Key Points
IL-2/mAb complexes expand Tregs and treat established murine cGVHD, but concurrent T-effector cell expansion can reduce their therapeutic index.
Prophylactic Treg infusions can prevent cGVHD, whereas therapeutic efficacy requires homing to and inhibition of the GC reaction.
Abstract
Chronic graft-versus-host disease (cGVHD) is a major complication of allogeneic hematopoietic stem cell transplantation. In cGVHD, alloreactive T cells and germinal center (GC) B cells often participate in GC reactions to produce pathogenic antibodies. Although regulatory T cells (Tregs) can inhibit GC reactions, Treg numbers are reduced in cGVHD, contributing to cGVHD pathogenesis. Here, we explored 2 means to increase Tregs in cGVHD: interleukin-2/monoclonal antibody (IL-2/mAb) complexes and donor Treg infusions. IL-2/mAb complexes given over 1 month were efficacious in expanding Tregs and treating established cGVHD in a multi-organ-system disease mouse model characterized by GC reactions, antibody deposition, and lung dysfunction. In an acute GVHD (aGVHD) model, IL-2/mAb complexes given for only 4 days resulted in rapid mortality, indicating IL-2/mAb complexes can drive conventional T-cell (Tcon)-mediated injury. In contrast, Treg infusions, which uniformly suppress aGVHD, increased Treg frequency and were effective in preventing the onset of, and treating, established cGVHD. Efficacy was dependent upon CXCR5-sufficient Tregs homing to, and inhibiting, GC reactions. These studies indicate that the infusion of Tregs, especially ones enriched for GC homing, may be desirable for cGVHD therapy. Although IL-2/mAb complexes can be efficacious in cGVHD, a cautious approach needs to be taken in settings in which aGVHD elements, and associated Tcon, are present.
Introduction
Chronic graft-versus-host disease (cGVHD) is the primary cause of long-term morbidity and mortality after allogeneic hematopoietic stem cell transplantation.1 The germinal center (GC) reaction between T-follicular helper cells (Tfh) and GC B cells plays a critical role in cGVHD pathogenesis, and inhibition of this reaction significantly reduces cGHVD in mouse models.2,3 A specialized subset of CD4+Foxp3+ regulatory cells (Tregs), T-follicular regulatory cells (Tfr), migrates to lymphoid follicles where they help quell GC reactions.4 However, Treg frequency is reduced in cGVHD patients,5 and this may contribute to cGVHD pathogenesis.2,6 Low-dose interleukin-2 (IL-2) therapy increases Tregs in some cGVHD patients, but does not always reverse all symptoms, and long-term dosing is required to maintain efficacy.7,8
IL-2 complexed with the JES6-1 clone of anti-IL-2 antibody (IL-2/monoclonal antibody [mAb] complexes) has a longer in vivo half-life compared with IL-2 alone.9 These complexes preferentially bind to CD25hi cells, which results in Treg expansion in a variety of disease models.9-11 As a result, IL-2/mAb complexes may be superior to IL-2 for Treg expansion in cGVHD. Treg infusions also increase Treg numbers, and, unlike IL-2-based therapies, only a single dose may be required.12,13 Prophylactic Treg infusions appear to reduce acute GVHD (aGVHD),14 but the efficacy of therapeutic Treg infusions in cGVHD has not yet been fully assessed.15 In this study, we analyzed the therapeutic efficacy of IL-2/mAb complexes and Treg infusions for preventing and treating cGVHD.
Study design
Mice and transplantation
C57BL/6 (B6) (Charles River), B10.BR, and B6-CXCR5−/− (Jackson Laboratory) mice were housed in a pathogen-free facility and used with Institutional Animal Care Committee approval. B6→B10.BR (cGVHD) and B6→BALB/c (aGVHD) models, including disease severity assessments, were used as described.16-18 For cGVHD, cyclophosphamide-treated (120 mg/kg/d, day −3, −2), irradiated (8.3Gy, day −1) recipients received B6 bone marrow (BM) ± 0.75 × 105 conventional T cells (Tcon) on day 0, ±0.5 × 106 Tregs on day 0 or day 28. For aGVHD, irradiated (7Gy, day −1) BALB/c recipients were given B6 BM ± 2 × 106 Tcon ± 1 × 106 Tregs on day 0. Tcon and Tregs were purified as described.19 IL-2 (0.5 µg)/JES6-1 anti-IL-2 mAb (25 µg) complexes were injected intraperitoneally days 0-3 (aGVHD) or days 28-56 (cGVHD).
cGVHD analyses
Flow cytometry for Tfh, Tfr, and GC B cells, immunofluorescence, and histopathology scoring were performed as described.16,20 Pulmonary function tests assessing cGVHD-associated bronchiolitis obliterans syndrome (BOS) were performed as described.16
Results and Discussion
IL-2/mAb complexes reduce cGVHD but worsen aGVHD
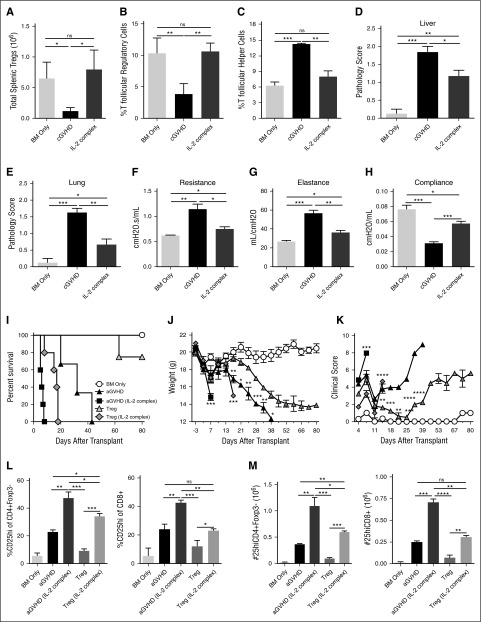
Consistent with patient data,5,21 cGVHD mice have significantly fewer Tregs and Tfr and more Tfh compared with no cGVHD (BM only) recipients (Figure 1A-C). Daily therapeutic dosing of IL-2/mAb complexes (days 28-56) increased Treg and Tfr levels (Figure 1A-B), reduced Tfh (Figure 1C) and tissue pathology scores (Figure 1D-E), and ameliorated cGVHD-associated BOS lung dysfunction16 (Figure 1F-H). Survival range was 90% to 100% (90% IL-2/mAb complex group), and neither survival nor weights differed among groups (not shown). These data suggest that therapeutic injections of IL-2/mAb complexes can expand Tregs, including Tfr, and reverse established cGVHD.
Figure 1.
IL-2/mAb complexes treat cGVHD but exacerbate aGVHD. (A-F) B10.BR mice were transplanted with either T-cell–depleted (TCD), B6 BM (BM Only), or B6 TCD BM with purified B6 Tcon to establish cGVHD. Mice given BM with Tcon were treated with 200 µL of phosphate-buffered saline (PBS) (cGVHD), or with 200 µL of IL-2/mAb complexes (0.5 µg IL-2/25 µg JES6-1 anti-IL-2 mAb) in PBS from days 28-56 after transplant. (A) Total number of splenic Tregs were measured on day 56 after transplant and found to be significantly higher in mice given IL-2/mAb complexes vs cGVHD mice. (B-C) Frequency of Tfr (CD4+Foxp3+PD1hiCXCR5hi) and Tfh (CD4+Foxp3-PD1hiCXCR5hi) out of total splenic CD4+ T cells were measured by flow cytometry on day 56 after transplant. (B) Tfr were found in a higher frequency, but (C) Tfh at a lower frequency in mice given IL-2/mAb complexes. (D-E) Histopathology scoring of the (D) liver and (E) lung based on H&E-stained cryopreserved sections of organs harvested on day 56 after transplant. Scores show reduced pathology in mice given IL-2/mAb complexes vs cGVHD. (F-H) Pulmonary function tests (PFTs) assessing (F) airway resistance, (G) total lung elastance, and (H) total lung compliance were performed on day 56 after transplant. PFTs demonstrate partial reversal of PFTs characteristic of cGVHD-associated BOS in mice given IL-2/mAb complexes. (A-F) Representative data from 2 independent experiments; n = 8-10 mice/group/experiment. Bar graphs show mean ± standard error of the mean (SEM). Multiple comparisons made using one-way ANOVA with Tukey’s posttest. Significance: *P < .05; **P < .01; ***P < .001; ****P < .0001. (I-M) BALB/c mice were transplanted with B6 BM (BM Only), BM with purified B6 Tcon (aGVHD), or BM, Tcon, and B6 Tregs (Tregs) on day 0. Groups were given 200 µL of PBS or 200 µL of IL-2/mAb complexes (0.5 µg IL-2/25 µg JES6-1 anti-IL-2 mAb) in PBS from days 0-3 after transplant. (I) Survival of recipient mice shows increased mortality rate in groups given IL-2/mAb complexes: aGVHD vs aGVHD/IL-2 complex (*); Tregs vs Tregs/IL-2 complex (***); aGVHD/IL-2 complex vs Tregs/IL-2 complex (*); aGVHD vs Tregs (**). (J) Recipient body weights show sharp declines for aGVHD/IL-2 complex group from days 3-7, and Treg/IL-2 complex group from days 13-17. Comparisons on graph: day 7: aGVHD vs aGVHD/IL-2 complex (***); day 17: Treg vs Treg/IL-2 complex (***); days 17-38: Treg vs aGVHD. (K) Clinical GVHD scores show sharp increases for aGVHD/IL-2 complex group from days 4-7, and for Treg/IL-2 complex group from days 11-14. Comparisons on graph: day 7: aGVHD vs aGVHD/IL-2 complex (****); day 17: Treg vs Treg/IL-2 complex (****); days 14-39: Treg vs aGVHD. (L-M) Flow cytometry analysis of splenic T cells on day 5 after transplant revealed a higher (L) frequency and greater (M) numbers of CD25hiCD4+Foxp3- and CD25hiCD8+Tcon in mice given IL-2 mAb complexes, compared with untreated groups. Survival differences analyzed by log-rank test. Bar graphs show mean ± SEM. Multiple comparisons made using one-way ANOVA with Tukey’s posttest. (I-M) Representative data from 3 independent experiments; n = 5-8 mice/group/experiment. Significance: *P < .05; **P < .01; ***P < .001; ****P < .0001.
As some cGVHD patients can have aGVHD overlap syndrome,22 we next assessed the efficacy of IL-2/mAb complexes in aGVHD. A 4-day course (days 0-3) of IL-2/mAb complexes accelerated mortality when given alone and when given in combination with otherwise highly effective Treg infusions (Figure 1I). IL-2/mAb complexes also increased severity of disease, as indicated by rapid recipient weight loss and increases in clinical GVHD scores from days 3-7 in mice given IL-2/mAb complexes alone, and days 13-17 when given with Tregs (Figure 1J-K). This exacerbation of aGVHD severity was due to expansion of CD25hiCD4+ and CD8+Tcon (Figure 1L-M). Therefore, although IL-2/mAb complexes may be effective in some cGVHD patients, they may not be ideal for cGVHD patients with aGVHD overlap, as IL-2/mAb complexes may expand CD25hi Tcon and overwhelm Treg expansion and GVHD suppression.
Treg adoptive transfer reverses established cGVHD
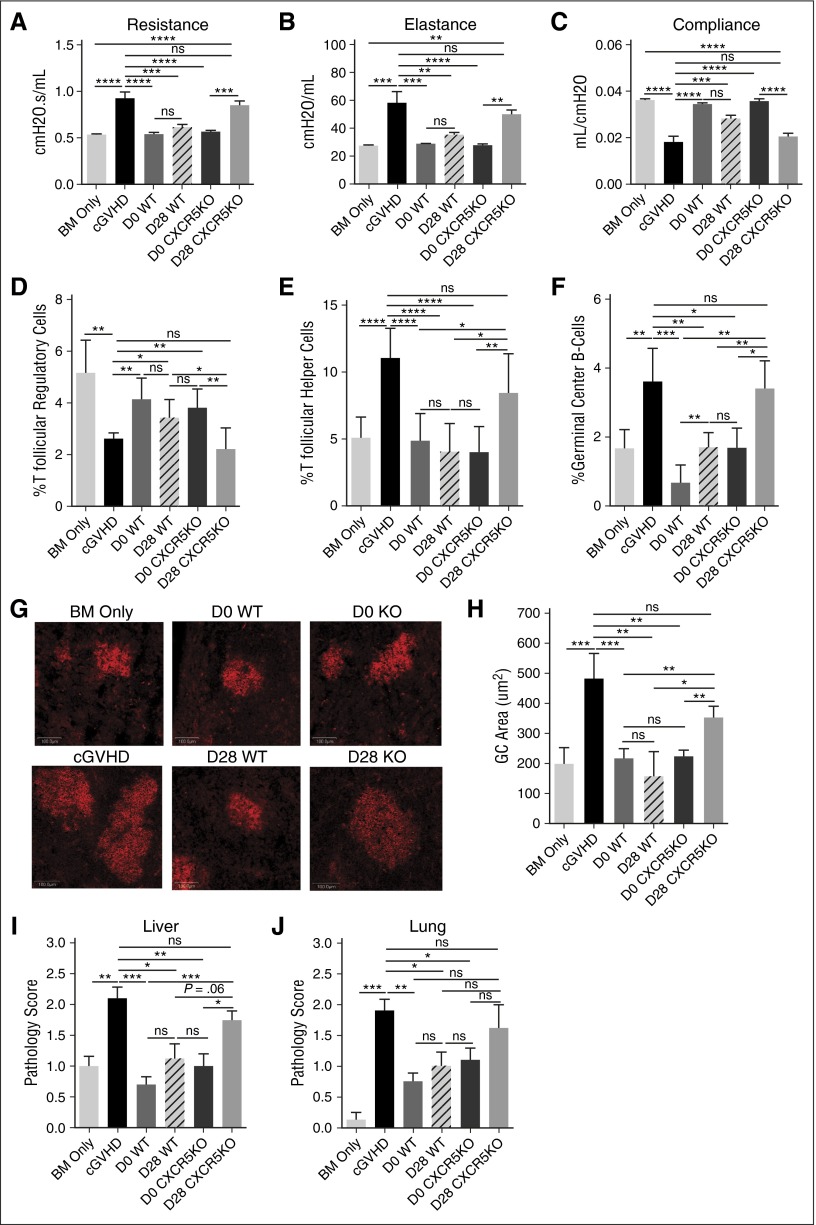
Because some, but not all, cGVHD patients may respond well to IL-2/mAb complexes, we tested Treg infusions in cGVHD. Treg infusions were given either therapeutically (day 28), or prophylactically (day 0). A single infusion with wild-type (WT) Tregs on day 28 or day 0 significantly reduced cGVHD-associated BOS (Figure 2A-C) and increased Treg frequency, including Tfr (Figure 2D). Tfh and GC B-cell frequency, GC size, and tissue pathology scores were also significantly reduced in mice given WT Tregs (Figure 2E-J). Survival range was 85% to 100%, and neither survival nor weights differed among groups (not shown).
Figure 2.
Treg infusions treat cGVHD in a CXCR5-dependent manner. B10.BR mice were transplanted with either T-cell–depleted (TCD), B6 BM (BM Only), or TCD B6 BM with purified B6 Tcon (cGVHD) as in Figure 1A-F. Some groups of mice given BM and Tcon were also given either WT or CXCR5KO Tregs on day 0 (prophylactic) or day 28 (therapeutic). (A-C) PFTs assessing (A) airway resistance, (B) total lung elastance, and (C) total lung compliance were performed on day 56 after transplant. PFTs demonstrate reversal of PFTs characteristic of cGVHD-associated BOS in all mice given WT Tregs, and CXCR5KO Tregs on day 0, but not mice given CXCR5KO Tregs on day 28. (D-F) Frequency of (D) Tfr and (E) Tfh out of total splenic CD4+ T cells, along with frequency of (F) GC B cells (CD19+GL7hiFAShi) out of total splenic B cells were measured by flow cytometry on day 56 after transplant. (D) Tfr frequency was found to be higher, but (E) Tfh and (F) GC B-cell frequency lower in mice given WT Tregs and CXCR5KO Tregs on day 0, but not mice given CXCR5KO Tregs on day 28. (G) Representative images (original magnification ×200) of frozen spleen tissues stained with Cy3-peanut agglutinin (red) to delineate GCs. (H) Quantification of GC area from frozen spleen tissues stained with Cy3-peanut agglutinin as in (G) from 5 spleens/group. WT CXCR5KO Tregs given on day 0 reduce GC area to levels near that of BM-only mice. CXCR5KO Tregs given on day 28 reduce GC area slightly, but not significantly, compared with cGVHD mice, and do not decrease GC area to the same degree as WT Tregs. (I) Histopathology scoring of the liver based on H&E-stained cryopreserved sections of organs harvested on day 56 after transplant. Scores are not significantly different between mice given CXCR5KO Tregs on day 28 and cGVHD mice. (J) Histopathology scoring of lung based on H&E staining as in (I). Scores are not significantly different between mice given CXCR5KO Tregs on day 28 and cGVHD mice. (A-J) Representative data from 2 independent experiments; n = 5-8 mice/group/experiment. Bar graphs show mean ± SEM. Multiple comparisons made using one-way ANOVA with Tukey’s posttest. Significance: *P < .05; **P < .01; ***P < .001; ****P < .0001.
Donor Tregs depend upon CXCR5 for therapeutic efficacy
Given the importance of the GC reaction in cGVHD pathogenesis,23 the finding that both therapeutic and prophylactic Treg infusions increased Tfr and reduced Tfh, GC B cells, and GC size suggests that Tregs may ameliorate or prevent cGVHD through GC reaction inhibition. The chemokine receptor CXCR5 is required for Tregs to home to GCs and inhibit B-cell responses.24 To determine whether homing to the GC was required for therapeutic or prophylactic Treg efficacy, we compared CXCR5 knockout (CXCR5KO) with WT Tregs. We hypothesized that CXCR5KO Tregs may be less effective than WT. However, like WT, CXCR5KO Tregs given prophylactically were able to prevent cGVHD-associated lung dysfunction (Figure 2A-C), increase Tfr, and reduce Tfh, GC B cells, GC size, and tissue pathology scores (Figure 2D-J). In contrast, CXCR5KO Tregs given therapeutically had no effect on lung function, Tfh, or GC B cells, and minimal effect on GC size or tissue pathology scores (Figure 2A-C,E-J). This lack of efficacy correlated with an absence of Tfr niche restoration, as Tfr levels were similar to cGVHD mice (Figure 2D). These data indicate that Tregs can suppress alloreactive T-cell responses required for cGVHD generation prior to the establishment of GC reactions, but once GCs are formed, Tregs must home to the GC to interrupt the pathogenic process of producing antibody-secreting cells.
In conclusion, our data indicate that both therapeutic Treg infusions and IL-2/mAb complexes can increase Tregs and treat established cGVHD. The therapeutic efficacy of Treg infusions and IL-2/mAb complexes may be driven by Treg-mediated inhibition of the GC reaction. Future Treg-modifying therapies in cGVHD may be augmented by targeting Treg homing to the GC, especially in patients with confirmed auto- or alloantibodies.25 Importantly, whereas Treg infusions may useful for all cGVHD patients, including those with aGVHD overlap syndrome, IL-2/mAb complexes may need to be used with more caution. Even though IL-2/mAb complexes have the capacity to expand Tregs in cGVHD, our data suggest that they also have the potential to expand activated CD25hi Tcon, and thus, may best be reserved for cGVHD patients with no aGVHD overlap. Alternatively, lower doses or different dosing schedules of IL-2/mAb complexes could result in less Tcon expansion, which could increase the therapeutic index for cGVHD patients, including those with aGVHD overlap.
Regardless, Treg infusions could prove to be advantageous over IL-2-based therapies, because IL-2-based approaches require long-term treatment to maintain efficacy, whereas Tregs might require more limited dosing. Considering that Tregs from a single autologous Treg infusion have been shown to persist at least 1 year in type 1 diabetes patients,13 it is possible that a single Treg dose would be sufficient. However, the half-life of infused Tregs in cGVHD is unknown, and long-term Treg survival may be limited by concurrent use of immunosuppressive agents that minimize IL-2 bioavailability. Therefore, it may be worthwhile in future studies to explore infusions of either nonenriched or CXCR5-enriched Tregs combined with low-dose IL-2, or other approaches that deliver IL-2 to drive Treg, but not Tcon, proliferation. Alternatively, Tregs and IL-2-based treatments could be given sequentially in cGVHD patients, including those with aGVHD overlap, treating first with Tregs, to ensure dampening of any aGVHD-like components, followed by IL-2-based therapies as a novel, potentially efficacious approach.
Acknowledgments
The authors thank Colby Feser for tissue preparation.
This study was funded by the National Institutes of Health, National Cancer Institute grants P01 CA142106-08A1 (B.R.B.) and R01 CA183560 (J.R.); National Institute of Allergy and Infectious Diseases grants P01 AI056299-13 (B.R.B.) and T32 AI007313 (C.M.-H. and R.F.); National Heart, Lung, and Blood Institute F30 HL121873 (C.M.-H.); and Leukemia and Lymphoma Society Translational Research grants 6458-15 (B.R.B.) and 6462-15 (I.M. and B.R.B.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.M.-H. and R.F. designed and performed experiments, discussed results, and wrote the paper; A.P.-M. and N.P. performed histologic analyses and edited the paper; K.P.A.M., G.R.H., L.L., J.S.S., W.J.M., I.M., D.H.M., L.A.T., J.K., C.S.C., J.H.A., R.J.S., and J.R. discussed results and edited the paper; B.R.B. contributed to experiments, discussed results, and edited the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
- 1.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657. doi: 10.1182/blood-2015-10-672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra143-179ra143. [DOI] [PMC free article] [PubMed]
- 8.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 10.Lee S-Y, Cho M-L, Oh H-J, et al. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology. 2012;137(4):305–316. doi: 10.1111/imm.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Tey S-K, Koyama M, et al. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J Immunol. 2013;191(10):5291–5303. doi: 10.4049/jimmunol.1301181. [DOI] [PubMed] [Google Scholar]
- 12.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7(280):280rv282-280rv282. [DOI] [PMC free article] [PubMed]
- 13.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189-315ra189. [DOI] [PMC free article] [PubMed]
- 14.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theil A, Tuve S, Oelschlägel U, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015;17(4):473–486. doi: 10.1016/j.jcyt.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichenbach DK, Schwarze V, Matta BM, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125(20):3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 19.Matta BM, Reichenbach DK, Zhang X, et al. Peri-alloHCT IL-33 administration expands recipient T regulatory cells that protect mice against acute GVHD [published online ahead of print May 24, 2016]. Blood. doi: 10.1182/blood-2015-12-684142. doi:10.1182/blood-2015-12-684142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn R, Allen JL, Luznik L, et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125(26):4085–4094. doi: 10.1182/blood-2014-08-595470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forcade E, Kim HT, Cutler C, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127(20):2489–2497. doi: 10.1182/blood-2015-12-688895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidala J, Vogelsang G, Martin P, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97(3):451–458. doi: 10.3324/haematol.2011.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]