (bars) and illness onset date (line). C, Number of ED and outpatient visits for ILI for the 3 hospitals servicing community A, by day. D, Proportion of students absent from school X in community A, by day. E, Proportion of students absent from the 4 other elementary schools in community A, by day.
Table 2.
Demographic Characteristics and Exposure Information for Surveyed Participants with Influenza-like Illness (ILI) versus those without ILI
| Survey participants with ILI (n=37) |
Survey participants without ILI (n=607) |
|||||
| Weighted proportions | Weighted proportions | |||||
| No. | (%) | [95% CI] | No. | (%) | [95% CI] | |
| Male | 21 | (44) | [26-63] | 303 | (53) | [52-54] |
| Age group | ||||||
| <5 years | 7 | (15) | [1-30] | 48 | (8) | [7-8] |
| 5–14 years | 8 | (28) | [4-52] | 54 | (11) | [10-12] |
| 15–24 years | 3 | (6) | [0-12] | 121 | (20) | [19-21] |
| 25–49 years | 17 | (43) | [21-65] | 255 | (45) | [44-46] |
| ≥ 50 years | 2 | (7) | [0-16] | 129 | (16) | [15-17] |
| White race | 32 | (68) | [44-92] | 433 | (48) | [47-49] |
| Hispanic ethnicity | 5 | (13) | [3-24] | 126 | (20) | [12-27] |
| Type of dwelling | ||||||
| Single family | 3 | (4) | [0-10] | 159 | (20) | [13-27] |
| Multifamily (2-10 units per building) | 21 | (44) | [22-66] | 230 | (33) | [25-42] |
| Apartment (>10 units per building) | 13 | (52) | [30-74] | 218 | (47) | [37-57] |
| Medical history | ||||||
| Chronic lung disease | 2 | (9) | [2-28] | 36 | (9) | [6-13] |
| Immunosuppressive condition | 2 | (5) | [1-19] | 7 | (1) | [0-2] |
| Metabolic disorder | 4 | (13) | [5-30] | 27 | (5) | [3-7] |
| Neurologic/neuromuscular condition | 1 | (5) | [1-27] | 9 | (2) | [1-4] |
| Attend or work at a school | ||||||
| Daycare/preschool | 3 | (7) | [0-15] | 17 | (2) | [1-4] |
| Elementary school (grades K-8) | 9 | (30) | [6-54] | 53 | (10) | [8-12] |
| High school (grades 9-12) | 1 | (1) | [0-3] | 25 | (5) | [3-7] |
| College | 4 | (10) | [0-21] | 96 | (15) | [10-19] |
| Health care worker | 2 | (4) | [0-15] | 37 | (4) | [4-8] |
| Visitor/travel history | ||||||
| Visitors from or travel to high risk statea | 2 | (4) | [1-18] | 24 | (3) | [1-5] |
| Visitors from or travel to Mexico | 0 | (0) | [ --- ] | 20 | (1) | [1-3] |
NOTE. CI=confidence interval.
One ILI case traveled to California and one traveled to Texas.
On univariate analysis, ILI cases were more likely to live in higher-density housing, have an underlying immunosuppressive condition, or attend or work at a primary school, and less likely to attend or work at a high school or college (Table 3). On multivariate analysis, higher-density housing (relative risk [RR], 4.8; 95% CI, 1.2–19) and school exposure (primary school, RR, 3.1; 95% CI, 1.2–8.3; and secondary school, RR 0.6; 95% CI, 0.2–2.1) remained significantly associated with risk of ILI.
Table 3.
Univariate Analysis of Demographic and Exposure Categories Comparing Influenza-like Illness (ILI) Cases to Those without Symptoms of ILI
| Weighted Univariate Analysis |
|||
| RR (95% CI) |
P value | ||
| Age group | 0.06 | ||
| <5 years | 4.1 | (0.9-19) | |
| 5-14 years | 5.2 | (0.9-31) | |
| 15-24 years | 0.7 | (0.1-4.5) | |
| 25-49 years | 2.1 | (0.5-8.6) | |
| ≥ 50 years | 1.0 | (referent) | |
| Multifamily or apartment dwelling | 5.0 | (1.2-21) | 0.01 |
| Immunosuppressive condition | 5.0 | (1.3-19) | 0.02 |
| Attend or work at a school | 0.02 | ||
| Primary schoola | 3.3 | (1.2-8.9) | |
| High school or college | 0.7 | (0.2-2.3) | |
| None | 1.0 | (referent) | |
NOTE. RR, relative risk; CI, confidence interval.
Daycare, preschool, or elementary school.
Acute-phase nasopharyngeal swab specimens were collected from 7 survey participants with ILI who reported fever within a week of the survey. The median number of days between illness onset and specimen collection was 5 days (range, 1–7 days). Only 1 sample, obtained 6 days after illness onset, was positive for 2009 H1N1 influenza viral RNA by rRT-PCR. The positive sample was collected from a 43-year-old white woman who developed a fever (temperature, 38.3°C) with cough, sore throat, and myalgias. She did not seek medical care for her illness.
Description of Confirmed Cases
A total of 43 laboratory-confirmed cases of pH1N1 among residents of community A were reported to CDPH with illness onset from 24 April through 15 May 2009 (Figure 1B). None of the confirmed pH1N1 case households were included in the community survey. There was a median delay of 3 days (range, 0–7 days) between illness onset and specimen collection date. None of the confirmed cases had specimens collected for testing during the baseline period (13 –26 April). In contrast, 36 (84%) survey participants with pH1N1 confirmed cases had a specimen collected during the school outbreak period (from 27 April through3 May), and the remaining 7 (16%) had a specimen collected during the post–school outbreak period (4–17 May).
The median age of case-patients was 8 years (range, 2 months-45 years). Approximately half of the survey participants were male, and 16 (37%) were Hispanic (Table 4). Thirty-six (84%) survey participants with ILI sought care in an ED or outpatient clinic, and 5 (12%) were hospitalized. Among the patients with exposure information, 20 (47%) were primary school students, including 15 (35%) students from school X. Another 6 survey participants with ILI (14%) had household contacts who attended school X. These data confirm a pH1N1 outbreak at school X.
Table 4.
Demographic Characteristics and Information on Medical Care and Exposures for Confirmed pH1N1 Cases in Community A
| Confirmed cases (n=43) |
||
| No. | (%) | |
| Male | 20 | (47) |
| Age group | ||
| <5 years | 7 | (16) |
| 5-14 years | 30 | (70) |
| 15-24 years | 3 | (7) |
| 25-49 years | 3 | (7) |
| 50-64 years | 0 | (0) |
| ≥ 65 years | 0 | (0) |
| Race/ethnicity | ||
| White | 5 | (12) |
| Black | 11 | (26) |
| Asian | 1 | (2) |
| Hispanic | 16 | (37) |
| Unknown | 10 | (23) |
| Medical care | ||
| Outpatient only | 36 | (84) |
| Hospitalized | 5 | (12) |
| Unknown | 2 | (5) |
| Attend or work at a school | ||
| Daycare/preschool | 2 | (5) |
| Elementary school (K-8) | 18 | (42) |
| High school (9-12) | 0 | (0) |
| College | 2 | (5) |
| None | 5 | (12) |
| Unknown | 16 | (37) |
Of the 43 community A patients with confirmed pH1N1 cases, 37 (86%) were <15 years of age, compared with an estimated 44% (95% CI, 21%–66%) of the ILI cases identified through the community survey. In addition, at least 50% of the confirmed cases had a direct connection to school X versus none of the ILI cases from the community survey. Comparisons of race and ethnicity were limited by both missing data and differences in the way that these data were classified.
Hospital ILI Data
From 13 April through 17 May, 402 visits for ILI were made by community A residents to 3 hospitals serving the area. During the baseline period, community A residents had a median of 5 ILI visits per day (range, 1–11 visits per day) (Figure 1C). During the school outbreak period, the median number of ILI visits increased to 16 per day (range, 7–78 visits per day), peaking on the first day of the school closure. During the post-school outbreak period, the median number of visits decreased to 11 per day (range, 3–19 visits per day).
Similar trends were seen in the absolute number of influenza tests performed and positive influenza test results obtained from community A residents at the 2 hospitals where this information was available. During the baseline period, a median of 1 influenza test was performed per day (range, 0–2 tests per day), and none of the results were positive. During the school outbreak period, this increased to a median of 12 tests per day (range, 2–38 tests per day) on community A residents and results of 27% of the influenza tests performed were positive. During the post–school outbreak period, a median of 3 influenza tests were performed per day (range, 0–11 tests per day) and results of 11% of the tests performed were positive.
Elementary School Absenteeism Data
There were 3133 students enrolled in the 5 elementary schools in community A, including 850 (27%) in school X, the elementary school that closed because of probable pH1N1 cases. For school X, a mean of 8% of the students were absent per day (range, 6%–13%) during the 2-week baseline period (Figure 1D). For the 2 days prior to the school closure (27–28 April), absenteeism increased to 15% per day. In the post–school outbreak period, absenteeism remained elevated at 13% per day but was higher for the first 3 days after the school reopened (18% absenteeism per day), compared with the following week (10% absenteeism per day).
Compared with school X, the other primary schools in the community had a slightly lower mean absenteeism rate of 5% per day (range, 3–8%) during the baseline period (Figure 1E). The rate increased to a mean of 9% absenteeism per day (range, 5–12%) during the week that school X was closed but quickly returned to normal during the following 2 weeks (mean, 6% per day; range, 5%–9%).
Estimated Impact of ILI in Community A
Using the ILI attack rate and 95% CI obtained in the survey, we estimate that, in this urban community of ∼38,000 persons, there were 915–2614 cases of ILI during the 5-week period from 13 April through 17 May 2009. Between 43% and 82% of these ILI cases may have sought medical care (360–1843 persons), 0–9% of these patients may have been hospitalized (0–154 hospitalizations), and there were no deaths. From the hospital ILI and influenza testing surveillance data, it was determined that 12% of the patients presenting with ILI had a positive test for influenza prior to changes in testing recommendations. Therefore, we estimate there were 110–314 influenza cases in community A during this period, for an incidence of 287–819 cases per 100,000 population.
DISCUSSION
Following the announcement in late April that the first 2009 pH1N1 case had been identified in Chicago, several area hospitals reported being inundated with patients seeking care for possible infection with the novel influenza virus [1]. Our findings from a community with one of the highest incidences of pH1N1 in Chicago substantiate a marked increase in the number of ILI visits to area hospitals following the closure of a neighborhood school. Within a few days, however, ILI visits decreased dramatically. In contrast, a household survey showed that the reported incidence of ILI among community residents was relatively stable throughout this timeframe and did not correspond to the dramatic peak seen in ED visits. These data suggest that hospital ILI visits may not reflect true ILI activity in the community.
This study helped to determine the potential impact of the pandemic in one of the first cities affected by the novel virus by establishing an estimated ILI attack rate of 4.6% in the community surrounding a school with a known outbreak of pH1N1. This rate suggests that despite the confirmed infection among school attendees and their household contacts, there initially was not widespread community transmission. Furthermore, the estimated ILI rate from this study is similar to other community surveys conducted for seasonal influenza [7, 8]. However, the comparator ILI rates were cumulative for a full influenza season while our estimated ILI rate was determined for a 5-week period and may not represent the full impact of the disease for a complete transmission season.
A comparison of ILI cases to survey participants without ILI identified possible risk factors for disease, including living in higher-density housing and primary school exposure. High school or college exposure was protective; most of these survey participants were college students who may have been less likely to interact with students, staff, or household contacts of the affected primary school. While additional studies are needed to confirm risk factors for pH1N1, these findings further support recommendations to mitigate transmission among household and school contacts through good hand-hygiene practices and targeting of primary school children for vaccination.
Similar to reports from other large metropolitan areas and Chicago as a whole, confirmed pH1N1 cases reported from community A occurred primarily among school-aged children [1, 9]. However, only 44% of the patients with ILI identified through the community survey were <15 years of age, compared with 86% of the patients with confirmed pH1N1. This difference may have been due to age dependent differences in health care seeking, or increased recognition and testing of sick children due to the school-based outbreak. In fact, at least half of the patients with confirmed pH1N1 had a direct connection to school X, compared with none of the survey participants with ILI. One day after the school closure in community A, CDPH revised previous guidelines and recommended that only patients hospitalized with ILI be tested for pH1N1. By that time, the brief surge in local ED visits for ILI and influenza testing, which was likely driven by students from school X and their household contacts, had already occurred. A similar survey in New York City found that a large proportion of the children tested for pH1N1 were identified primarily due to their association with a school-based outbreak [10]. Despite these differences in the proportions of school-aged children, none of the confirmed pH1N1 or ILI cases in Community A were >60 years of age. The low disease burden in older adults is likely due to higher levels of cross-protective antibodies that have been observed in this population secondary to their previous exposure to other influenza H1N1 viruses [11, 12].
Although the community survey overcomes some of the problems that result from determining pH1N1 disease burden through laboratory-based surveillance for confirmed cases or provider-based surveillance for ILI, the estimates derived from the survey still have limitations. First, we cannot account for how the closure of school X may have impacted the ILI rate in community A [13–15], and these findings may not be applicable to a similar setting where schools remain open. In addition, while the survey provides an estimated ILI attack rate, it is unknown how many of the ILI cases were actually due to pH1N1. Only one (14%) of the 7 nasal swabs obtained from recently ill patients was positive for the novel viral RNA. A follow-up serosurvey to evaluate for 2009 pH1N1 virus-specific antibody among ILI cases is planned to help address this issue. Finally, the population estimates derived from the raw data are dependent on statistical calibrations made to account for nonresponse. Since only 2000 Census estimates were available at the resolution required for these calculations, changes in community demographics such as age, race, or ethnicity may have impacted the population estimates and could explain some of the differences between the confirmed pH1N1 cases and ILI cases identified through the survey.
Together with electronic surveillance data and information on confirmed pH1N1 cases, the household survey provided a more complete picture of the incidence and epidemiology of ILI in this Chicago community. Trends in hospital ILI visits, influenza testing, and school absenteeism were each correlated with numbers of confirmed pH1N1 cases reported to the health department. Because of the lag between illness onset and the testing and reporting of a confirmed case, electronic surveillance data could be used prospectively by health departments as a timely way to track and predict short-term trends in expected pH1N1 cases. However, both electronic surveillance data and diagnosis of confirmed influenza cases are influenced by other factors such as public awareness, health care utilization patterns, and testing practices. Community-based surveys are a valuable tool to help assess the burden and epidemiology of ILI in a given area. Finally, similar evaluations should be considered as a model for assessing severity and community attack rates in the future.
Chicago H1N1 Epidemiology Team: John F. Beltrami, Kashmira A. Date, Miatta Dennis, Mary E. Fournier, Lauren Gallagher, Tracie J. Gardner, Susan L. Hills, Farah Husain, Martha Iwamoto, Surbhi V. Modi, Thai-An Nguyen, Philip M. Ricks, Tove K. Ryman, and Wendy C. Westbrooks.
Acknowledgments
We thank Julio Fernandez, Loretta Miller, Vanessa Smiley, Enrique Ramirez, Kingsley Weaver, Shamika Smith, William Wong, Juan Elias, and Phil Battaglia, Chicago Department of Public Health; Rose Wang, Meredith Deutscher, Ricardo Beato, Otilio Oyervides, Christopher Gregory, Joanna Regan, Anthony Fiore, and Lyn Finelli, Centers for Disease Control and Prevention; Elizabeth Weber, Children's Memorial Hospital, Chicago; Megan Patel, Cook County Department of Public Health; Ari Robicsek, Northshore University Health System; Charisse Coulombe, Resurrection Health Care, Chicago; John Nawrocki and Terry Oldfield, Illinois Department of Public Health, Division of Laboratories; Joseph Moore and Betsy Vandercook, City of Chicago; Chicago Public School System.
Supplement sponsorship. Published as part of a supplement entitled “The 2009 H1N1 Influenza Pandemic: Field and Epidemiologic Investigations,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Centers for Disease Control and Prevention. 2009 pandemic influenza A (H1N1) virus infections – Chicago, Illinois, April-July 2009. MMWR Morb Mortal Wkly Rep. 2009;58:913–8. [PubMed] [Google Scholar]
- 2.Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–4. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 3.Chicago Public Schools. Geographic information systems. Available at: http://schoollocator.cps.k12.il.us/. Accessed 15 September 2009. [Google Scholar]
- 4.Thomas DR, Rao JNK. Small-sample comparisons of level and power for simple goodness-of-fit statistics under cluster sampling. J Am Stat Assoc. 1987;82:630–6. [Google Scholar]
- 5.Särndal CE, Lundström S. West Sussex, United Kingdom: John Wiley & Sons, Ltd; 2005. Estimation in surveys with nonresponse. [Google Scholar]
- 6.World Health Organization. CDC protocol of realtime RTPCR for influenza A (H1N1) Available at: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Accessed 14 October 2009. [Google Scholar]
- 7.Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmaco Econ. 2008;26:911–24. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- 8.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Dawood FS, Jain S, Finelli L, et al. and the Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Swine-origin influenza A (H1N1) virus infections in a school: New York City, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1–3. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–4. [PubMed] [Google Scholar]
- 12.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 13.Cauchemez S, Valleron AJ, Boelle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452:750–4. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 14.Heymann AD, Hoch I, Valinsky L, Kokia E, Steinberg DM. School closure may be effective in reducing transmission of respiratory viruses in the community. Epidemiol Infect. 2009;137:1369–76. doi: 10.1017/S0950268809002556. [DOI] [PubMed] [Google Scholar]
- 15.Glass K, Barnes B. How much would closing schools reduce transmission during an influenza pandemic? Epidemiology. 2007;18:623–8. doi: 10.1097/EDE.0b013e31812713b4. [DOI] [PubMed] [Google Scholar]