Abstract
Background
The complications in healthcare systems associated with antibiotic-resistant microorganisms have resulted in an intense search for new effective antimicrobials. Attractive substances from which novel antibiotics may be developed are the bacteriocins. These naturally occurring peptides are generally considered to be safe and efficient at eliminating pathogenic bacteria. Among specific keystone pathogens in periodontitis, Porphyromonas gingivalis is considered to be the most important pathogen in the development and progression of chronic inflammatory disease. The aim of the present study was to investigate the antimicrobial effects of different Lactobacillus species and the two-peptide bacteriocin PLNC8 αβ on P. gingivalis.
Results
Growth inhibition of P. gingivalis was obtained by viable Lactobacillus and culture media from L. plantarum NC8 and 44048, but not L. brevis 30670. The two-peptide bacteriocin from L. plantarum NC8 (PLNC8 αβ) was found to be efficient against P. gingivalis through binding followed by permeabilization of the membranes, using Surface plasmon resonance analysis and DNA staining with Sytox Green. Liposomal systems were acquired to verify membrane permeabilization by PLNC8 αβ. The antimicrobial activity of PLNC8 αβ was found to be rapid (1 min) and visualized by TEM to cause cellular distortion through detachment of the outer membrane and bacterial lysis.
Conclusion
Soluble or immobilized PLNC8 αβ bacteriocins may be used to prevent P. gingivalis colonization and subsequent pathogenicity, and thus supplement the host immune system against invading pathogens associated with periodontitis.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-016-0810-8) contains supplementary material, which is available to authorized users.
Keywords: Periodontitis, P. gingivalis, Lactobacillus, Bacteriocin, PLNC8
Background
There is today an intense search for new antimicrobials with effective activity and with less possibility to induce antimicrobial resistance [1]. Attractive substances from which novel antibiotics may be developed are the bacteriocins, a group of bacterially produced compounds used to fight other bacteria [2]. Bacteriocins have a net positive charge and express amphipathic structures and kill microbes through interaction with negatively charged microbial membrane structures, such as Lipopolysaccharide and Lipoteichoic Acid. These mechanisms are more difficult to evade by developing resistance, compared to metabolic enzymes which usually are targets for conventional antibiotics.
Periodontitis is a common chronic inflammatory disease caused by an accumulation of different pathogenic biofilm-forming bacteria in dental pockets, leading to an exaggerated immune response that destructs periodontal ligament and causes alveolar bone loss. P. gingivalis is considered a keystone pathogen among these pathogenic bacteria and is well associated with the development of periodontitis. P. gingivalis is an anaerobic gram-negative, black-pigmented bacterium that has been widely associated as a putative organism in causing aggressive periodontitis and progression into systemic diseases [3]. The ability of P. gingivalis to invade host cells, including gingival epithelial cells [4] and heart and aortic endothelial cells [5] demonstrate a possible mechanism for its establishment and subsequent pathogenesis by evading the host immune system. P. gingivalis express multiple virulence factors (fimbriae, capsule, LPS, proteinases and toxic end-products) that contribute significantly to their pathogenicity by altering host immune responses [6]. However, P. gingivalis virulence is primarily associated with the elevated proteolytic activity of cysteine proteinases (reviewed in [7]) that are divided into arginine-specific (Rgp) and lysine-specific (Kgp) [8]. Besides the immunomodulatory effects of P. gingivalis, the bacterium is also able to form biofilms containing extracellular polymeric substances that provides antibiotic resistance and a niche for long-term survival within the host by evading host immune responses. These biofilms are difficult to treat and conventional methods are still being used to treat patient with periodontitis, including mechanical removal of hard and soft subgingival biofilms, scaling and chemical treatment to control plaque (metallic salts, antibiotics and phenols). These methods are less efficient since within a few hours new biofilms are formed that are prone to cause infections [9]. Furthermore, periodontitis has been associated with other systemic conditions, such as atherosclerosis, following identification of periodontal pathogens, including P. gingivalis, in atherosclerotic plaques [3, 10]. We have recently shown that P. gingivalis induces gene expression of angiopoietin 2 in aortic smooth muscle cells, which increases the migration of the cells that is associated with the pathogenesis of atherosclerosis [11].
The oral cavity harbours a wide variety of bacteria from the genus Lactobacillus that play an important role in the maintenance of a healthy balance of the oral ecosystem and are suggested to have protective effects in the pathogenesis of periodontal disease [12, 13]. However, many Lactobacillus species are outcompeted by gram-negative pathogens, including P. gingivalis, during disease progression. It has previously been reported that certain Lactobacillus species, including L. rhamnosus and L. salivarius, possess antimicrobial activity against periodontal pathogens, such as P. gingivalis [14]. Pangsomboon and colleagues [15] reported that bacteriocins from L. paracasei were able to kill P. gingivalis at a minimal concentration of 0.14 mM. Bacteriocins are divided into three classes depending on their characteristics. Class I (lantibiotics) are small peptides (<5 kDa) that contain unusual amino acids, lanthionine and three-methyllanthionine, introduced by post-translational modifications. Class II bacteriocins are synthesized in precursor forms that are processed after two glycine residues and display structural stability against proteolysis, heat and a wide range of pH. This group also includes bacteriocins composed of two different peptides that dimerize to form an active poration complex. Class III includes bacteriocins with large molecules that are sensitive to heat. Bacteriocins are antimicrobial compounds secreted by bacteria as part of their defence mechanism. Likewise, L. plantarum NC8 has previously been shown to produce a two-peptide bacteriocin, composed of PLNC8 α and β, which is classified as a class II bacteriocin. PLNC8 αβ are heat-stable and reported to possess antimicrobial activity towards gram-positive bacteria [16].
It is important to determine the anti-bacterial activity of Lactobacillus species and their bacteriocins, as an alternative method to antibiotic therapy, in preventing the colonization of P. gingivalis. We hypothesize that specific Lactobacillus species are able to suppress P. gingivalis growth, which is primarily due to expression and secretion of bacteriocins, and that these substances may be successful in the prevention of periodontitis. The aim of the present study was to elucidate the properties and antimicrobial effects of different Lactobacillus species and the two-peptide bacteriocin PLNC8 αβ on P. gingivalis.
Methods
Bacterial culture conditions
P. gingivalis wild type strains ATCC 33277 (ATCC, Manassas, VA) and W50, and the W50-derived Kgp proteinase and Rgp proteinase mutant strains (K1A and E8, respectively) were a kind gift from Dr. M.A. Curtis (Molecular Pathogenesis Group, Queen Mary, University of London). P. gingivalis strains were grown in anaerobic conditions (80 % N2, 10 % CO2, and 10 % H2) at 37 °C in an anaerobic chamber (Concept 400 Anaerobic Workstation; Ruskinn Technology Ltd., Leeds, United Kingdom). The bacterial concentration was adjusted to correlate with approximately 109 CFU/ml, which was determined by viable count by culturing the bacteria on fastidious anaerobe agar (45.7 g/l, pH 7.2, Acumedia, Neogen, Lansing, USA), supplemented with 5 % defibrinated horse blood for 5 days.
The Lactobacillus strains L. plantarum NC8, L. plantarum 44048 and L. brevis 30670 (Culture Collection, University of Gothenburg, Sweden) were grown on deMan Rogosa Sharp (MRS, BD Science) supplemented with agar (Difco) at 37 °C for 24 h in a jar containing an oxygen-free environment (Anaerobic pouch system EZ, BD Biosciences, CA, USA). Fresh cultures were used to inoculate MRS broth (Difco, BD Biosciences, CA, USA), and grown statically and anaerobically for 24 h at 37 °C. Lactobacillus were grown from a 0.5 % inoculum for 24 h at 37 °C under anaerobic and static growth conditions, and were then used for further experiments. The two-peptide bacteriocin from L. plantarum NC8, PLNC8 α and β, were purchased from GL Biochem (Shanghai) Ltd, China. Amino acid sequences for the peptides are:
PLNC8α- DLTTKLWSSWGYYLGKKARWNLKHPYVQF and
PLNC8β- SVPTSVYTLGIKILWSAYKHRKTIEKSFNKGFYH [16]
Liposome preparation
Liposomes were prepared according to methods that are well established in the field [17, 18]. Briefly, liposomes were prepared by dry film formation, hydration and finally extrusion through a polycarbonate membrane to form monodisperse large unilamellar vesicles. The lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) (Avanti Polar Lipids, Alabaster, USA) were mixed at molar ratios 1:99, 5:95 and 10:90 while dissolved in chloroform. A dry lipid film was formed by evaporation of the chloroform by nitrogen flow and overnight lyophilization. The film was hydrated with either 10 mM phosphate buffer (PB) pH 7 or 10 mM phosphate buffer saline (PBS) pH 7, and the solution was vortexed for 1 min and put on a shaker for 1 h before extruded 21 times through a 100 nm pore-sized polycarbonate membrane. For fluorescence leakage assay the lipid film was hydrated with buffer (PBS) containing self-quenching concentration (50 mM) of 5 (6)-carboxyfluorescein (CF) (Sigma Aldrich) and liposomes were prepared as described above. Removal of unencapsulated CF was done by gel filtration using a PD-25 column (GE Healthcare, Uppsala, Sweden) and liposomes with encapsulated CF were eluted with PBS.
Dynamic light scattering and Zeta potential
In order to mimic P. gingivalis membrane composition and potential, the hydrodynamic radius and zeta potential was measured on liposomes suspended in 10 mM PB pH 7 and suspensions containing microvesicles from P. gingivalis W50, using a Malvern ZetaSizer Nano S (Malvern Instruments Ltd, UK) and a disposable cuvette.
Carboxyfluorescein (CF) release assay
Leakage of the liposome encapsulated fluorophore CF due to additions of the bacteriocins was recorded using a fluorescence plate reader (Infinite 200, Tecan, Austria) where λex = 485 nm and λem = 520 nm. CF was encapsulated at self-quenching concentration, and CF release results in an increased fluorescence signal. Liposomes were diluted to 25 μM (total lipid concentration) in PBS, followed by additions of 0, 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1 and 2 μM of the peptides PLNC8 α and β, separately and combined. In order to estimate the maximum release from each sample, a final addition of 0.5 % Triton X-100 was made at the end of all measurements and the total amount of CF (100 % release) was estimated after 15 min incubation. The CF release is presented as percentage release for each time interval (measurements taken every minute). The percentage CF release is calculated as 100 × (F – F0)/(FT – F0) where F0 is the initial fluorescence intensity of CF before peptide addition, F is the fluorescence intensity of CF at time point t and FT is the maximum fluorescence after the addition of Triton X-100.
Circular Dichroism (CD) spectroscopy
Bacteriocins are often unstructured in solution but typically adopt a well-defined secondary structure when bound to the bacterial cell membrane as a result of membrane partitioning [19]. The secondary structure of the bacteriocins was investigated using CD spectroscopy. CD spectra were recorded using a Chirascan spectropolarimeter (Applied Photophysics, UK) and a 1 mm quartz cuvette at 20 °C with a sampling interval of 0.5 nm. All measurements were done in triplicates and averaged before converted to mean residue ellipticity (MRE) and curves smoothened using Savitzky-Golay algorithm.
Antimicrobial activity of Lactobacillus
The ability of different Lactobacillus strains to inhibit P. gingivalis growth was assessed on fastidious anaerobe agar plates. Briefly, different P. gingivalis strains (108 CFU in 100 μl) were spread onto fastidious anaerobe agar plates and allowed to dry. Lactobacillus were diluted in MRS broth and 10 μl drops (106 CFU) were placed onto the P. gingivalis layer. The plates were incubated for 4 days, after which images were acquired with Olympus SZX9 at 10× magnification and the zone of inhibition was measured using the software ImageJ.
Antimicrobial activity was also assessed using Lactobacillus culture media (MRS broth), in which different Lactobacillus strains were cultured for 24 h. The bacteria were removed by centrifugation at 7000 × g and the supernatants were sterile filtered (0.2 μm). The pH was measured and the supernatants were used to determine antimicrobial activity against P. gingivalis on fastidious anaerobe agar plates (10 μl drops), as mentioned above.
Antimicrobial activity of bacteriocin PLNC8 αβ
The antimicrobial activity of PLNC8 αβ on P. gingivalis was visualized using the fluorescent dye Sytox® Green, which can only cross damaged membranes and fluoresce upon binding to nucleic acids. P. gingivalis were washed and resuspended in Krebs-Ringer Glucose buffer (KRG) (120 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, 1.7 mM KH2PO4, 8.3 mM Na2HPO4, and 10 mM glucose, pH 7.3) and incubated in the presence or absence of PLNC8 αβ in 96-well microtiter plates. Images were captured with Olympus BX41 at 40× magnification.
Transmission electron microscopy (TEM) was used to visualize the damage of P. gingivalis, caused by PLNC8 αβ. Briefly, P. gingivalis ATCC 33277 were pelleted and washed with KRG. The bacteria were then treated with 280 nM of PLNC8 αβ in a molar ratio of 1:2 for 2 min and 10 min, followed by fixation in 2.5 % glutaraldehyde in 0.1 M phosphate buffer, pH 7.3. Specimens were washed in 0.1 M phosphate buffer, postfixed in 2 % osmium tetroxide in 0.1 M phosphate buffer for 2 h and embedded into LX-112 (Ladd, Burlington, Vermont, USA). Ultrathin sections (approximately 50-60 nm) were cut by a Leica ultracut UCT/ Leica EM UC 6 (Leica, Wien, Austria). Sections were contrasted with uranyl acetate followed by lead citrate and examined in a Hitachi HT 7700 (Tokyo, Japan). Digital images were taken by using a Veleta camera (Olympus Soft Imaging Solutions, GmbH, Münster, Germany).
SPR analysis
Surface plasmon resonance analysis was performed by immobilizing PLNC8 αβ peptides in 1:2 molar ratio onto a carboxymethylated dextran (CM-5 sensor chip, GE-Healthcare GmbH, Uppsala, Sweden) using biacore 2000 instrument equipped with four flow cells (GE-Healthcare GmbH, Uppsala, Sweden). Each channel of the chip was immobilized with 3 different concentrations of PLNC8 αβ (2.8, 28, 280 nM) respectively, and the fourth channel was used as a blank for negative reference subtraction between the channels. HBS-EP (0.01 M HEPES, pH7.4, 0.15 M NaCl, 3 mM EDTA, 0.005 % surfactant P20) (GE-Healthcare GmbH) was used as running buffer and the flow cell temperature was set to 25 °C in all experiments. Immobilization was a 3-step process performed using amine coupling kit (GE-Healthcare GmbH, Uppsala, Sweden) where the chip surface was activated using 200 mM N-ethyl-N0-(3 diethylaminopropyl) carbodimide (EDC) and 50 mM N-hydroxysuccinimide (NHS) mixture. PLNC8 αβ peptides diluted in acetate 4.5 buffer (GE-Healthcare GmbH, Uppsala, Sweden) were immobilized to the activated surface. Ethanolamine-HCl (pH 8.5) was used to deactivate the surface to enable an efficient binding of samples to the immobilized ligand. The contact time was 7 min, which resulted in immobilization levels between 1350 and 1750 response units (RU). One thousand RU corresponds to a surface peptide concentration of about 1 ng/mm2.
P. gingivalis were washed and prepared as described above after which the bacteria were pre-incubated with 2.8, 28 or 280 nM of PLNC8 αβ for 5 min at room temperature before analysis. P. gingivalis without PLNC8 αβ was used as positive control. The binding affinity of the bacteria to the peptides immobilized on each channel of the chip was measured in response units (RU). 1RU = 1 pg/mm2 using Bia-evaluation software [20].
Immobilization of surfaces with P. gingivalis-specific antibodies produced and tested as described in our previous studies [21], were used to study the binding affinity of P. gingivalis with and without PLNC8 αβ to the immobilized antibodies. Samples were prepared as described above and the responses obtained from Bia-evaluation software were plotted. The immobilization response for anti-P. gingivalis antibodies was 6400 RU.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). One-way ANOVA with Tukey’s multiple comparison test was used for the comparisons between the different treatments. P-values are referred to as *,#p < 0.05; **,##p < 0.01; ***,###p < 0.001.
Results
Lactobacillus spp. suppress P. gingivalis growth
Antimicrobial activity of different Lactobacillus strains (L. brevis 30670, L. plantarum 44048 and L. plantarum NC8) was assessed by co-culture with different P. gingivalis strains. L. brevis 30670 did not affect the growth of any of the P. gingivalis strains, while L. plantarum 44048 and NC8 could significantly inhibit all P. gingivalis strains (Fig. 1). Interestingly, P. gingivalis that lack Kgp proteinase activity was more susceptible to growth inhibition than the wild-type (WT) and the Rgp-deficient strain E8.
Fig. 1.
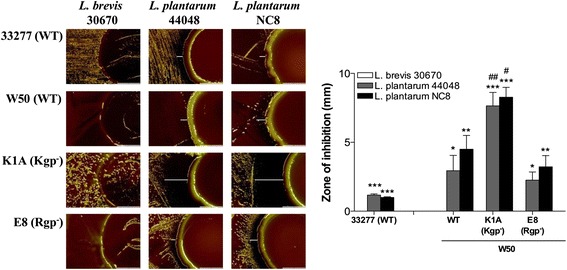
Lactobacillus suppresses P. gingivalis growth. Images were acquired after coculture of P. gingivalis with Lactobacillus for 4 days, using Olympus SZX9 at 10× magnification. The zone of inhibition was measured using ImageJ, n = 3. White lines indicate the inhibition zone. Prevention of P. gingivalis growth by Lactobacillus was shown to be species dependent. */#p < 0.05; **/##p < 0.01; ***/###p < 0.001, *- significance from L. brevis 30670 within each P. gingivalis strain, #- significance from WT W50 between the same Lactobacillus strain
Since Lactobacillus are able to lower the pH in their environment, we have investigated if the antimicrobial activity of lactic acid bacteria was due to acidic pH. The growth media from all three Lactobacillus strains were found to be acidic (pH 3.4-4.1). However, antimicrobial activity against P. gingivalis was only found in media derived from L. plantarum 44048 and L. plantarum NC8 (data not shown). This prompted us to determine if these effects are due to bacteriocins, since acidic pH was not a contributing factor for the observed antimicrobial activity.
PLNC8 α and β are two peptides produced by L. plantarum NC8 that form an active poration complex with antimicrobial activity. Thus, the effect of PLNC8 αβ on P. gingivalis viability was investigated by utilizing the fluorescent dye Sytox Green, which can only cross damaged membranes and fluoresce upon binding to nucleic acids. The uptake of Sytox Green by wild type P. gingivalis ATCC 33277 (Fig. 2a) and W50 (Fig. 2b) was significantly enhanced with increasing concentrations of PLNC8 αβ, which indicates a disordered integrity of P. gingivalis membranes. Interestingly, the effect of PLNC8 αβ bacteriocins was observed to be instant, as fluorescence was detected immediately after addition of PLNC8 αβ, reaching maximum intensity already after 1 min.
Fig. 2.
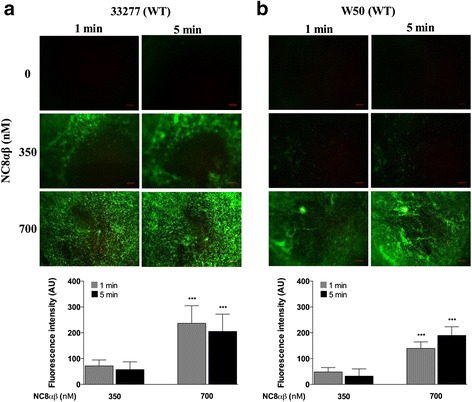
Bacteriocin PLNC8 αβ from L. plantarum NC8 is efficient against P. gingivalis. The antimicrobial activity of PLNC8 αβ on wild type (WT) P. gingivalis ATCC 33277 (a) and W50 (b), respectively, was visualized using the fluorescent dye Sytox® Green. Images were acquired using Olympus BX41 at 40× magnification. The antimicrobial effect of PLNC8 αβ was rapid and a significant number of P. gingivalis cells could fluoresce already after 1 min, indicating damaged membranes. Representative images and quantitative data of at least three independent experiments are shown. Quantitative data were normalized and the controls at each time point were set to 1. ***p < 0.001, *- significance from the control at each time point. Scale bar = 300 μm
Influence of PLNC8 αβ on model lipid membrane integrity
A carboxyfluorescein (CF) release assay was used in order to investigate the effect of the bacteriocins on the integrity of model lipid membranes. Extrusion of liposomes resulted in large unilamellar vesicles (LUVs) with a hydrodynamic diameter of about 123 nm and a zeta potential of -24 mV. The lipid composition (5:95 POPS: POPC) was chosen to mimic the zeta potential of microvesicles present in the supernatant retrieved cultures of P. gingivalis W50 (-24.8 mV) (Fig. 3).
Fig. 3.
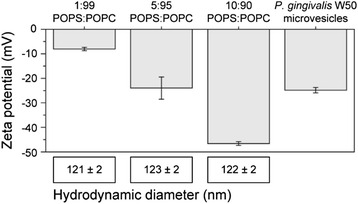
Zeta potential and size of liposomes and W50 microvesicles. The zeta potential and size of liposomes with different lipid composition was measured to identify the best match with microvesicles from P. gingivalis W50. All measurements are done in triplicates
The CF release was monitored over time using concentrations of bacteriocins ranging from 0.005 to 2 μM. The relative release after 30 min incubation with PLNC8 α, PLNC8 β and PLNC8 αβ (molar ratio 1:2) is shown in Fig. 4a. While CF release without addition of bacteriocins were < 1 %, a 30 min incubation with 2 μM PLNC8 α caused about 70 % release of CF and the same concentration of PLNC8 β induced almost complete release of the liposomal content (97.5 %). It is noteworthy that even for low concentrations of PLNC8 β the release of CF was high. As little as 0.05 μM (~0.002 peptide/lipid) PLNC8 β caused about 80 % CF release in 30 min. For PLNC8 αβ, the release kinetics and extent of release was similar to PLNC8 β alone for concentrations < 0.2 μM, whereas higher concentrations resulted in slightly higher release for PLNC8 αβ. The CF release assay thus indicates that both peptides bind to lipid membranes resulting in a significant reduction in membrane integrity. Interestingly, in model membranes the effect of PLNC8 β was comparable to the effect of PLNC8 αβ and the contribution from PLNC8 α thus appeared to be almost negligible. PLNC8 α alone did however cause significant leakage of CF albeit at a higher concentration than PLNC8 αβ. The release kinetics for PLNC8 α and β (Fig. 4b and c respectively) show that membrane disruption was almost immediate after exposure to the bacteriocins. Within the first couple of minutes, the CF release had already saturated. The maximum release increased with increasing concentration of bacteriocin. At lower concentrations a large fractions of liposomes were unaffected.
Fig. 4.
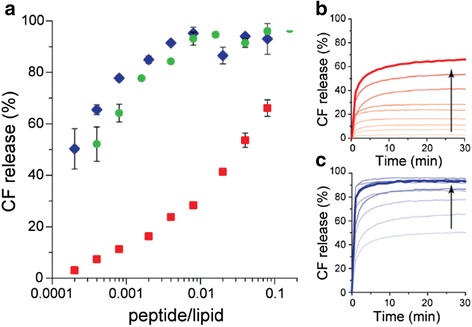
Dose- and time dependent release of CF in response to bacteriocin PLNC8 αβ. a Release of 5 (6)-carboxyfluorescein (CF) from liposomes (5:95 POPS: POPC) after 30 min with addition of PLNC8 α (red), β (blue) and αβ 1:2 (green). The interaction kinetics was recorded every minute for PLNC8 α (b) and PLNC8 β (c) with the liposomes and is displayed in increasing bacteriocin concentration (0.005–2 μM) indicated by the arrow. The total lipid concentration was kept constant at 25 μM. CF release without addition of bacteriocins were < 1 % and all data points are average of n = 3. The thick red and blue lines in B and C indicate the highest peptide concentration
Bacteriocin secondary structure
By using CD spectroscopy we found that PLNC8 α showed no signs of ordered secondary structure (i.e. random coil) in the absence of lipid membranes (Fig. 5). PLNC8 β, on the other hand, showed CD spectra indicative of α-helical secondary structure. In the presence of POPS:POPC (5:95) LUVs, both peptides underwent a structural transition, which was quite prominent for PLNC8 β that adopted a distinct β -sheet conformation. PLNC8 α demonstrated a shift in the CD spectrum indicating a certain amount of α-helical structure.
Fig. 5.
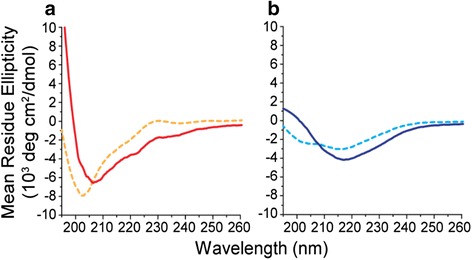
Secondary structure change of PLNC8 α and β. CD spectra of 100 μM PLNC8 α (a) and PLNC8 β (b) in solution (dashed lines) and with 1 mM (total lipid concentration) 5:95 POPS:POPC liposomes (solid lines) in 10 mM PB pH 7, 20 °C
The bacteriocin PLNC8 αβ binds to P. gingivalis
The antimicrobial activity of bacteriocins is dependent on binding to common epitopes on bacterial surfaces. Although binding of bacteriocins alone does not attribute for damage of bacterial membranes, we show that the antimicrobial effect of PLNC8 αβ on P. gingivalis is evident (Fig. 2). It is therefore important to determine if these effects are specific through binding of PLNC8 αβ to P. gingivalis. Both P. gingivalis ATCC 33277 (Fig. 6a) and W50 (Fig. 6b) were shown to bind to immobilized bacteriocin PLNC8 αβ (280 nM). Furthermore, pre-incubation of P. gingivalis with increasing concentrations of PLNC8 αβ caused a dose-dependent decrease in binding of the bacteria to the immobilized PLNC8 αβ peptides. Interestingly, pre-incubation of the bacteria with low concentrations of PLNC8 αβ (2.8 nM) were able to significantly decrease the binding, compared to untreated bacteria, indicating high specificity and binding affinity. Similar results were obtained with channels immobilized with 2.8, 28 and 2800 nM of PLNC8 αβ and with similar pre-treatments (data not shown).
Fig. 6.
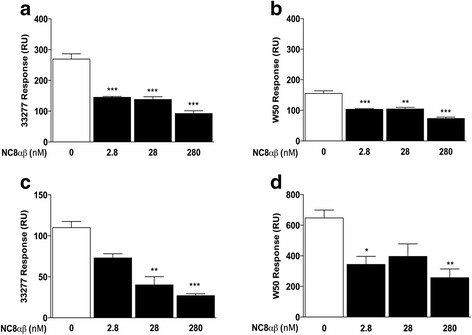
PLNC8 αβ binds to P. gingivalis in a dose-dependent manner. Binding of P. gingivalis to PLNC8 αβ and to anti- P.gingivalis antibodies was analyzed by SPR. Both P. gingivalis ATCC 33277 (a) and W50 (b) were found to bind to immobilized PLNC8 αβ (280 nM). The binding was verified by pre-incubating the bacteria with different concentrations of soluble PLNC8 αβ prior to analysis, which resulted in a significantly reduced binding to the immobilized bacteriocins. Pre-incubation of P. gingivalis ATCC 33277 (c) and W50 (d) with increasing concentrations of soluble PLNC8 αβ prior to analysis reduced the bacterial binding to anti-P. gingivalis antibodies in a dose-dependent manner. Results are presented from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
The binding of PLNC8 αβ to P. gingivalis was verified using channels immobilized with anti-P. gingivalis antibodies. Both ATCC 33277 (Fig. 6c) and W50 (Fig. 6d) bound to the immobilized antibodies. This binding was significantly reduced in a dose-dependent manner following pre-incubation of the bacteria with increasing concentrations of PLNC8 αβ. The results suggest that the damage caused by PLNC8 αβ bacteriocins alters the integrity of P. gingivalis membranes, resulting in modified epitopes with reduced binding to the antibodies, compared to untreated bacteria.
Rapid rupture of the outer membrane
The binding and antimicrobial effect of PLNC8 αβ on P. gingivalis is evident, which prompted us to visualize the nature of the damage caused by these peptides. P. gingivalis ATCC 33277 were either left untreated or treated with 280 nM of PLNC8 αβ for 2 and 10 min, followed by morphological studies using TEM. Untreated P. gingivalis cells showed typical coccobacillus morphology in which the outer and inner membrane are apparent and can be distinguished (Fig. 7). Interestingly, treatment of the bacteria with PLNC8 αβ for 2 min resulted in leakage of intracellular content, which may be due to pore formation. Furthermore, bacteriocin treatment caused detachment of the outer membrane. After 10 min of treatment, a large number of distorted and completely separated membranes were observed. These findings confirm that the effects of PLNC8 αβ on P. gingivalis are specific and instant.
Fig. 7.
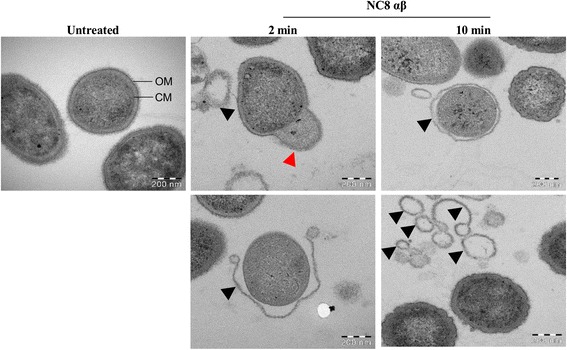
The bacteriocin PLNC8 αβ damages the membrane of P. gingivalis. Bacterial ultrastructure was examined using a Hitachi HT 7700 transmission electron microscope and showed typical coccobacillus shapes of untreated P. gingivalis, where the outer membrane (OM) and cytoplasmic membrane (CM) could be clearly distinguished. Treatment with PLNC8 αβ (1:2) efficiently damaged ccells by causing rupture of the bacterial membrane and leakage of intracellular content (red arrow head), eventually resulting in completely detached outer membrane (black arrow heads). Scale bar = 200 nm
Discussion
The overuse of antibiotics have increased the occurrence of complications in healthcare systems due to bacterial resistance, which in turn have resulted in an intense search for new and effective antimicrobials with less possibility to induce antimicrobial resistance. This study suggests that Lactobacillus and bacteriocin PLNC8 αβ can be used as an alternative method to antibiotic therapy in the prevention and treatment of infectious diseases, including periodontitis.
Lactobacillus species are part of the normal flora of humans, and have been reported to be important contributors to the balance of oral microflora by preventing pathogen colonization and thus maintaining a healthy state [22]. However, the mechanisms by which Lactobacillus interacts with periodontal pathogens and promotes health benefits are sparsely investigated. The oral cavity harbors a wide variety of Lactobacillus species, including L. gasseri, L. salivarius, L. brevis, L. plantarum and L. rhamnosus [22, 23]. Although the majority of these species are frequently found in the mouth, we show that they possess different specificity towards inhibition of P. gingivalis. While L. brevis 30670 was ineffective at inhibiting growth of the four P. gingivalis strains investigated in this study, both L. plantarum NC8 and 44048 were able to significantly suppress P. gingivalis growth. Furthermore, although inhibition zones correlate with the ability of an antimicrobial agent to diffuse through a semi-solid matrix, the Kgp-deficient P. gingivalis strain K1A was more susceptible to growth inhibition compared to its parent wild type strain W50 and Rgp-deficient strain E8, indicating an essential role for Kgp in P. gingivalis survival. The inhibition may possibly be due to secretion of active antimicrobial peptides, as the cell-free conditioning media from L. plantarum NC8 and 44048, but not L. brevis 30670, caused obvious inhibition, independent of the low pH.
L. plantarum NC8 has previously been reported to express a two-peptide bacteriocin, composed of PLNC8 α and β, in the presence of other bacteria [24, 25]. We show that these peptides are effective against P. gingivalis by damaging the membrane integrity and allowing the fluorescent dye Sytox Green to enter. By considering the amino acid sequences of PLNC8 αβ (Additional file 1: Figure S1), it is evident that lysine residues (PLNC8 α-4; PLNC8 β-5) are more abundant than arginine residues (PLNC8 α-1; PLNC8 β-1). It is therefore reasonable to suggest a role for the proteinase Kgp in cleaving PLNC8 α and β. This is supported by the fact that P. gingivalis lacking Kgp was more susceptible to growth inhibition by L. plantarum NC8, compared to WT W50. The proteinases Kgp and RgpA/B account for >80 % of the proteolytic activity of P. gingivalis [26]. Studies have established the potent enzymatic activity of cysteine proteinases through degradation of cytokines [27], epithelial junctional proteins [28] and immune receptors [29, 30]. We have recently shown that Kgp, but not Rgps, was associated with suppression of CXCL8 and IL-6 accumulation, induction of TGF-β1 and fibroblast cell viability [31]. The possible degradation of PLNC8 α and β by P. gingivalis-derived proteinases is currently under investigation.
Although bacteriocins are generally considered to target bacteria that are closely related to the bacteriocin-producing species, our results suggest that PLNC8 αβ may be used to suppress P. gingivalis growth and prevent its establishment. The reported narrow-spectrum activity of most bacteriocins [2] could suggest that their mechanism of action is primarily mediated through binding to specific receptors. Diep and colleagues [32] showed that the class II bacteriocin lactococcin A from Lactococcus lactis, binds to the membrane-located components IIC and IID of the mannose phosphotransferase system on target bacteria. Although P. gingivalis contains functional glucose/galactose transporters, these processes have mainly been associated with the LPS synthesis pathway [33]. Genetic analysis of P. gingivalis suggest that amino acids are the primary source for growth, while the uptake/metabolism of carbohydrates is limited [34]. Furthermore, plantaricin EF (two-peptide bacteriocin, subclass IIb) from L. plantarum C11 did not exploit the mannose phosphotransferase system to kill susceptible bacteria [32], suggesting that the two-peptide bacteriocins have a different mechanism of action. The calculated net charge for PLNC8 α and β at pH 7 is 4.1 and 5.2, respectively. These positively charged peptides may be attracted to the negatively charged components lipid A and phosphate groups of the LPS molecule, which initiates the initial interaction prior to pore formation. By incorporating LPS or lipid A into liposomes, to resemble the outer membrane of gram-negative bacteria, Matsuzaki et al. [35] were able to show that the antimicrobial peptide magainin was attracted to the negatively charged lipid A and formed a helix upon binding. This appears to be a common mechanism applied by a range of different naturally occurring and synthetic cationic antimicrobial peptides [36–38], and could thus be used to reduce LPS-induced inflammation and sepsis. Scott and colleagues [39] showed that neutralization of the inflammatory effects of LPS by human α-defensin-1, human β-defensin-2 and other synthetic peptides, was due to prevention of LPS interaction with the acute-phase LPS-binding protein.
Our studies with model membranes suggest that the bacteriocins may interact with lipid membranes without a specific target epitope. The interaction is most likely due to an initial electrostatic attraction between the cationic bacteriocins (Additional file 1: Figure S1) and the anionic lipid membrane, which is commonly seen in the mechanisms of many antimicrobial peptides [40]. It has previously been shown that P. gingivalis cells are highly negatively charged at pH > 3 [41]. An increased concentration of bacteriocins on the liposome surface will in turn cause an increase in permeability of the liposomes. This increased permeability is related to the structural changes that were observed when the bacteriocins interact with the liposome surface, likely due to partition-folding coupling due to their amphipathic properties (Additional file 1: Figure S1) [40]. The studies on the model membrane showed that both bacteriocins individually cause disruption of the membrane integrity, although the effect was more pronounced for PLNC8 β.
Efforts have been made to screen for and synthesize antimicrobial peptides against P. gingivalis, considering its role as a keystone pathogen in periodontitis. In a study where 236 lactic acid bacterial isolates from food were included, none of the tested strains was able to affect the growth of gram-negative periodontal pathogens, including P. gingivalis [42]. Antimicrobial agents from L. paracasei HL32 have been shown to suppress P. gingivalis growth [43, 44], and a short biosynthetic peptide, Pep-7, was produced and demonstrated to be selectively active against P. gingivalis [45]. We show that the antimicrobial effect of the two-peptide bacteriocin PLNC8 αβ occur rapidly and is very efficient, using liposomes and viable P. gingivalis.
Conclusions
In this study, we show that L. plantarum NC8 and 44048 are able to suppress P. gingivalis growth. Furthermore, bacteriocin PLNC8 αβ from L. plantarum NC8 are able to bind, at the nanomolar range, to P. gingivalis and cause cellular distortion through detachment of the outer membrane and bacterial lysis. Soluble or immobilized PLNC8 αβ bacteriocins may be used to prevent P. gingivalis colonization and pathogenicity, and thus supplement the host immune system against invading pathogens associated with periodontitis and other systemic inflammatory diseases, including atherosclerosis.
Acknowledgements
We would like to thank Professor M.A. Curtis, Molecular Pathogenesis Group, Queen Mary, University of London, for providing us with P. gingivalis WT W50 and the proteinase-deficient strains K1A and E8.
Funding
This work was supported by the Swedish Heart-Lung Foundation, the Foundation of Magnus Bergvall, the Foundation of Olle Engkvist and by the Knowledge Foundation, Sweden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated and analysed during this study are included in this published article [and its supplementary information files].
Authors’contributions
Study design: HK, DA, TB. Acquisition of data: HK, SSN, CS, AS, KH, NS. Analysis and interpretation: HK, SSN, CS, AS, KH, NS, DA, TB. Drafting and revising the manuscript: HK, SSN, CS, AS, KH, NS, DA, TB. Final approval to publish: HK, SSN, CS, AS, KH, NS, DA, TB. All authors read and approved the final manuscript.
Competing interests
The authors declares that they have no competing interests.
Consent for publication
No applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- CF
5 (6)-carboxyfluorescein
- EDC
N-ethyl-N0-(3 diethylaminopropyl) carbodimide
- Kgp
Lysine gingipain
- KRG
Krebs-ringer glucose buffer
- NHS
N-hydroxysuccinimide
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine
- POPS
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine
- Rgp
Arginine gingipain
- SPR
Surface plasmon resonance
- TEM
Transmission electron microscopy
Additional file
Hydropathy scores of PLNC8 α and β. The amino acid sequence of PLNC8 α (A) and PLNC8 β (B) and their corresponding hydropathy score [46]. (TIF 369 kb)
Contributor Information
Hazem Khalaf, Email: hazem.khalaf@oru.se.
Sravya Sowdamini Nakka, Email: sravya.nakka@oru.se.
Camilla Sandén, Email: camsa@ifm.liu.se.
Anna Svärd, Email: annsv796@student.liu.se.
Kjell Hultenby, Email: Kjell.Hultenby@ki.se.
Nikolai Scherbak, Email: nikolai.scherbak@oru.se.
Daniel Aili, Email: danai@ifm.liu.se.
Torbjörn Bengtsson, Email: torbjorn.bengtsson@oru.se.
References
- 1.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11(2):95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 3.Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, Madianos P, Sotres D, Chang YL, Koch G, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25(7):1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- 4.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63(10):3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66(11):5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer CL, Walters KS, Drake DR, Dawson DV, Blanchette DR, Brogden KA, Wertz PW. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int J Oral Sci. 2013;5(3):130–140. doi: 10.1038/ijos.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moelants EA, Loozen G, Mortier A, Martens E, Opdenakker G, Mizgalska D, Szmigielski B, Potempa J, Van Damme J, Teughels W, et al. Citrullination and proteolytic processing of chemokines by Porphyromonas gingivalis. Infect Immun. 2014;82(6):2511–2519. doi: 10.1128/IAI.01624-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuramitsu HK. Proteases of Porphyromonas gingivalis: what don’t they do? Oral Microbiol Immunol. 1998;13(5):263–270. doi: 10.1111/j.1399-302X.1998.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 9.Chandki R, Banthia P, Banthia R. Biofilms: a microbial home. J Indian Soc Periodontol. 2011;15(2):111–114. doi: 10.4103/0972-124X.84377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138(5 Pt 2):S534–536. doi: 10.1016/S0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Khalaf H, Sirsjo A, Bengtsson T: Gingipains from the periodontal pathogen Porphyromonas gingivalis play a significant role in regulation of Angiopoietin 1 and Angiopoietin 2 in human aortic smooth muscle cells. Infect Immun. 2015; IAI.00498-15. [DOI] [PMC free article] [PubMed]
- 12.Badet C, Thebaud NB. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. 2008;2:38–48. doi: 10.2174/1874285800802010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szkaradkiewicz AK, Karpinski TM, Zeidler A, Wyganowska-Swiatkowska M, Szkaradkiewicz A. Protective effect of oral Lactobacilli in pathogenesis of chronic periodontitis. J Physiol Pharmacol. 2011;62(6):685–689. [PubMed] [Google Scholar]
- 14.Prathibha RS. Comparing the reinforcing effects of a resin modified glassionomer cement, flowable compomer, and flowable composite in the restoration of calcium hydroxide-treated immature roots in vitro. Contemp Clin Dent. 2011;2(1):21–26. doi: 10.4103/0976-237X.79298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komiyama M, Ishiguro T, Terada A, Murakami Y. Transcardiac cerebral angiography in a child. J Neurosurg Pediatr. 2013;11(1):95–99. doi: 10.3171/2012.10.PEDS12170. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado A, Ruiz-Barba JL, Jimenez-Diaz R. Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Appl Environ Microbiol. 2003;69(1):383–389. doi: 10.1128/AEM.69.1.383-389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Methods in Enzymology, Liposomes, vol. 391. London: Elsevier Academic Press; 2005.
- 18.Lim SK, Sanden C, Selegard R, Liedberg B, Aili D. Tuning liposome membrane permeability by competitive peptide dimerization and partitioning-folding interactions regulated by proteolytic activity. Sci Rep. 2016;6:21123. doi: 10.1038/srep21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ennahar S, Sashihara T, Sonomoto K, Ishizaki A. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev. 2000;24(1):85–106. doi: 10.1111/j.1574-6976.2000.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 20.Stenberg E, Persson B, Roos H, Urbaniczky C. Quantitative-determination of surface concentration of protein with surface-plasmon resonance using radiolabeled proteins. J Colloid Interf Sci. 1991;143(2):513–526. doi: 10.1016/0021-9797(91)90284-F. [DOI] [Google Scholar]
- 21.Nakka SS, Lönn J, Johansson CS, Bengtsson T, Nayeri F: Antibodies produced in vitro in the detection of periodontal bacteria by using surface plasmon resonance analysis. Clinical and experimental dental research (in press) 2015. [DOI] [PMC free article] [PubMed]
- 22.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354–361. doi: 10.1111/j.1399-302X.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 23.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004;42(7):3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado A, Jimenez-Diaz R, Ruiz-Barba JL. Induction of plantaricin production in Lactobacillus plantarum NC8 after coculture with specific gram-positive bacteria is mediated by an autoinduction mechanism. J Bacteriol. 2004;186(5):1556–1564. doi: 10.1128/JB.186.5.1556-1564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado A, Ruiz-Barba JL, Jimenez-Diaz R. Production of plantaricin NC8 by Lactobacillus plantarum NC8 is induced in the presence of different types of gram-positive bacteria. Arch Microbiol. 2004;181(1):8–16. doi: 10.1007/s00203-003-0606-8. [DOI] [PubMed] [Google Scholar]
- 26.de Diego I, Veillard F, Sztukowska MN, Guevara T, Potempa B, Pomowski A, Huntington JA, Potempa J, Gomis-Ruth FX. Structure and mechanism of cysteine peptidase gingipain K (Kgp), a major virulence factor of Porphyromonas gingivalis in periodontitis. J Biol Chem. 2014;289(46):32291–32302. doi: 10.1074/jbc.M114.602052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. The host cytokine response to Porphyromonas gingivalis is modified by gingipains. Oral Microbiol Immunol. 2009;24(1):11–17. doi: 10.1111/j.1399-302X.2008.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz J, Yang QB, Zhang P, Potempa J, Travis J, Michalek SM, Balkovetz DF. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect Immun. 2002;70(5):2512–2518. doi: 10.1128/IAI.70.5.2512-2518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura Y, Matono S, Aida Y, Hirofuji T, Maeda K. Gingipains in the culture supernatant of Porphyromonas gingivalis cleave CD4 and CD8 on human T cells. J Periodontal Res. 2002;37(6):464–468. doi: 10.1034/j.1600-0765.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 30.Duncan L, Yoshioka M, Chandad F, Grenier D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb Pathog. 2004;36(6):319–325. doi: 10.1016/j.micpath.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson T, Khalaf A, Khalaf H. Secreted gingipains from Porphyromonas gingivalis colonies exert potent immunomodulatory effects on human gingival fibroblasts. Microbiol Res. 2015;178:18–26. doi: 10.1016/j.micres.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A. 2007;104(7):2384–2389. doi: 10.1073/pnas.0608775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazumdar V, Snitkin ES, Amar S, Segre D. Metabolic network model of a human oral pathogen. J Bacteriol. 2009;191(1):74–90. doi: 10.1128/JB.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, Daugherty SC, Dodson RJ, Durkin AS, Gwinn M, et al. Complete genome sequence of the oral pathogenic Bacterium porphyromonas gingivalis strain W83. J Bacteriol. 2003;185(18):5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzaki K, Sugishita K, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with lipopolysaccharide-containing liposomes as a model for outer membranes of gram-negative bacteria. FEBS Lett. 1999;449(2-3):221–224. doi: 10.1016/S0014-5793(99)00443-3. [DOI] [PubMed] [Google Scholar]
- 36.Gough M, Hancock RE, Kelly NM. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64(12):4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott MG, Yan H, Hancock RE. Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect Immun. 1999;67(4):2005–2009. doi: 10.1128/iai.67.4.2005-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63(4):1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164(2):549–553. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- 40.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 41.Cowan MM, van der Mei HC, Rouxhet PG, Busscher HJ. Physicochemical and structural investigation of the surfaces of some anaerobic subgingival bacteria. Appl Environ Microbiol. 1992;58(4):1326–1334. doi: 10.1128/aem.58.4.1326-1334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoumpopoulou G, Pepelassi E, Papaioannou W, Georgalaki M, Maragkoudakis PA, Tarantilis PA, Polissiou M, Tsakalidou E, Papadimitriou K. Incidence of bacteriocins produced by food-related lactic acid bacteria active towards oral pathogens. Int J Mol Sci. 2013;14(3):4640–4654. doi: 10.3390/ijms14034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pangsomboon K, Kaewnopparat S, Pitakpornpreecha T, Srichana T. Antibacterial activity of a bacteriocin from Lactobacillus paracasei HL32 against Porphyromonas gingivalis. Arch Oral Biol. 2006;51(9):784–793. doi: 10.1016/j.archoralbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Pangsomboon K, Bansal S, Martin GP, Suntinanalert P, Kaewnopparat S, Srichana T. Further characterization of a bacteriocin produced by Lactobacillus paracasei HL32. J Appl Microbiol. 2009;106(6):1928–1940. doi: 10.1111/j.1365-2672.2009.04146.x. [DOI] [PubMed] [Google Scholar]
- 45.Suwandecha T, Srichana T, Balekar N, Nakpheng T, Pangsomboon K. Novel antimicrobial peptide specifically active against Porphyromonas gingivalis. Arch Microbiol. 2015;197(7):899–909. doi: 10.1007/s00203-015-1126-z. [DOI] [PubMed] [Google Scholar]
- 46.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analysed during this study are included in this published article [and its supplementary information files].


