A retrospective analysis was performed to determine the time to seroreversion in human immunodeficiency virus–exposed but uninfected infants and to examine possible correlates of this timing based on biological and clinical factors of both mother and infant.
Abstract
Background. Since the introduction of highly active antiretroviral therapy (HAART) for prevention of mother-to-child transmission of human immunodeficiency virus (HIV) in pregnancy in the United States, the time of seroreversion in infants born to HIV-infected mothers has not been documented. The objective of this study was to determine the timing of clearance of HIV antibodies and to identify any associated biological and clinical factors.
Methods. A retrospective analysis of infants who remained uninfected after perinatal HIV exposure was performed. Infant and maternal medical records from January 2000 to December 2007 were reviewed and the time of seroreversion was estimated using methods for censored survival data.
Results. In total, 744 infants were included in the study, with prenatal data available for 551 mothers. The median age of seroreversion was 13.9 months, and 14% of infants remained seropositive after 18 months, 4.3% after 21 months, and 1.2% after 24 months. Earlier age of seroreversion was associated with higher immunoglobulin G (IgG) levels at 3–7 months of age (P = .0029) and a higher rate of IgG change over the next 6 months of life (P = .003). Infants born by vaginal delivery were more likely to serorevert at a younger age (P = .0052), and maternal exposure to protease inhibitors was associated with a later age of seroreversion (P = .026).
Conclusions. Clearance of HIV antibodies in uninfected infants was found to occur at a later age than has been previously reported. Fourteen percent of the infants had persistence of HIV antibodies at or beyond 18 months of age.
Perinatal transmission of human immunodeficiency virus (HIV) has decreased to <2% in the United States as a result of multiple interventions, such as antiretroviral therapy (ART) during pregnancy, cesarean delivery for high maternal viral load, and exclusive formula feeding [1, 2]. In 2009, there were approximately 185 new cases of HIV infection in pediatric population <13 years of age; among them, 82% (152 cases) were attributed to the perinatal route of transmission in the United States [3].
Infants born to HIV-infected women have passive transfer of HIV antibodies that persist after birth and are detected by serologic tests; however, the definitive diagnosis of HIV infection in the infant is based on virologic tests to detect HIV DNA or RNA using polymerase chain reaction (PCR) assays. Infants in high-resource settings are tested repeatedly during the first 6 months of life to establish their HIV status and serologic testing is usually deferred until ≥12 months of age [4]. Current guidelines indicate that antibody testing should not be used for diagnosis in infants ≤18 months of age, suggesting that HIV antibodies may be detectable in HIV-exposed infants up to 18 months of age [4].
The timing of clearance of HIV antibodies in infants born in the United States has not been documented in the era of treatment of pregnant women undergoing highly active antiretroviral therapy (HAART). Most of the available data on time to seroreversion in HIV-exposed children have been described in European [5, 6], US [7–9], and African [10] studies on infants followed prior to 1995 and more recent figures have been derived from studies in Malawi [10] and Vietnam [11] in 2007 and 2009, respectively. The most current data for infants born and clinically followed in the United States are from 1995, prior to the introduction of HAART [7–9].
Studies on the decay of maternally derived antibodies to HIV indicate that most children serorevert by 12 months of age [6, 12–14]. However, the median time to clearance of maternal antibody, measured by enzyme-linked immunosorbent assay (ELISA) has varied in different studies, for example, 9.4 months [7], 11.6 months [8], and 13 months [15], and a small proportion of uninfected children were found to remain HIV antibody positive at 15 months [13] or 18 months [6].
The maximal age reported by the Centers for Disease Control and Prevention (CDC) at which detection of serum HIV antibody is indicative of HIV infection has changed over the years. In 1987, CDC guidelines stated that persistence of HIV antibodies beyond 15 months of age was an indicator of HIV infection [16], but in 1994, the age limit for antibody detection was extended to 18 months [17]. This recommendation remains in the most recent CDC revised surveillance case definition for HIV infection in 2008 [18]; however, this contrasts with anecdotal observations that a small number of HIV perinatally exposed uninfected infants followed at the University of Miami did not lose maternal HIV antibodies until after 18 months of age.
The objective of this study was to describe the timing of seroreversion by ELISA test in a large population of HIV-exposed uninfected infants clinically followed at the University of Miami/Jackson Memorial Hospital Center in Miami, Florida, in the post-HAART era and to identify possible biological and clinical factors associated with the age of seroreversion.
MATERIALS AND METHODS
Study Population and Data Collection
A retrospective analysis of infants born to HIV-infected mothers and who remained uninfected after perinatal HIV exposure was performed. These infants were delivered at Jackson Memorial Hospital or other hospitals in Miami, Florida, with clinical follow-up at the University of Miami HIV Screening Clinic between 1 January 2000 and 31 December 2007. Infants included in the study were defined as uninfected using criteria published in 1998 by the CDC that require at least 2 negative HIV DNA or RNA virologic tests from separate specimens, both of which were obtained at >1 month and 1 of which was obtained at >4 months [4, 18]. In addition, at least 1 HIV ELISA test performed at a minimal age of 12 months was required for inclusion in the study. Any infant not meeting the above criteria was excluded from the analysis.
Infant demographic, laboratory, and HIV ELISA results were obtained from infant medical records. Maternal demographic, clinical, and laboratory data were gathered from the HIV perinatal database maintained at the University of Miami/Jackson Memorial Medical Center for women who received prenatal care in this center. Data for women who received prenatal care or delivered in other facilities were not included.
Determination of HIV Status
Diagnostic HIV testing for infants was initiated within 48 hours of birth prior to hospital discharge using an HIV DNA PCR assay. Infants with a negative PCR result at birth were retested at 2 weeks, 4−6 weeks, and 4–6 months of age. The infants received 6 weeks of zidovudine prophylaxis followed by trimethoprim/sulfamethoxazole according to the guidelines for the use of antiretroviral agents in pediatric HIV infection [4]. HIV ELISA testing was initiated between 10 and 12 months of age for the majority of the infants; subsequently, ELISA tests were repeated at 15 months of age with sequential tests performed at 1-month intervals until negative results were attained or until the last positive ELISA test was recorded. Seroreversion was defined as documentation of a negative HIV ELISA test that was performed using the Vironostika HIV-1 Plus O Microelisa System (bioMérieux, Durham, North Carolina), whose reported sensitivity and specificity were 99.57% and 99%–100%, respectively.
HIV ELISA test results, CD4 lymphocyte counts, and immunoglobulin G (IgG) levels were obtained according to standard protocol followed by the Pediatric HIV Screening Clinic at the University of Miami (Supplementary Table 1).
Analysis
To estimate the survival function (ie, time to event) for age of seroreversion, statistical methods that accounted for data censoring were used. Survival curves were calculated using both nonparametric Kaplan-Meier product limit survival estimates and parametric methods designed for left-censored (infants who seroreverted by their first ELISA test), interval-censored (infants who had ≥1 positive ELISA test with an eventual negative test), and right-censored (infants who had repeated positive ELISA tests with no eventual negative test) data. Subsequent to model screening, the log-normal parametric survival model was chosen to compare survival functions across risk factors. The log-normal model proved to be the best fit for the observed Kaplan-Meier data as indicated by several goodness-of-fit statistics including the Bayesian and Akaike information criteria.
Infant variables used in the analysis of possible associations with time to seroreversion included ethnicity, mode of delivery, birth weight, gestational age, CD4 lymphocyte count, IgG levels, and IgG rate of change. Maternal risk factors included age at delivery, mode of delivery, clinical stage of HIV/AIDS, the first and last viral load during pregnancy, last CD4 count before delivery, antenatal maternal antiretroviral exposure (categorized as monotherapy, combination therapy with protease inhibitor, or combination therapy without protease inhibitor), gestational age at the start of ART, and duration of rupture of membranes.
The estimation of the rate of change of IgG level was based on the difference between the infant's first and last IgG values divided by the interval of time between both measurements. IgG values obtained at 3–7 months of age and the rate of change over the next 6 months were divided into 3 quantiles corresponding to the following: ≤Q1, lowest values; Q1–Q3, medium values; and ≥Q3, highest values.
Human Subjects Protection
The Institutional Review Board at the University of Miami approved the study protocol.
RESULTS
Study Population
Of the 1095 infants screened between January 2000 and December 2007, 744 (67.9%) met the inclusion criteria for the study and 351 were excluded (Supplementary Figure 1). Among the 351 excluded infants, 188 (53.6%) were found to be HIV uninfected by HIV DNA PCR testing but did not have an ELISA test done, and 163(46.4%) infants did not meet virologic criteria for exclusion of HIV infection. Most of the infants belonged to minority groups: black, non-Hispanic (70%), and Hispanic (15.7%); the majority of infants were born at term (80%). Furthermore, 60% were born by cesarean delivery and 85% of the mothers received zidovudine during labor. All the infants received zidovudine prophylaxis and none reported breastfeeding (Table 1). Data were available for 551 mothers of infants included in the study.
Table 1.
Characteristics of the Cohort of HIV-Exposed Uninfected Infants in Miami, Florida
| Characteristics | No.a | Percentage |
|---|---|---|
| Sex | ||
| Male | 400 | 54.20 |
| Female | 338 | 45.80 |
| Race/Ethnicity | ||
| Black, non-Hispanic | 519 | 69.70 |
| White, non-Hispanic | 27 | 3.60 |
| Hispanic | 117 | 15.70 |
| Unknown | 81 | 10.80 |
| Birth weight, g | ||
| <1500 | 17 | 2.37 |
| 1500–<2500 | 105 | 14.66 |
| 2500–<3500 | 467 | 65.22 |
| >3500 | 127 | 17.74 |
| Gestational age | ||
| <32 weeks | 23 | 3.20 |
| 32–<37 weeks | 119 | 16.55 |
| 37–42 weeks | 577 | 80.25 |
| Mode of delivery | ||
| Vaginal | 289 | 39.53 |
| Cesarean | 443 | 60.47 |
| Intrapartum zidovudine | ||
| Nob | 103 | 14.05 |
| Yes | 630 | 85.95 |
| Postpartum zidovudine prophylaxis | ||
| Yes | 744 | 100 |
| Breastfeeding | ||
| No | 744 | 100 |
a Infant data were not available for some variables.
b Majority had no prenatal care, presented in rapid labor, or were delivered outside our facility.
Seroreversion
Antibody loss occurred by their first ELISA test in 273 (36.7%) infants (left censored), during an interval between last positive and first negative test in 407 (54.7%) infants (interval censored) and after the last positive test in 64 (8.6%) infants (right censored).
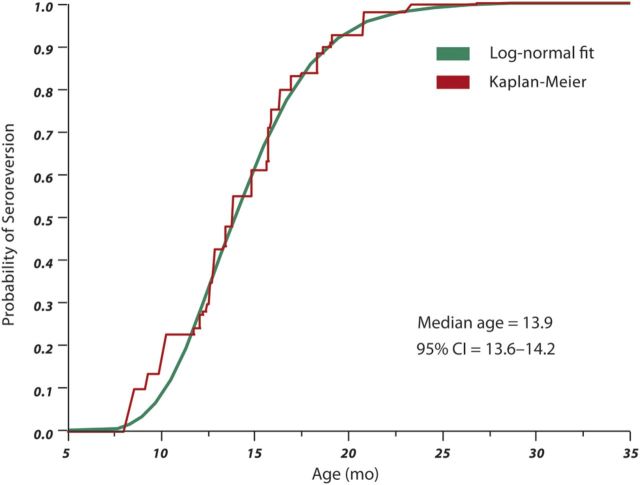
The Kaplan-Meier curve and the parametric log-normal fit for age of seroreversion are shown in Figure 1. It is based on the data from 744 infants and displays the pattern of ELISA seroreversion at a specific age, with the exception of <12 months of age due to the absence of ELISA tests early during the screening process. This analysis allowed the estimation of the probability of seroreversion (P.S.) at a given age as follows:
Figure 1.
Survival estimate of the probability of seroreversion by age, based on 744 infants. Abbreviation: CI, confidence interval.
Table 2 shows the distribution of seroreversion at a certain age by quantiles. The median age of seroreversion was 13.9 months (95% confidence interval [CI], 13.6–14.2). Twenty-five percent of HIV-exposed uninfected infants seroreverted by 12 months (95% CI, 11.45–12.5) and 75% of the subjects seroreverted by 16.4 months (95% CI, 16.5–16.7). Among infants with early seroreversion, 2.5% lost antibodies by 8.7 months, whereas for those with later seroreversion, 14% remained seropositive after 18 months, 4.3% after 21 months, and 1.2% after 24 months of age.
Table 2.
Distribution of Probability of Seroreversion at a Certain Age, by Quantile
| Age (Months) | Probability of Seroreversiona | 95% CI Age ± | |
|---|---|---|---|
| 8.7 | .025 | .83 | |
| 9.4 | .05 | .65 | |
| 10.2 | .10 | .50 | |
| 11.8 | Quartile | .25 | .35 |
| 13.9 | Median | .50 | .30 |
| 16.4 | Quartile | .75 | .30 |
| 18.9 | .90 | .35 | |
| 20.6 | .95 | .37 | |
| 22.3 | .975 | .38 |
Abbreviation: CI, confidence interval.
a Probability of seroreversion = Negative infinity to z cumulative area under the normal curve [(Loge Age − 2.632)/0.24].
Factors Associated With Age of Seroreversion
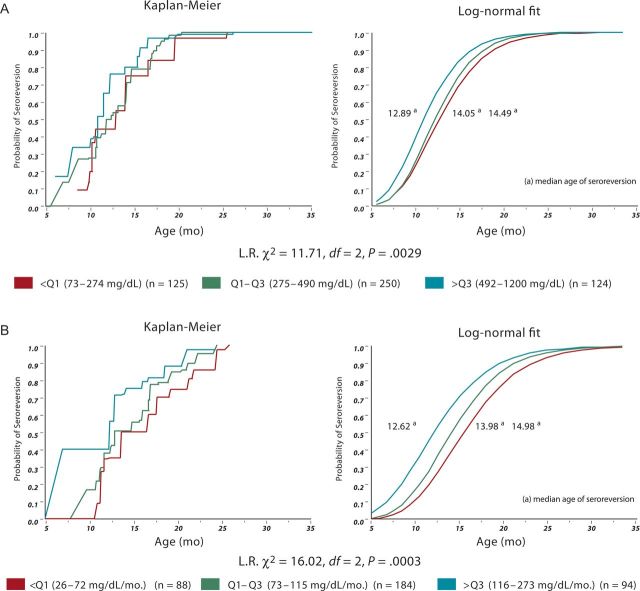
As shown in Figure 2, the age of seroreversion was found to be associated with the IgG value (P = .0029) and IgG rate of change (P = .003). The higher the IgG values were, the earlier the clearance of antibodies, as shown by the median time of seroreversion of 12.89 months for the group with the highest IgG values (≥Q3), 14.05 months for the group with medium values (Q1–Q2), and 14.49 months for the group with the lowest values (≤Q1). Additionally, the higher the IgG rate of change, the earlier the age of antibody loss, as shown by the median ages of seroreversion of 12.62, 13.98, and 14.98 months, corresponding to the highest, medium, and lowest rates of change, respectively. Furthermore, 732 subjects in our cohort showed a positive rate of change (ie, increase) of IgG level over the 6 months after the first measure.
Figure 2.
A, Survival curve of immunoglobulin G (IgG) level at 3–7 months of age and age of seroreversion. B, Survival curves of IgG rate of change and age of seroreversion. Abbreviation: L.R., likelihood ratio.
Statistically significant associations between the age of seroreversion and infant as well as maternal variables were found for the type of delivery, maternal antiretroviral regimen, first maternal viral load during pregnancy, and last maternal CD4 count before delivery (Table 3).
Table 3.
Correlation of Age of Seroreversion With Infant and Maternal Variables
| Age of Seroreversion, Median mo | P Value | |
|---|---|---|
| Infant Variables | ||
| Ethnicity | .0915 | |
| Non-Hispanic | 14.04 | |
| Hispanic | 14.65 | |
| Race | .2509 | |
| Black | 14.04 | |
| White | 14.50 | |
| Gestational age | .5504 | |
| <37 weeks | 13.59 | |
| ≥37 weeks | 13.28 | |
| Type of delivery | .0052 | |
| Vaginal | 13.43 | |
| Cesarean | 14.23 | |
| Rupture of membranes | .4636 | |
| <4 hours | 13.34 | |
| ≥4 hours | 14.36 | |
| CD4 lymphocyte count | .4259 | |
| Maternal variables | ||
| Age at delivery | .0836 | |
| 12–17 years | 12.10 | |
| 18–34 years | 13.97 | |
| >34 years | 14.35 | |
| HIV stage | .4917 | |
| HIV | 13.84 | |
| AIDS | 14.10 | |
| Maternal ART | .0263 | |
| ART + PI | 14.42 | |
| ART − PI | 13.63 | |
| First viral load during pregnancy | .0209 | |
| <1000 copies/mL | 13.62 | |
| 1000–49 999 copies/mL | 14.29 | |
| ≥50 000 copies/mL | 13.33 | |
| Last CD4 count before delivery | .0108 | |
| <200 cells/mm3 | 13.50 | |
| 200–500 cells/mm3 | 14.46 | |
| >500 cells/mm3 | 13.38 | |
| Zidovudine during labor | .7954 | |
| Yes | 13.89 | |
| No | 14.06 | |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PI, protease inhibitor.
Infants born by vaginal delivery were more likely to serorevert at a younger age (13.43 vs 14.23 months; P = .0052); conversely, maternal exposure to protease inhibitors was associated with later seroreversion (14.42 vs 13.63 months; P = .026). When the first maternal viral load and last maternal CD4 count were analyzed, an unordered effect was observed for both variables, with earlier ages of seroreversion associated with lower viral loads (13.62 vs 14.29 vs 13.33 months; P = .02) and lower CD4 counts (13.5 vs 14.46 vs 13.38 months; P = .01).
DISCUSSION
This is the most recent and the largest single site study to determine the age of seroreversion in HIV exposed but uninfected infants born to HIV-infected women since the introduction of HAART for the prevention of mother-to-child HIV transmission in the United States.
Uninfected HIV-exposed children followed in our cohort demonstrated later ages of seroreversion when compared with prior reports in the literature, with a median age of antibody loss of 13.9 months compared with 9.4 [7], 10.3 [5], and 10.9 months [8] described in cohorts followed during the late 1980s and early 1990s, when HIV-infected women did not receive ART during pregnancy. In 2009, a Vietnamese cohort presented a median age of seroreversion of 18.3 months but apparently this study was limited by the low specificity of the ELISA assay [11].
Our study showed a greater proportion of infants seroreverting at later ages. Seroreversion was 86% at 18 months and 95.7% at 21 months. These represent later ages of seroreversion when compared with the findings in Malawi with seroreversion of 98% at 18 months and 98.2% at 21 months of age for the period between 2000 and 2003 [10]. This lower percentage of seroreversion at 18 and 21 months described in our cohort might be explained by the fact that pregnant women who received HAART antenatally have a higher degree of HIV immunity, thus promoting a higher HIV-specific IgG response, subsequent higher HIV antibody transfer to their infants, and delayed clearance of antibodies.
Several factors may have an effect on the time of seroreversion. In our study, higher levels of IgG and its accelerated rate of change were found to be associated with earlier antibody loss in infants that could theoretically be explained by an increased consumption of immunoglobulins possibly secondary to infections during the first 15 months of life. However, we did not collect information regarding history of infections requiring hospitalization or outpatient evaluation to support this hypothesis.
The mechanisms through which maternal- and infant-related factors affect clearance of HIV-specific immunoglobulins are not fully understood. Plausible theories include a possible link between earlier age of seroreversion and decrease of maternal hypergammaglobulinemia induced by ART [19–21]; conversely, another theory suggests that later age of seroreversion may be explained by maternal immune reconstitution induced by ART [10].
Overall, there is not a single theory able to explain how vaginal delivery, low maternal viral loads, and maternal exposure to protease inhibitors may be associated with the age of seroreversion. A Vietnamese study [11] found an association between maternal exposure to protease inhibitors and later age of seroreversion; to that end, it could be postulated that women entering pregnancy with high viral loads would have been prescribed protease inhibitors with subsequent greater reductions of peripartum viral load and later seroreversion on their infants. There were no other significant infant and maternal factors affecting the time of seroreversion in other studies [8, 10].
One limitation of our study is that given the observational nature of the data, it was not possible to obtain an exact time of seroreversion. Although accounted for by the statistical methodologies, ELISA tests were performed approximately every 3 months after 12 months of age and seroreversion may have occurred at any point during this 3-month interval with shorter seroreversion intervals at older ages since ELISA testing was performed on a more frequent basis after 18 months of age. Prospective studies in which the timing of each ELISA tests are fixed and at frequent intervals are required for more accurate estimation of seroreversion time. Additionally, there were only a limited number of infants measured prior to 12 months of age (10%); therefore, the lower end of the survival curve could not be estimated with the same precision as the higher end. Despite the limitations in the timing of the measurements, it should be noted that the statistical log-normal model represents an almost exact fit to the observed data. Maternal data were available for 75% of the infants with the remainder including mothers who did not receive prenatal care or had received care outside our facility. Although there were statistical differences in seroreversion times between risk factors, it is difficult to conclude if these findings are of clinical relevance given that, in most cases, time differences were <1 month when evaluating time of seroreversion against IgG value, IgG rate of change, vaginal delivery, and maternal exposure to protease inhibitors. Further replications of these associations are needed before their validity can be determined.
This study clearly demonstrates a delay in clearance of HIV antibodies in a cohort of infants born in the United States. Fourteen percent of nonbreastfed infants with no prior virologic evidence of HIV infection may have residual antibody at ≥18 months of age. Although the last CDC surveillance case definition for HIV infection requires a positive HIV antibody test result at age ≥18 months [18], HIV pediatric guidelines consider that on rare occasions, HIV-exposed infants may have residual antibody at 18 months of age and these may be late seroreverters [4]. The main concern is that persistence of a positive HIV ELISA antibody test may cause confusion among pediatricians, causing children to be misclassified as HIV infected. However, if the child is seroreverting, although the HIV ELISA test may still be positive, the Western blot test should show an indeterminate antibody pattern.
In our cohort, late seroreversion is more frequent than previously reported [4] (14% of infants seroreverting later than 18 months). These results suggest that HIV ELISA tests performed in perinatally exposed infants and children between 18 and 24 months of age may remain positive, but this does not necessarily indicate HIV infection in the child. Although indeed rare, there can be reasons to explain antibody persistence after standard virologic testing results excluded perinatal HIV infection, such as premastication of food, percutaneous injuries, receipt of transplanted tissues, or sexual abuse with persons infected with HIV in the household. However, none of the children who had HIV excluded by HIV DNA PCR testing during the first 6 months of life and had persistence of HIV antibody past 18 months of age were infected. This observation emphasizes the importance of repeating HIV DNA PCR testing or documenting disappearance of Western blot bands for children with long persistence of HIV antibodies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Maria Marin and Sunil Mathews for creating the infant database. We also thank Lunthita Duthely for providing the HIV perinatal database.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Reduction in perinatal transmission of HIV infection—United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–7. [PubMed] [Google Scholar]
- 2.Creek T, Sherman G, Nkengasong J. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197(3 suppl):S64–71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV surveillance report, 2010. Published March. 2012 Available at: http://www.cdc.gov/hiv/topics/surveillance/reports/ Accessed April 2012. [Google Scholar]
- 4.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 16 August 2010. Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf. Accessed on 10 June 2011. [Google Scholar]
- 5.European Collaborative Study. Mother-to-child transmission of HIV infection. Lancet. 1988;332:1039–43. [PubMed] [Google Scholar]
- 6.European Collaborative Study. Children born to women with HIV-1 infection: natural history and risk of transmission. Lancet. 1991;337:253–60. [PubMed] [Google Scholar]
- 7.Simpson BJ, Andiman WA. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention. Pediatrics. 1994;93:840–2. [PubMed] [Google Scholar]
- 8.Chantry CJ, Cooper ER, Pelton SI, et al. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J. 1995;14:382–7. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Van Dyke RB, Korber BT, Popek E, et al. The Ariel Project: a prospective cohort study of maternal-child transmission of human immunodeficiency virus type 1 in the era of maternal antiretroviral therapy. J Infect Dis. 1999;179:319–28. doi: 10.1086/314580. [DOI] [PubMed] [Google Scholar]
- 10.Gulia J, Kumwenda N, Li Q, Taha Taha E. HIV seroreversion time in HIV- 1-uninfected children born to HIV-1-infected mothers in Malawi. J Acquir Immune Defic Syndr. 2007;46:332–7. doi: 10.1097/QAI.0b013e3181576860. [DOI] [PubMed] [Google Scholar]
- 11.Sohn A, Chi T, Quoc L, et al. Failure of human immunodeficiency virus enzyme immunoassay to rule-out infection among polymerase chain reaction-negative Vietnamese infants at 12 months of age. Pediatr Infect Dis J. 2009;28:273–6. doi: 10.1097/INF.0b013e31818e03b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moodley D, Bobat RA, Coutsoudis A, et al. Predicting perinatal human immunodeficiency virus infection by antibody patterns. Pediatr Infect Dis J. 1995;14:850–2. doi: 10.1097/00006454-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Moodley D, Coovadia HM, Bobat RA, Madurai S, Sullivan JL. The relationship between maternal-infant antibody levels and vertical transmission of HIV-1 infection. J Trop Pediatr. 1997;43:75–9. doi: 10.1093/tropej/43.2.75. [DOI] [PubMed] [Google Scholar]
- 14.Sherman GG, Stevens WS, Stevens G, Galpin JS. Diagnosis of human immunodeficiency virus infection in perinatally exposed orphaned infants in a resource-poor setting. Pediatr Infect Dis J. 2000;19:1014–5. doi: 10.1097/00006454-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Alcantara K, Pereira G, Albuquerque M. Seroreversion in children born to HIV positive and AIDS mothers from Central West Brazil. Trans R Soc Trop Med Hyg. 2009;103:620–26. doi: 10.1016/j.trstmh.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Current trends classification system for human immunodeficiency virus (HIV) infection in children under 13 years of age. MMWR Morb Mortal Wkly Rep. 1987;36:225–30. 235–6. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43:1–10. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(RR-10):1–12. [PubMed] [Google Scholar]
- 19.Bunders M, Pembrey L, Kuijpers T, Newell ML. Evidence of impact of maternal HIV infection on immunoglobulin levels in HIV-exposed uninfected children. AIDS Res Hum Retroviruses. 2010;26:967–75. doi: 10.1089/aid.2009.0241. [DOI] [PubMed] [Google Scholar]
- 20.Morris L, Binley JM, Clas BA, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–45. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Italian Register for HIV Infection in Children. Combined antiretroviral therapy reduces hyperimmunoglobulinemia in HIV-1 infected children. AIDS. 2004;18:1423–8. doi: 10.1097/01.aids.0000125985.94527.b2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.