This study described the spatial distribution and trend of HIV/AIDS among older adults in China, and we tried to identify the clustering of infections and translocation of hotspots during the study period.
Keywords: HIV/AIDS, older adult, spatial analysis
Abstract
Background. Recent studies have indicated an increasing burden of human immunodeficiency virus (HIV)/AIDS among older adults.
Methods. All identified people living with HIV/AIDS (PLWHA) recorded through the Chinese HIV/AIDS CRS during 2005–2012 were included in the study, except for the cases that lacked specific spatial information. Trend tests and spatial analyses were conducted.
Results. Information about 73,521 PLWHA (aged ≥50 years) was collected during 2005–2012. Three provinces—Guangxi, Henan, and Yunnan—accounted for 54.4% of the identified cases during the study period. Compared with 2005, the ratio between residents and migrants among the study population decreased to 40.1% in 2012. The ratio of HIV-infected patients to AIDS patients and the ratio of males to females increased gradually among older infected adults. Results of spatial analysis indicate a clustered distribution of HIV/AIDS among older adults throughout the country. Hot spots were observed in 4 provinces (Guangxi, Henan, Yunnan, and Sichuan) and 1 municipality (Chongqing). A trend from central provinces toward southern provinces was also identified.
Conclusions. The number and proportion of HIV/AIDS among older adults have increased in recent years. The hot spots showed movement from central to southern China. A focused intervention strategy targeting the older PLWHA is urgently required in China.
The majority of epidemiologic research in the field of human immunodeficiency virus (HIV)/AIDS involves individuals aged 15–49 years [1]. However, burgeoning evidence suggests that a considerable burden of HIV/AIDS exists among older adults aged ≥50 years [2–4]. The number of people living with HIV/AIDS (PLWHA) who belong to this age group has been rising [3–6] because of increased survival that is the result of an improved and comprehensive medical system, including wider availability of antiretroviral therapy (ART) [3, 7, 8]. Nonetheless, this vulnerable population is seldom the target of HIV risk–reduction interventions, despite having a high propensity of acquiring HIV/AIDS [9–11].
According to the National Bureau of Statistics of China, the overall population approached 1.35 billion at the end of 2011 [12]. As the most populated country in the world, China is facing an emerging challenge of excessive HIV/AIDS among its elderly populace for reasons mentioned above. Some studies have reported a high risk of HIV/AIDS among older adults in China, and a potential upsurge of an HIV/AIDS epidemic in this age group has also been indicated [13, 14]. Moreover, because of residency system reforms in the 1980s, a large proportion of the elderly population has migrated away from their birthplaces [15]. The subsequent lack of social support might have had an influence on the risk of acquiring HIV/AIDS among the older generation [16–18].
There have been a few efforts to understand the HIV/AIDS epidemic among older adults in isolated geographical areas of China, and a nationwide picture regarding the distribution and trend of the HIV/AIDS burden in this age group is not available. The objectives of this study were to describe the epidemiological and spatial distribution of HIV/AIDS among older adults in China from 2005 to 2012.
MATERIALS AND METHODS
Data Collection
China established the HIV/AIDS case reporting system (CRS) in 1985. Every new infection identified at local hospitals or clinics run by the Chinese Centers for Disease Control and Prevention (CDC) are reported through this web-based system. The principles of HIV/AIDS diagnostics and national HIV/AIDS test technology regulation and criteria were used to detect AIDS patient and HIV infection, respectively [19]. In accordance with the prevailing system, demographic (age, gender, occupation, address, registered place of residency [Hukou], and similar information) and risk behavior–specific information were collected using standardized case report forms through interviews conducted in private. After the uniqueness of newly identified cases was ascertained, completed case reports were communicated to the National Center for AIDS/STD Control and Prevention (NCAIDS) for data quality monitoring and logic checks. To minimize the probability of duplication of reported cases, local CDC personnel conducted field-level inquiries, which consisted of checking ID numbers, names, and addresses when infected patients were being identified. NCAIDS staff then conducted a second level of database evaluation in order to identify mistakes in logic and duplication at the national level. Through this elaborate CRS procedure, we obtained information on PLWHA and their spatial distribution throughout the country. If duplicate records were detected, a single record was kept for each duplicated subject.
In our study, “HIV” referred to the presence of HIV infection at the time of reporting, “AIDS” referred to diagnosed AIDS patients, and “HIV/AIDS” implied either HIV infection or an AIDS case.
Data Management
All identified cases of HIV/AIDS captured through the CRS between 2005 and 2012 were included in our study, with the exception of those lacking spatial information. All personal identifiers were removed from the database before it was analyzed in order to protect participants’ privacy. To identify the current residential location of reported cases, corresponding national standard geocodes at the provincial and city levels were included in the analysis. In terms of age, PLWHA were categorized into older (aged ≥50 years) and younger (≤49 years) adult groups. Electronic maps were obtained from the China CDC. ArcGIS 10.1 software (ESRI Inc., Redlands, CA, USA) was used to create maps. SPSS21.0 software (IBM Inc., Armonk, NY, USA) was used to process and analyze the data.
Trend Analysis
The trends in demographic characteristics and disease types among people who were reported to be infected with HIV/AIDS during the study period were analyzed to identify any changes in the trends during the study period. Further, we compared the changing trends between younger (aged ≤49 years) and older age groups (aged ≥50 years). Relative distributions (using ratios) of demographic characteristics (eg, Hukou [resident, migrant], gender [male, female]) were assessed. In our study, “resident” referred to those with Hukou registration for residency; the remainder were termed “migrant.” The proportion of disease types (HIV infected [not progressing to AIDS when diagnosed]/AIDS patient [at the time of diagnosis]) across age groups (≤49 years/≥50 years) was calculated, while gender distribution among reported PLWHA as per Hukou status was also determined by cross-tabulation. The Cochran–Armitage test was used to analyze the changing trends (α = 0.05).
General Spatial Autocorrelation
In the present study, Moran's I was used to detect the presence of HIV/AIDS clustering among older adults under the assumption that provinces throughout the country did not differ from each other statistically in terms of the spatial distribution of infection; the Moran's I value was set between −1 and 1. If the Moran's I value was > 0 with a Z value > 1.96 or if the Moran's I value was < 0 with a Z value <−1.96, we concluded that the infection distribution was clustered. Otherwise, infection was considered to be distributed randomly or diversely [20].
Local Spatial Autocorrelation
Local spatial autocorrelation was initially hypothesized and developed in order to test for clustering of cases of rare diseases [21]. The local spatial autocorrelation is usually used to detect specific local clusters of cases without any preconception about their locations, making it possible to recognize clusters that may not have been identifiable using general spatial autocorrelation [21]. We used this statistic to identify the local (city) clusters in the present study. Local clusters with a Z value ≥1.96 were defined as hot spots, signifying high clustering of infections in those places. We implemented a local spatial autocorrelation method at the city level to detect the hot spots of infection among older adults.
Spatial Median Point
The spatial median point analysis was conducted following local spatial autocorrelation. The spatial site of the median point of hot spots was calculated using the weighted number of identified hot spots for HIV/AIDS and spatial matrix. Although the spatial median point could be considered an effective tool for observing spatial changes in the infection hot spots among older adults directly, it did not necessarily mean that the spatial site of the median point represented the key epidemic.
RESULTS
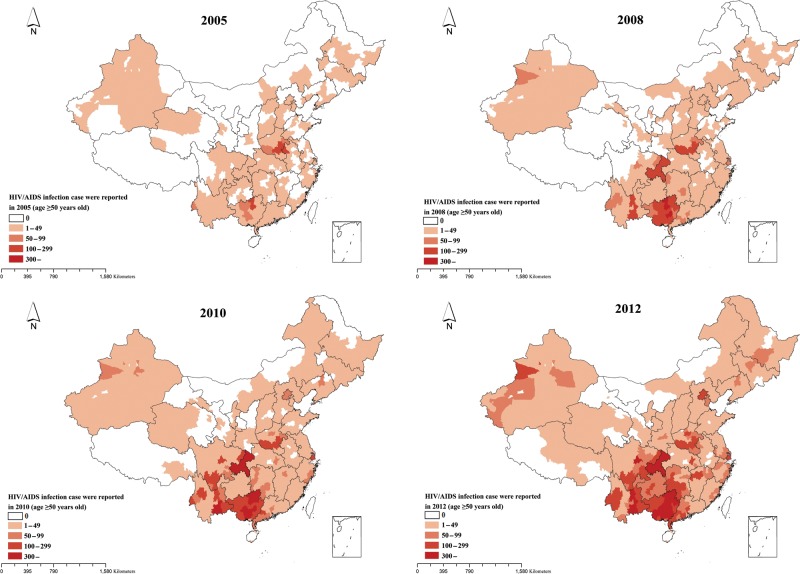
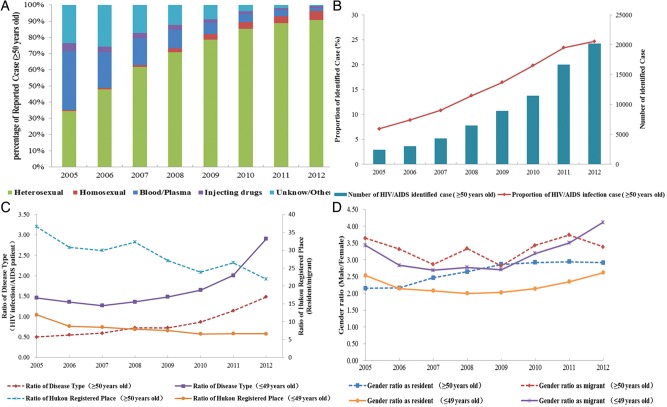
Between 2005 and 2012, 73 521 PLWHA belonging to the older age group (aged ≥50 years) and whose records included spatial information were reported. Of all identified cases in this age group, 0.79% of cases were excluded due to lack of specific spatial information at the city level. In terms of geographical distribution, identified PLWHA among older adults were found to be spread throughout China. In total, 204 cities reported PLWHA among older adults in 2005 and 316 cities reported PLWHA among older adults in 2012. Guangxi, Henan, and Yunnan provinces accounted for 54.4% of the identified cases (Figure 1). Among the identified cases, 73.7% were males, 3.75% were migrants, and 50.7% suffered from AIDS. The proportion of individuals infected through a sexual route gradually increased over time, whereas opposite trends were observed for bloodborne and injecting drug use–mediated transmissions (Figure 2A).
Figure 1.
Geographical distribution of the number of people living with human immunodeficiency virus/AIDS reported among older adults at the city level in the years 2005, 2008, 2010, and 2012 in China. Abbreviation: HIV, human immunodeficiency virus.
Figure 2.
A, Proportional distribution of transmission route of identified people living with human immunodeficiency virus (HIV)/AIDS (PLWHA) among older adults by year. B, Number and proportion of identified PLWHA among older adults by year. C, Ratio of disease type and Hukou status among younger and older HIV-infected cases. D, Gender distribution (male:female) among resident and migrant HIV-infected persons in younger and elderly age groups.
Changes in Age Distribution
The ratio of younger cases to older cases decreased over time from 13.06 in 2005 to 3.05 in 2012 (Z value = −103.92; P < .001; Figure 2B). Meanwhile, the ratio between resident and migrant cases showed a decreasing trend among both younger and older age groups, with a 40.10% decrease observed in the older group. Compared with the younger group, older adults had a significantly higher ratio between resident and migrant (Figure 2C). Although the ratio of disease types (HIV infection/AIDS patient) increased gradually over time in both age groups, older adults were more likely to be AIDS patient at the time of first diagnosis of their HIV infection. In the older age group, this ratio was 0.50 in 2005 and 1.48 in 2012, while in the younger group it was 1.46 in 2005 and 2.90 in 2012 (Figure 2C). The proportion of males in both resident and migrant groups also increased over time (Figure 2D). The results of the Cochran–Armitage trend test for groups are presented in Table 1.
Table 1.
Result of Cochran–Armitage Trend Test for 9 Groups of People Living with Human Immunodeficiency Virus/AIDS in China, 2005–2012
| Proportional Distribution of Variables | Ratio Between | Z Value | P Value |
|---|---|---|---|
| Number of identified people living with HIV/AIDS | (≤49 y):(≥50 y) | −103.92 | <.001 |
| Hukou status among those aged ≥50 y | Resident:Migrant | −6.11 | <.001 |
| Hukou status among those aged ≤49 y | Resident:Migrant | −28.23 | <.001 |
| Disease type among those aged ≥50 y | HIV infection:AIDS patient | 43.85 | <.001 |
| Disease type among those aged ≤49 y | HIV infection:AIDS patient | 64.59 | <.001 |
| Gender among residents aged ≥50 y | Male:Female | 9.35 | <.001 |
| Gender among residents aged ≤49 y | Male:Female | 0.80 | .42 |
| Gender among migrants aged ≥50 y | Male:Female | 9.34 | <.001 |
| Gender among migrants aged ≤49 y | Male:Female | 9.27 | <.001 |
Abbreviation: HIV, human immunodeficiency virus.
Spatial Analysis
We used general spatial autocorrelation with the distance matrix in order to determine the cumulative number of PLWHA belonging to the older adult group (Table 2). The Moran's I value obtained was 0.27 (P < .01). Further, general spatial autocorrelation tests were conducted for older adult PLWHA identified during each year of the study period. The results indicate the presence of HIV/AIDS epidemic clusters spread throughout the country among older adults. This clustering was revealed when we analyzed data from the entire study period, as well as cases reported each year.
Table 2.
Results of General Spatial Autocorrelation for Human Immunodeficiency Virus/AIDS Infection Among Old Adults in China
| Year | Moran's Index | Z Value | P Value |
|---|---|---|---|
| 2005 | 0.20 | 15.90 | <.001 |
| 2006 | 0.18 | 14.361 | <.001 |
| 2007 | 0.26 | 19.84 | <.001 |
| 2008 | 0.30 | 22.68 | <.001 |
| 2009 | 0.29 | 22.13 | <.001 |
| 2010 | 0.29 | 22.11 | <.001 |
| 2011 | 0.28 | 21.51 | <.001 |
| 2012 | 0.24 | 18.91 | <.001 |
| Accumulation (2005–2012) | 0.27 | 21.05 | <.001 |
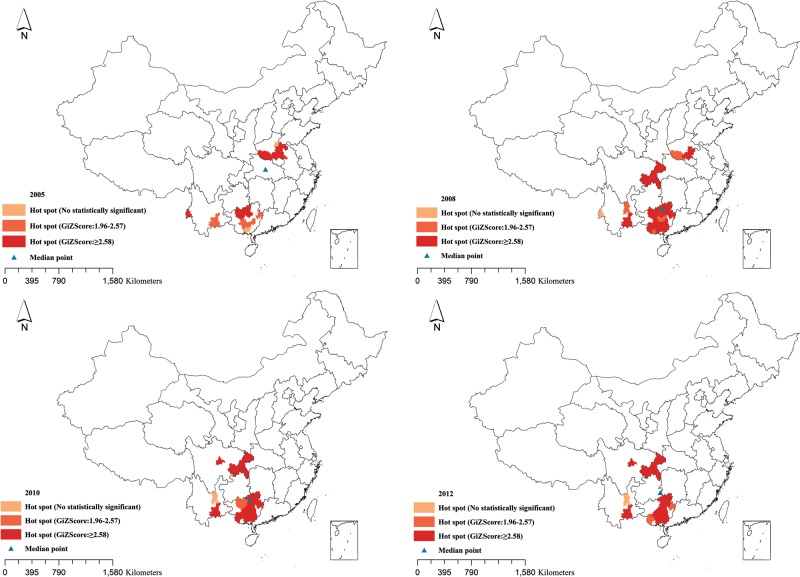
In order to determine the year-wise distribution of hot spots using the same matrix, we implemented local spatial autocorrelation. During the early years of our study, hot spots were primarily located in and around Henan province. Post-2009, the hot spots started spreading to Guangxi, Yunnan, and Sichuan provinces and Chongqing municipality.
The spatial median points of the identified cases are presented in Figure 3 and Supplementary material. The median points were located near the center of China in 2005 and started moving toward southern China during 2006–2007. Between 2008 and 2012, the median points were located mainly around the intersections of Guizhou, Guangxi, and Sichuan provinces.
Figure 3.
Hot spots and spatial median points of human immunodeficiency virus/AIDS infection among older adults at the city level in the years 2005, 2008, 2010, and 2012.
DISCUSSION
Our study revealed an upsurge in the HIV/AIDS epidemic among older adults in China, with a relative decrease in the number of younger cases over time (during 2005–2012) compared with older cases. Additionally, spatial analysis indicated the spread of infection hot spots to Guangxi, Henan, Yunnan, and Sichuan provinces and Chongqing municipality.
Older adults accounted for a growing share of identified PLWHA during 2005–2012. The total number of identified PLWHA also increased during this period. Expanded coverage of HIV antibody tests in China in recent years could explain this increase in the number of cases. However, the increasing proportion of older adults among all identified PLWHA suggests an increasing epidemic of HIV/AIDS among older adults in China. This increase may give rise to an enormous public health challenge in the near future, with a tremendous impact on the healthcare infrastructure and social structure [22].
The reasons for this upsurge in HIV/AIDS epidemic among older adults remain unclear. Previous reports have identified sexual route, both homosexual and heterosexual, as the main mode of transmission of HIV/AIDS among these older adults. In China, the sexual demands of older adults are often neglected by their partners as well as by the society, frequently leading to unsafe sexual practices. Unprotected sexual exposures remain very common among older adults, which in turn increases the risk of HIV/AIDS acquisition. Indirect evidence to support this mechanism of exposure is based on the increasing number of PLWHA who are older adult males compared with females among both residents and migrants.
Since the 1980s, following policy reforms, migrants were allowed to seek jobs and education and were entitled to receive public services at places other than their Hukou. As a consequence, a large portion of the population migrated to prosperous cities in search of better jobs and opportunities. Compared with nonmigrants, the migrants had a higher propensity to indulge in sexually risky behaviors [16], resulting in exacerbation of the HIV/AIDS epidemic among them [23]. In the present study, contrary to expectation, the proportion of PLWHA was detected to be higher among older adults residents than older adults migrants. There could be several reasons for this finding. Most of the HIV testing sites were located in urban areas where residents likely had better access compared with migrants, especially among older adults. Owing to poor educational status compared with residents, migrants might have lacked the necessary awareness to seek diagnostic services. Older migrants were likely to be less mobile and less motivated to get tested than younger migrants, and this could have further limited their access to HIV diagnostic services. However, we should also note that although mobility was higher among younger migrants, the difference in the ratio of younger PLWHA to older PLWHA decreased over time (from 24.6 to 15.3 during the study period), indicating an even greater increase in HIV/AIDS burden among older migrants.
The increase in the ratio of those infected with HIV only to those with AIDS among both younger and older age groups possibly indicates an increase in the number of earlier diagnoses of HIV infections. Expanded coverage of the HIV antibody testing program could be the principal reason for this finding. A larger proportion of males in the immigrant population probably skewed gender ratios toward males among migrants than among residents, which is consistent with findings from studies conducted in other countries [24–26].
From the perspective of geographical distribution, the spatial analysis indicated that PLWHA among older adults were mainly concentrated in and around central and southern China. The results of general spatial autocorrelation suggest the presence of infections clusters throughout the country during each year of the study. Moreover, the local spatial autocorrelation recognized Guangxi, Henan, Yunnan, and Sichuan provinces and Chongqing municipality as disease hot spots during the study period.
Unlike most other provinces in China,(such as Yunan, Xinjiang, Guangxi, and Guizhou, Sichuan, where intravenous drug use was initially the predominant route of HIV transmission, the HIV/AIDS epidemic in Henan province was initially more concentrated among paid-blood/plasma donors during the 1990s. Since implementation of a blood donation law and strengthening of blood supply management in 1998, 100% of blood products used in clinical settings in this province are now procured from voluntary donors, leading to greater control of the epidemic. However, most people who are infected through the bloodborne route were identified 10 years after implementation of this new testing policy. Also, after almost 20 years since becoming infected, the surviving PLWHA have aged considerably. This could explain why Henan turned into a hot spot between 2005 and 2008. In Yunnan, Guangxi, and Sichuan provinces, the HIV/AIDS epidemic followed the trafficking routes of illicit drugs [27]. Drug transportation was associated with many high-risk behaviors including injecting drugs and sharing needles among peddlers. However, with initiation of the National Methadone Maintenance Treatment Program and the Needle Exchange Program, which occurred around 2004 [28, 29], injecting drug use ceased to be the primary route of HIV/AIDS transmission in these areas, being replaced by sexual transmission. Additionally, a large number of medical and health institutions were established during this time, increasing the accessibility to HIV antibody testing for older adults. This improvement was relatively more marked in the southern region (eg, Sichuan, Guangxi, and Yunnan provinces), perhaps because this region was most affected by the early phases of the epidemic, in turn, imposing a higher burden of infected older survivors. Thus, improved access to testing among older HIV-infected adults in these provinces probably drove the concentration of hot spots from central China to the southern provinces during the study period.
Large sample size, efficient use of existing infrastructures that culminated in lower expenditures, access to records from a broad geographical region, and use of a geographic information system for identifying the epidemic hot spots were the important strengths of this study.
As with most record-based studies, our study suffered from several limitations. First, the number of identified PLWHA might have been affected by the intensity and coverage of HIV-antibody testing, the efficiency of policies and intervention strategies, and the study design. Available data did not allow us to control for these factors. Further, the unidentified cases (not detected or not reported) might have influenced the method of determination of hot spots in our study. However, we expect these drawbacks to be partially accounted for by the country-wide expansion and intensification of HIV/AIDS reporting system and sentinel surveillance system.
Even with these limitations, we can conclude that this study provides evidence of an upsurge in the HIV/AIDS epidemic among older adults. We also determined the presence of a trend in terms of geographical distribution of the epidemic. Focused attention, development of specialized interventional strategies, and implementation of prevention programs targeted toward older adults are urgently needed in China. Further research, including analysis of available data to explore the potential correlates of increased prevalence of HIV/AIDS and clustering of infection among older adults, can provide valuable insight into the HIV/AIDS epidemic in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors acknowledge the staff of the HIV/AIDS case reporting system in China and all related individuals. We give special thanks to Tanmay Mahapatra and Weiming Tang for revising this paper.
Financial support. This work was supported by the mega-projects of national science research under the 12th Five-Year Plan of China (2012ZX10001001).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chin J. Current and future dimensions of the HIV/AIDS pandemic in women and children. Lancet. 1990;336:221–4. doi: 10.1016/0140-6736(90)91743-t. [DOI] [PubMed] [Google Scholar]
- 2.Smith G. Aging hearing: HIV over fifty, exploring the new threat. Washington, DC: Senate Committee on Aging; 2006. [Google Scholar]
- 3.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88:847–53. doi: 10.2471/BLT.10.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longo B, Camoni L, Boros S, Suligoi B. Increasing proportion of AIDS diagnoses among older adults in Italy. AIDS Patient Care and STDs. 2008;22:365–71. doi: 10.1089/apc.2007.0168. [DOI] [PubMed] [Google Scholar]
- 5.Program and Policy Implication Prb. HIV/AIDS and older adults in the United States. USA: Today's Research on Aging; 2009. [Google Scholar]
- 6.HIV E. HIV/AIDS Surveillance in Europe, European Centre for Disease Prevention and Control, Sweden. 2005.
- 7.Greenbaum AH, Wilson LE, Keruly JC, Moore RD, Gebo KA. Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. AIDS. 2008;22:2331. doi: 10.1097/QAD.0b013e32831883f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogueras M, Navarro G, Antón E, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperman NA, Arnsten JH, Klein RS. Current sexual activity and risky sexual behavior in older men with or at risk for HIV infection. AIDS Educ Preven. 2007;19:321. doi: 10.1521/aeap.2007.19.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emlet CA. A comparison of HIV stigma and disclosure patterns between older and younger adults living with HIV/AIDS. AIDS Patient Care and STDs. 2006;20:350–8. doi: 10.1089/apc.2006.20.350. [DOI] [PubMed] [Google Scholar]
- 11.Crystal S, Akincigil A, Sambamoorthi U, et al. The diverse older HIV-positive population: a national profile of economic circumstances, social support, and quality of life. J Acquir Immune Defic Syndr. 2003;33:S76–83. [PubMed] [Google Scholar]
- 12.Zhang NJ, Guo M, Zheng X. China: Awakening giant developing solutions to population aging. Gerontologist. 2012;52:589–96. doi: 10.1093/geront/gns105. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Lin X, Xu Y, Chen S, Shi J, Morisky D. Emerging HIV epidemic among older adults in Nanning, China. AIDS Patient Care and STDs. 2012;26:565–7. doi: 10.1089/apc.2012.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Jia M, Ma Y, et al. The changing face of HIV in China. Nature. 2008;455:609–11. doi: 10.1038/455609a. [DOI] [PubMed] [Google Scholar]
- 15.Chan KW, Liu T, Yang Y. Hukou and non-hukou migrations in China: comparisons and contrasts. Int J Popul Geogr. 1999;5:425. doi: 10.1002/(SICI)1099-1220(199911/12)5:6<425::AID-IJPG158>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Hesketh T, Li L, Ye X, Wang H, Jiang M, Tomkins A. HIV and syphilis in migrant workers in eastern China. Sex Transm Infect. 2006;82:11–4. doi: 10.1136/sti.2004.014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Stanton B, Fang X, et al. HIV/STD risk behaviors and perceptions among rural-to-urban migrants in China. AIDS Educ Preven. 2004;16:538. doi: 10.1521/aeap.16.6.538.53787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong Y, Stanton B, Li X, et al. Rural-to-urban migrants and the HIV epidemic in China. AIDS Behav. 2006;10:421–30. doi: 10.1007/s10461-005-9039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Disease Control and Prevention. National HIV/AIDS test technology regulation and criteria. Chinese Journal of Drug Dependence. 2004;13:318. [Google Scholar]
- 20.Zhi-Hang P, Yue-Jia C, Reilly KH, et al. Spatial distribution of HIV/AIDS in Yunnan province, People's Republic of China. Geospatial Health. 2011;5:177–82. doi: 10.4081/gh.2011.169. [DOI] [PubMed] [Google Scholar]
- 21.Ord JK, Getis A. Local spatial autocorrelation statistics: distributional issues and an application. Geogr Anal. 1995;27:286–306. [Google Scholar]
- 22.Newsom JT, Schulz R. Social support as a mediator in the relation between functional status and quality of life in older adults. Psychol Aging. 1996;11:34. doi: 10.1037/0882-7974.11.1.34. [DOI] [PubMed] [Google Scholar]
- 23.Jia Z, Wang L, Chen RY, et al. Tracking the evolution of HIV/AIDS in China from 1989–2009 to inform future prevention and control efforts. PLoS One. 2011;6:e25671. doi: 10.1371/journal.pone.0025671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagarde E, Van Der Loeff MS, Enel C, et al. Mobility and the spread of human immunodeficiency virus into rural areas of West Africa. Int J Epidemiol. 2003;32:744–52. doi: 10.1093/ije/dyg111. [DOI] [PubMed] [Google Scholar]
- 25.Lurie MN, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis. 2003;30:149–56. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kane F, Alary M, Ndoye I, et al. Temporary expatriation is related to HIV-1 infection in rural Senegal. AIDS. 1993;7:1261–6. doi: 10.1097/00002030-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Beyrer C, Razak MH, Lisam K, Chen J, Lui W, Yu X-F. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14:75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin W, Hao Y, Sun X, et al. Scaling up the national methadone maintenance treatment program in China: achievements and challenges. Int J Epidemiol. 2010;39(Suppl 2):ii29–37. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.