Abstract
To comprehensively assess conventional vs. some alternative laying-hen housing systems under U.S. production conditions, a multi-institute and multi-disciplinary project, known as the Coalition for Sustainable Egg Supply (CSES) study, was carried out at a commercial egg production farm in the Midwestern United States over two single-cycle production flocks. The housing systems studied include a conventional cage house (200,000 hen capacity), an aviary house (50,000 hen capacity), and an enriched colony house (50,000 hen capacity). As an integral part of the CSES project, continual environmental monitoring over a 27-month period described in this paper quantifies indoor gaseous and particulate matter concentrations, thermal environment, and building ventilation rate of each house. Results showed that similar indoor thermal environments in all three houses were maintained through ventilation management and environmental control. Gaseous and particulate matter concentrations of the enriched colony house were comparable with those of the conventional cage house. In comparison, the aviary house had poorer indoor air quality, especially in wintertime, resulting from the presence of floor litter (higher ammonia levels) and hens’ activities (higher particulate matter levels) in it. Specifically, daily mean indoor ammonia concentrations had the 95% confidence interval values of 3.8 to 4.2 (overall mean of 4.0) ppm for the conventional cage house; 6.2 to 7.2 (overall mean of 6.7) ppm for the aviary house; and 2.7 to 3.0 (overall mean of 2.8) ppm for the enriched colony house. The 95% confidence interval (overall mean) values of daily mean indoor carbon dioxide concentrations were 1997 to 2170 (2083) ppm for the conventional cage house, 2367 to 2582 (2475) ppm for the aviary house, and 2124 to 2309 (2216) ppm for the enriched colony house. Daily mean indoor methane concentrations were similar for all three houses, with 95% confidence interval values of 11.1 to 11.9 (overall mean of 11.5) ppm. The 95% confidence interval values (overall mean) of daily mean PM10 and PM2.5 concentrations, in mg/m3, were, respectively, 0.57 to 0.61 (0.59) and 0.033 to 0.037 (0.035) for the conventional cage house, 3.61 to 4.29 (3.95) and 0.374 to 0.446 (0.410) for the aviary house, and 0.42 to 0.46 (0.44) and 0.054 to 0.059 (0.056) for the enriched colony house. Investigation of mitigation practices to improve indoor air quality of the litter-floor aviary housing system is warranted.
Keywords: indoor air quality, ammonia, greenhouse gas, particulate matter, alternative hen housing
INTRODUCTION
Ammonia (NH3), greenhouse gases (including carbon dioxide (CO2), nitrous oxide (N2O), methane (CH4), and particulate matter (PM) are among the aerial pollutants of concern in poultry houses because of their potential impact on the health of the birds, the caretakers, and the environmental footprint. A considerable amount of work has been done to collect baseline concentration data for typical, conventional production facilities. Derived from a review of literature, Appendixes 1 and 2 summarize findings of various studies concerning indoor concentrations of gases (particularly NH3) and PM in laying-hen houses. It is apparent that large variations exist among the study results, which are subject to the influence of housing type, management practice, local climatic conditions, and to some extent, the associated measurement methods. The much-needed research information concerning the viability of certain alternative laying-hen housing systems vs. conventional housing systems for U.S. egg production led to the formation of a public-private partnership that enabled the development and implementation of a multi-institute and multi-disciplinary commercial-scale research project (Swanson et al., 2014). The project, known as the Coalition for Sustainable Egg Supply (CSES), was to systematically evaluate three laying-hen housing systems–conventional cage (CC), aviary (AV), and enriched colony (EC) houses (Zhao et al., 2014a) with regards to animal behavior and well-being, egg safety and quality, environment impact, food affordability, and worker health and ergonomics.
As a part of the CSES publication series in Poultry Science, this paper deals with the environmental impact component of the project, with emphasis on description of the environmental monitoring system and presentation and comparison of indoor air quality (i.e., gaseous and PM concentrations), thermal environment (air temperature and relative humidity or RH), and building ventilation rate (VR) among the three monitored houses. A companion paper of the publication series by Shepherd et al. (2014) delineates and compares the gaseous and PM emissions from each of the housing systems.
MATERIALS AND METHODS
The environmental monitoring was carried out with three hen housing systems (CC, AV, and EC) located at the same farm in the Midwest United States, involving two single-cycle flocks of Lohmann LSL White laying-hens (78 wk of hen age per flock). The CC house had a nominal capacity of 200,000 hens and was equipped with manure belts that conveyed the accumulated manure out of the house every 3 to 4 d. The AV house had a nominal capacity of 50,000 hens and was provided with colonies and litter area accessible by the hens part of a day to perform foraging and dust-bathing behaviors. Manure belts were installed in all hen colonies to remove manure out of the house every 3 to 4 d, while the manure deposited/accumulated on the litter floor was only removed at the end of each flock. The EC house also had a nominal capacity of 50,000 hens, and all manure was disposed onto the manure belts and was removed out of the house every 3 to 4 d. For each flock, the three houses were populated with hens at the same age. The monitoring periods were June 2011 to May 2012 for flock 1 and July 2012 to August 2013 for flock 2, which covered the majority of the flock lifetime. There was a 3-week downtime between flocks during which no monitoring was performed. Detailed description of the housing systems, manure storage and management practices was provided by Zhao et al. (2014a).
House Environment and Emissions Monitoring
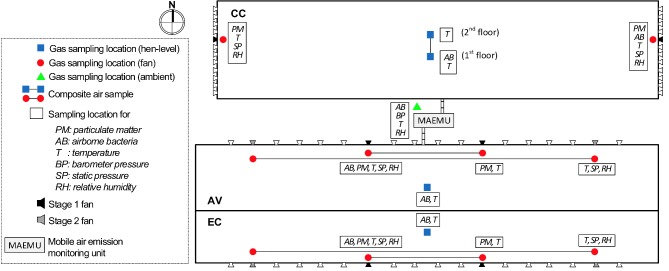
A mobile air emission-monitoring unit (MAEMU) was installed on-site to perform the continuous monitoring of the three housing systems. Moody et al. (2008) provided a full description of the MAEMU system and the standard operating procedures (SOPs). The MAEMU was modified to meet the site-specific monitoring needs by the CSES project, integrating multiple gas analyzers and a data acquisition system (Compact Fieldpoint, National Instruments, Austin, TX) to automatically collect and analyze sequential air samples from nine in-house locations (three locations per house) and one ambient location (Figure 1). The MAEMU simultaneously recorded data on the thermal environment, operational status of ventilation fans (used to derive building VR), gaseous and PM concentrations, electricity use, and propane use. Figure 2 shows outside and inside photographs of the MAEMU; and Figure 3 shows the schematic representation of the sampling system.
Figure 1.
A schematic representation of the house layout and sampling locations for the environmental monitoring of the conventional cage (CC), enriched colony (EC), and aviary (AV) houses. Air samples from two sampling locations connected with a line is combined as one composite sample.
Figure 2.
Photographs of the environmental monitoring system: (A) mobile air emissions monitoring unit (MAEMU); (B) data acquisition system (DAQ) and gas analyzers; (C) positive-pressure gas sampling system (GSS).
Figure 3.
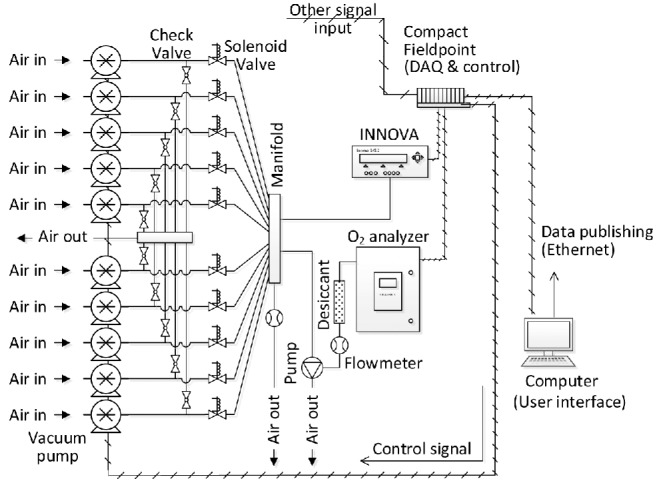
A schematic representation of the gas sampling system and data acquisition (DAQ) system. ‘Other signal input’ includes those for particulate matter concentrations, air temperature, relative humidity, static pressure, barometric pressure, fan operation status, and temperature of heat trace and heat tape.
Concentrations of NH3, CO2, CH4, N2O, and dew-point temperature (DP) were measured with a fast-response and precision photoacoustic multi-gas analyzer (Innova 1412, LumaSense Technologies A/S, Ballerup, Denmark). Oxygen (O2) concentration was measured with a paramagnetic gas analyzer (model 755a, Rosemount Analytical, Irvine, CA). To prevent data loss from long-term interruptions of the primary gas analyzer (Innova 1412), backup instruments were installed, including a single-gas infrared NH3 analyzer (Chillgard RT, MSA, Pittsburgh, PA), a CO2 probe of 0 to 7000 ppm (±1.5% range + 2% reading uncertainty) (GMT222, Vaisala Inc., Woburn, MA), and a DP probe (DewTrak II, EdgeTech Moisture and Humidity, Marlborough, MA).
To account for in-house spatial variation, two exhaust air samples and one hen-level location (between two colony/cage rows in the middle of the house) were sampled in each house along with one ambient air location (Figure 1). Exhaust air sample locations in the CC house were placed near the stage-1 ventilation fan of the east and west end-walls, while sampling in the AV and EC houses provided a composite sample of the two stage-1 ventilation fans and a composite sample of the two stage-2 ventilation fans (Figure 1). Hen-level sample locations were placed in the middle of each house, with a composite sample of the upper and lower tiers collected in the CC house. Fluorinated ethylene propylene (FEP) Teflon tubing (9.5 mm outside diameter, and 6.4 mm inside diameter) was used for the air sampling lines to avoid NH3 absorption to the sampling lines. Sample lines running between the MAEMU and hen houses were maintained at 32 to 38°C using heat trace and heat tapes to avoid in-line moisture condensation. Each in-house sampling location was equipped with a Y-shaped sampling port with two dust filters (3011 NAPA, Atlanta, GA) to keep large particles from plugging the air tubes, and with two inline Teflon filters (47mm filter membrane, 5 to 6 μm, Savillex, Eden Prairie, MN) to protect the gas sampling systems (GSS) and gas analyzers from fine particulate matter.
Because the same gas analyzers were used to measure all 10 locations, sequential air sampling was implemented using a positive-pressure GSS (Figure 2c). Each location was sampled for 6 to 8 min (flock 1) and 8 min (flock 2). To maximize measurement accuracy of the concentration values, with the response time of the gas analyzers being 5 to 7 min, the last minute readings were used as the measured values. In addition, every two cycles of the sequential samplings the outside air was drawn and analyzed. The less frequent sampling and analysis of the outside air was because of its relatively constant compositions, as consistently demonstrated in our previous field monitoring studies. This sequential measurement yielded one gas concentration measurement per sampling location every 54 or 72 min.
Air temperature was measured with type-T thermocouples (Cole-Parmer, Vernon Hills, IL). The RH was measured with capacitance-type humidity sensors (HMP 61U, Vaisala Inc., Woburn, MA). Concentrations of PM10 and PM2.5 inside the houses were measured with real-time Tapered Element Oscillating Microbalances (TEOM, Model 1400a, Thermo Fisher Scientific Inc., Waltham, MA) that were set to a 300-s integration time. The filters of the TEOM units were changed weekly. Daily data following the farm visit were manually verified with respect to overloading of the TEOM filters in all houses. Within the AV, because of the much higher PM levels, only 2 to 4 d of valid data were obtained following a filter change, as compared to 3 to 5 d of valid data for the CC or EC house following the filter change. Two TEOM units were co-located near the stage-1 ventilation fans and were respectively equipped with the PM10 and PM2.5 separation heads. Over a 3-week period in flock 2, the co-located TEOM units in all houses were set to sample PM10 simultaneously to verify consistency of measurement units. Comparison of average daily concentrations over this period revealed <7% differences between co-located TEOMs. From April 2013 to August 2013, TEOMs in the AV house were relocated to separate stage-1 ventilation fans to characterize spatial variations in PM10 concentrations. Over this period the difference in average daily PM10 concentrations was 9%.
Building VR was derived from in situ fan calibration with a 1.37 m (54 inch) fan assessment numeration system (FANS) (Gates et al., 2004). Individual fan airflow curves were developed for each ventilation stage by calibrating at least one fan from each stage at about half way and at the end of each flock cycle, for a total of five calibration events throughout the study. Over 50% of the fans, representing each ventilation stage in each house, were assessed during each calibration event; and all fans in ventilation stages 1 to 3 were calibrated to achieve more accurate VR determination at low ventilation rates. Additionally, the impact of light trap cleanliness was quantified and accounted for in the calibration events, as dirty light traps in the AV and EC systems were found to cause a 15 to 25% reduction in the fan airflow. Runtime of the fans in each ventilation stage was continuously monitored with inductive current switches (CR9321-PNP, CR Magnetics, St. Louis, MO) as described by Muhlbauer et al. (2011). In total, 24 of the 44 fans in the CC house and 10 of the 18 fans in the EC and AV houses each were monitored. Building static pressure (SP), which is the pressure difference between inside and outside of the building, was continuously measured with a SP sensor (model 264, Setra, Boxborough, MA) at two locations in each house, along with barometric pressure (WE100, Global Water, Gold River, CA). Overall building VR was calculated at 30-s increments based on the fan curves for each stage, fan runtime, SP, and environmental conditions.
Measurements of the environmental conditions (temperature, RH, and barometric pressure), ventilation conditions (fan status and SP), PM concentrations, and propane use were continuously sampled with the DAQ system at 1-s intervals, and averaged to 30-s values corresponding to the sample integration time of the Innova 1412 multi-gas analyzer.
Environmental Monitoring Quality Assurance/Quality Control (QA/QC)
Rigorous SOPs and quality assurance project plan (QAPP), as described by Moody et al. (2008), were followed in the data collection and processing to attain the highest data quality possible. This was accomplished through weekly site visits for on-site equipment check and calibration, daily inspection of the system via remote access of the DAQ computer, timely processing and auditing of the recorded data, regular collaboration with the farm managerial staff, and mid-flock quality control audits performed by an experienced engineer versed in the design and management of comparable environmental monitoring systems. During each site visit, the Innova 1412 gas analyzer was challenged with zero gas (ultra-high purity nitrogen gas, 99.999%, Praxair Inc., Danbury, CT) and span reference gases with certified concentrations (NH3: 25 ppm; CO2: 3000 ppm; CH4: 100 ppm; N2O: 5.1 ppm). The span gas levels were close to the expected maximal indoor gas concentrations. Successful challenges required all gas readings to fall within 5% of the expected concentration values; a failed challenge would trigger recalibration of the gas analyzer, resulting in its temporary removal or replacement. The Rosemount 755a O2 analyzer was calibrated weekly with two certified span gasses (20.4% and 20.9% O2, Praxair Inc., Danbury, CT). The TEOM filters and cyclone heads were changed weekly and tested for leaks and required air flow rates. The GSS pumps, valves, and sample lines were checked biweekly for leaks and flow rates. Temperature, SP, and RH sensors were calibrated prior to each flock cycle; mechanical failures required the replacement of the unit with a new calibrated sensor. Table 1 provided the information on instrumentation maintenance to maximize measurement accuracy.
Table 1.
Maintenance schedules of the environmental monitoring instruments for the study.
| Instrument | Function | Maintenance | Frequency of |
|---|---|---|---|
| Maintenance | |||
| Innova 1412 | Gas analyzer (NH3, CO2, CH4, N2O, dew-point temperature) | Challenge | Weekly |
| Calibration | Reading is 5% off the reference | ||
| Chillgard RT | O2 analyzer | Calibration | Weekly |
| Thermocouple | Temperature sensor | Calibration | Once (start of flock) |
| Vaisala HMP 61U | Relative humidity transmitter | Calibration | Once (start of flock) |
| Setra 264 | Static pressure sensor | Calibration | Once (start of flock) |
| WE100 | Barometric pressure sensor | Calibration | Once (start of flock) |
| Desiccant | H2O removal for O2 analyzer | Change | Weekly |
| Heat trace and tape | Condensation prevention | Temperature check | Weekly |
| Vacuum pump | Gas sampling | Leakage check | Biweekly |
| Flow check | Weekly | ||
| Teflon tubing | Gas sampling line | Leakage check | Biweekly |
| Flow meters | Gas sampling line | Flow check | Weekly |
| Filter | Sample line dust filtration | Change | Every two months |
| TEOM | PM sampler | Filter change | Weekly |
| Clean of cyclone head | Weekly | ||
| Leakage check | Weekly | ||
| Flow check | Once (start of flock) | ||
| Mass transducer calibration constant factor check | Once (start of flock) | ||
| Pump check | Weekly |
Three months of data (July 2011 to September 2011) were selected for validating and refining the data processing programs. Part of the selected data (one month) was analyzed by two Excel-based Macro programs that were independently developed by two data analysts. The program code was scrutinized and errors identified and corrected when any discrepancy was detected between the results obtained from the two programs. The other part of the data (two months) was used to validate the corrected programs.
Measurement of CH4 concentration is inherently interfered with environment moisture (a common issue of the INNOVA 1412 gas analyzer). In this study, the interference was minimized by correction for moisture during challenge/calibration.
Data Processing and Analysis
Daily mean temperature, RH, VR, and PM concentrations were calculated using 30-s data; and daily mean gaseous concentrations were calculated using either 54-min or 72-min interval data. Each datum point presented in this paper is the mean of all sampling locations within the hen house. A valid day of data was considered as having 75% or greater of the continuously recorded dynamic data passing the QA/QC.
Statistical analysis was performed to compare the daily mean gaseous and PM concentrations among the three houses and under different ambient temperature ranges, using the GLIMMIX model in Statistical Analysis System version 9.3 (SAS 9.3, SAS Institute Inc., Cary, NC). Based on average daily ambient temperature, the ambient temperature was categorized into six ranges, i.e., ≤−10°C, −10 to 0°C, 0 to 10°C, 10 to 20°C, 20 to 25°C, and >25°C. The concentration (or ‘Y’ in equation 1) was transformed into a logarithmic scale for even residual distribution. The model included house, ambient temperature range, house×ambient temperature range, and flock as fixed effects (equation 1). A random term of house×flock was included to account for dependency of measurements taken from the same house in the same flock. The effects were considered significant at a probability level of P < 0.05.
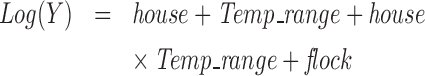 |
(1) |
RESULTS AND DISCUSSION
The numbers of valid days and completeness for temperature, RH, VR, gaseous, and PM concentrations over the entire monitoring period for both flocks are listed in Table 2. These numbers of valid days also represent the sample sizes of the environmental variables presented in the summary tables (Tables 3 and 4).
Table 2.
Number of days with valid data and completeness for ambient environment, conventional cage (CC), aviary (AV), and enriched colony (EC) houses.
| Variable | Ambient | CC | AV | EC | ||||
|---|---|---|---|---|---|---|---|---|
| No. of valid day | Compl. | No. of valid day | Compl. | No. of valid day | Compl. | No. of valid day | Compl. | |
| Temp. | 556 | 67% | 551 | 66% | 556 | 67% | 552 | 66% |
| (259/297) | (63%/71%) | (254/297) | (62%/70%) | (259/297) | (63%/71%) | (257/295) | (62%/62%) | |
| RH | 547 | 66% | 554 | 67% | 555 | 67% | 551 | 66% |
| (255/292) | (62%/70%) | (257/297) | (62%/71%) | (258/297) | (62%/71%) | (259/292) | (63%/70%) | |
| Vent. rate | - | - | 540 | 65% | 519 | 62% | 524 | 63% |
| (255/285) | (62%/68%) | (243/276) | (59%/66%) | (248/276) | (60%/66%) | |||
| NH3 conc. | 549 | 66% | 550 | 66% | 546 | 66% | 550 | 66% |
| (259/290) | (63%/69%) | (259/291) | (63%/69%) | (255/291) | (62%/69%) | (259/291) | (63%/69%) | |
| CO2 conc. | 549 | 66% | 550 | 66% | 546 | 66% | 550 | 66% |
| (259/290) | (63%/69%) | (259/291) | (63%/69%) | (255/291) | (62%/69%) | (259/291) | (63%/69%) | |
| CH4 conc. | 335 | 40% | 337 | 40% | 336 | 40% | 337 | 40% |
| (149/186) | (36%/44%) | (149/188) | (36%/45%) | (148/188) | (36%/45%) | (149/188) | (36%/45%) | |
| PM10 conc. | - | - | 332 | 40% | 261 | 31% | 371 | 45% |
| (109/223) | (26%/53%) | (116/145) | (28%/35%) | (133/238) | (32%/57%) | |||
| PM2.5 conc. | - | - | 142 | 17% | 190 | 23% | 296 | 36% |
| (42/100) | (10%/24%) | (48/142) | (12%/34%) | (48/248) | (12%/59%) | |||
Note: A valid day must have 75% or greater of the continuously recorded dynamic data passing the quality assurance and quality control (QA/QC). Values outside the parenthesis are combined numbers of valid days for both flocks, and those in the parenthesis are the respective numbers of valid days for flock 1 (before slash) and flock 2 (after slash). ‘-’ means the variable was not monitored for ambient.
Table 3.
Summary of ambient and indoor temperature, relative humidity (RH), and ventilation rate (VR) in the conventional cage (CC), aviary (AV), and enriched colony (EC) houses.
| Variable | Ambient | CC | AV | EC |
|---|---|---|---|---|
| Temperature, °C | 8.9 ± 11.2 | 24.6 ± 1.9 | 26.7 ± 1.1 | 25.2 ± 1.3 |
| (9.9 ± 10.6 / 8.1 ± 11.8) | (24.7 ± 1.9/24.4 ± 2.0) | (26.9 ± 1.2/26.6 ± 1.0) | (25.1 ± 1.5/25.3 ± 1.1) | |
| RH,% | 71 ± 14 | 57 ± 9 | 54 ± 7 | 56 ± 9 |
| (68 ± 14/73 ± 14) | (54 ± 8/60 ± 8) | (52 ± 8/55 ± 7) | (54 ± 9/58 ± 8) | |
| VR, m3/h/hen | - | 1.9 ± 1.6 | 1.9 ± 1.8 | 2.2 ± 2.0 |
| (1.9 ± 1.6/1.8 ± 1.5) | (1.8 ± 1.8/1.9 ± 1.8) | (2.1 ± 1.9/2.2 ± 2.0) |
Note: Values outside the parenthesis are mean±SD for both flocks, and those in the parenthesis are respective mean±SD values for flock 1 (before slash) and flock 2 (after slash).
Table 4.
Summary of ammonia (NH3), carbon dioxide (CO2), particulate matter (PM10 and PM2.5) concentrations for ambient environment and in the conventional cage (CC), aviary (AV), and enriched colony (EC) houses.
| Variable | Ambient | CC | AV | EC |
|---|---|---|---|---|
| NH3, ppm | 0.4 ± 0.5 | 4.0a,b ± 2.4 | 6.7a ± 5.9 | 2.8b ± 1.7 |
| (0.4 ± 0.7/0.3 ± 0.2) | (4.4 ± 2.6 / 3.6 ± 2.1) | (7.8 ± 6.8 / 5.8 ± 4.9) | (3.1 ± 1.9 / 2.6 ± 1.5) | |
| CO2, ppm | 452 ± 25 | 2084c ± 1034 | 2475a ± 1280 | 2216b ± 1112 |
| (443 ± 24/461 ± 23) | (2019 ± 987 / 2141 ± 1072) | (2337 ± 1132 / 2596 ± 1388) | (2172 ± 1062 / 2256 ± 1155) | |
| CH4, ppm | 5.7 ± 5.1 | 10.9a ± 5.7 | 11.7a ± 5.4 | 11.9a ± 5.9 |
| (6.3 ± 5.5/5.2 ± 4.8) | (14.8 ± 4.3 / 7.9 ± 4.7) | (15.6 ± 4.0 / 8.6 ± 4.3) | (16.2 ± 4.3 / 8.5 ± 4.7) | |
| PM10, mg/m3 | - | 0.59b ± 0.16 | 3.95a ± 2.83 | 0.44c ± 0.18 |
| (0.46 ± 0.14/0.65 ± 0.14) | (3.23 ± 2.16/4.53 ± 3.16) | (0.30 ± 0.11/0.52 ± 0.16) | ||
| PM2.5, mg/m3 | - | 0.035b ± 0.013 | 0.410a ± 0.251 | 0.056b ± 0.021 |
| (0.019 ± 0.006 / 0.042 ± 0.009) | (0.285 ± 0.159 / 0.452 ± 0.262) | (0.020 ± 0.005 / 0.063 ± 0.015) |
Note: Values outside the parentheses are mean±SD for both flocks, and those inside the parentheses are respective mean ± SD values for flock 1 (before slash) and flock 2 (after slash). a,b,cThe means of gas or PM concentration in three housing systems (CC, AV or EC) with different superscript letters significantly differ (P < 0.05). Ambient concentrations are not included in the comparison.
Temperature, Relative Humidity (RH), and Ventilation Rate (VR)
The average indoor temperatures were 24.6°C for CC, 25.2°C for EC, and 26.7°C for AV (Table 3). Concerns and speculations have been raised that the alternative hen-housing systems may have a difficult time maintaining indoor temperatures during wintertime because of their considerably reduced stocking densities as compared to the CC housing system. The data from the current study show that the indoor temperatures in all three houses during wintertime were maintained above 20°C (Figure 4), i.e., within the thermoneutral zone for laying hens. While supplemental heat contributed to maintaining the desired indoor temperature of the AV house, the small amount of liquid propane fuel use was indicative that such contribution or need was minor, at least for the climatic conditions encountered during the study period. The fundamental reason for being able to maintain the desired indoor temperature without supplemental heating at the lower stocking density in the EC house is that when ammonia level is not an issue, building VR is designed and used to remove moisture production by hens in the house during cold weather. A lower number of hens in the house leads to lower moisture production, which in turn requires lower VR (Chepete and Xin, 2004; Zhao et al., 2013a). The lower VR helps conserve the ventilation loss of the hen body heat, hence, maintaining the desired indoor temperature.
Figure 4.
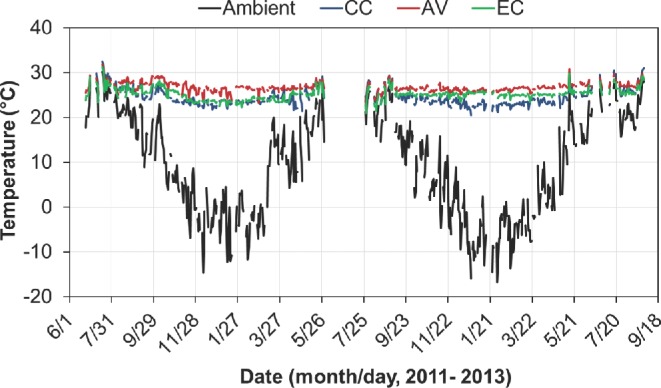
Daily mean ambient temperature and indoor temperatures of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses during the 2-flock production period.
Indoor RH values of the hen houses were generally in the acceptable range of 40% to 70% (Figure 5), averaging 57% for CC, 56% for EC, and 54% for AV (Table 3). There was no significant difference in RH among the houses.
Figure 5.
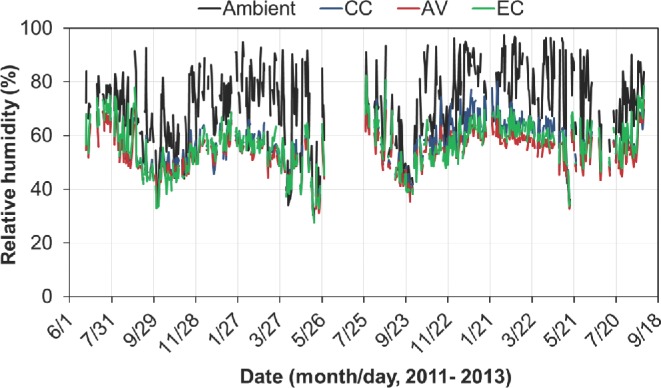
Daily mean ambient relative humidity (RH) and indoor RH of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses during the 2-flock production period.
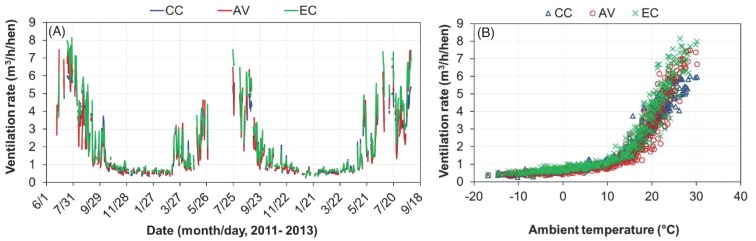
Building VR showed clear seasonal patterns in all cases, with higher VR on warm/hot days and lower VR on cool/cold days (Figure 6). The VR ranged from 0.3 to 6.0 m3/h/hen for the CC house, 0.3 to 8.1 m3/h/hen for the EC house, and 0.3 to 7.5 m3/h/hen for the AV house. The lower maximal VR for the CC house was possibly due to deterioration of fan performance over the 5-year usage. Because the CC house was tunnel-ventilated, its ventilation air traveled faster through the house, thus, providing a similar or greater cooling effect for the hens in the summertime, as compared to the two cross-ventilated alternative (EC and AV) houses. The maximal VR of the AV house, 7.8 m3/h/hen, was considerably lower than those of similar AV houses we had previously worked with (11 to 12 m3/h/hen) (Hayes et al., 2013 for brown hens; Zhao et al., 2013b for white hens). Each fan of the CSES AV house was installed with a light trap at the upper air stream, which can increase the pressure drop and reduce the fan airflow.
Figure 6.
Daily mean ventilation rate (VR) of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses. (A) Daily mean VR; (B) Daily mean VR vs. ambient temperature.
Gaseous Concentrations
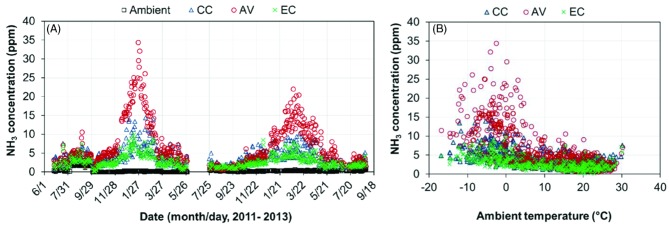
The ambient daily mean NH3 concentration was generally below 1 ppm, and the daily mean indoor NH3 concentration was highest in the AV house (6.7 ppm; 95% C.I. of 6.2 to 7.2 ppm), followed by the CC house (4.0 ppm; 95% C.I. of 3.8 to 4.2 ppm) and the EC house (2.8 ppm; 95% C.I. of 2.7 to 3.0 ppm) (Table 4). During the entire monitoring period, indoor daily mean NH3 concentrations in the CC and EC houses never exceeded 25 ppm, which is the threshold recommended in the United Egg Producers hen welfare guidelines (UEP, 2014), while daily mean NH3 concentrations exceeded 25 ppm on 12 winter days of flock 1 in the AV house (Figure 7). This finding was consistent with the previous observation on NH3 concentrations in two AV houses with brown hens in the Midwest (Hayes et al., 2013). The higher-than-threshold NH3 concentrations in the AV house were believed to arise from the accumulated floor litter coupled with lower building VR.
Figure 7.
Daily mean ammonia (NH3) concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses. (A) Daily mean NH3 concentration; (B) Daily mean NH3 concentration vs. ambient temperature.
The indoor daily mean NH3 concentrations in all three hen houses are inversely related with ambient temperature (Figure 7). Table 5 compares the gaseous and PM concentrations among the houses under different ranges of ambient temperature. It can be seen that the NH3 concentration of the EC houses was the lowest of the three for all ambient temperature conditions. At ambient temperature below 10°C, the AV house had significantly higher NH3 levels than the CC house (P < 0.05); however the difference diminished at higher ambient temperature (i.e., >10°C). This outcome primarily arose from the dilution effect of greater VR at higher air temperatures when there was a finite NH3 generation from the sources (houses). Higher VR coupled with warmer indoor air also leads to greater drying effect on the manure, and drier manure gives off less NH3. During cold weather, the low VR and humid air resulted in greater moisture content of the litter accumulated on the floor in the AV house, being more favorable for microbial decomposition of uric acid to NH3.
Table 5.
Air pollutant concentrations in the conventional cage (CC), aviary (AV) and enriched colony (EC) houses under different ranges of ambient temperature conditions.
| Gas or PM | Daily mean ambient | Daily mean concentration (mean ± SD) | ||
|---|---|---|---|---|
| temperature range °C | CC | AV | EC | |
| NH3, ppm | <−10 | 6.3a,B ± 2.7 (18) | 14.4a,A ± 5.3 (16) | 4.8a,C ± 1.3 (18) |
| −10 to 0 | 6.2a,B ± 2.6 (130) | 12.7a,A ± 6.3 (128) | 4.5a,B ± 1.8 (130) | |
| 0 to 10 | 4.1b,B ± 1.9 (132) | 7.4b,A ± 5.4 (132) | 2.8b,C ± 1.3 (132) | |
| 10 to 20 | 2.7cd,A ± 1.4 (151) | 3.5c,A ± 1.9 (151) | 1.8c,B ± 0.9 (151) | |
| 20 to 25 | 2.4d,A ± 1.2 (89) | 2.8d,A ± 1.6 (89) | 1.9c,B ± 1.1 (89) | |
| >25 | 3.0c,A ± 1.4 (30) | 2.5d,A ± 1.3 (30) | 2.4b,A ± 1.4 (30) | |
| CO2, ppm | <−10 | 4052a,B ± 161 (18) | 4787a,A ± 362 (16) | 4309a,AB ± 195 (18) |
| −10 to 0 | 3359b,C ± 291 (130) | 4027b,A ± 400 (128) | 3537b,B ± 360 (130) | |
| 0 to 10 | 2448c,C ± 309 (132) | 3016c,A ± 413 (132) | 2672c,B ± 360 (132) | |
| 10 to 20 | 1402d,C ± 285 (151) | 1680d,A ± 389 (151) | 1480d,B ± 335 (151) | |
| 20 to 25 | 891e,B ± 110 (89) | 972e,A ± 163 (89) | 931e,AB ± 127 (89) | |
| >25 | 722f,A ± 95 (30) | 721f,A ± 77 (30) | 746f,A ± 107 (30) | |
| CH4, ppm | <−10 | 8.4d,A ± 5.8 (10) | 8.2d,A ± 4.7 (10) | 9.3d,A ± 6.4 (10) |
| −10 to 0 | 8.7d,A ± 5.5 (105) | 9.7d,A ± 5.1 (104) | 9.8d,A ± 6.0 (105) | |
| 0 to 10 | 10.1d,A ± 4.7 (87) | 11.4d,A ± 4.6 (87) | 11.5d,A ± 5.1 (87) | |
| 10 to 20 | 12.5c,A ± 6.0 (86) | 13.0c,A ± 6.1 (86) | 13.1c,A ± 6.3 (86) | |
| 20 to 25 | 14.4b,A ± 4.2 (39) | 14.3b,A ± 4.2 (39) | 15.0b,A ± 4.5 (39) | |
| >25 | 16.8a,A ± 3.0 (10) | 16.5a,A ± 3.0 (10) | 17.1a,A ± 3.0 (10) | |
| PM10, mg/m3 | <−10 | 0.68ab,B ± 0.11 (12) | 7.38a,A ± 1.69 (7) | 0.59a,B ± 0.14 (10) |
| −10 to 0 | 0.68a,B ± 0.11 (83) | 6.80a,A ± 1.66 (52) | 0.56a,B ± 0.15 (85) | |
| 0 to 10 | 0.69a,B ± 0.15 (68) | 6.11a,A ± 1.72 (50) | 0.58a,B ± 0.13 (69) | |
| 10 to 20 | 0.56b,B ± 0.13 (99) | 3.33b,A ± 1.85 (75) | 0.41b,C ± 0.12 (110) | |
| 20 to 25 | 0.42c,B ± 0.12 (53) | 1.14c,A ± 0.89 (55) | 0.28c,C ± 0.10 (70) | |
| >25 | 0.39c,A ± 0.10 (17) | 0.38d,A ± 0.33 (22) | 0.21d,B ± 0.12 (27) | |
| PM2.5, mg/m3 | <−10 | - | 0.762a,A ± 0.039 (7) | 0.073a,B ± 0.017 (13) |
| −10 to 0 | 0.047a,B ± 0.011 (10) | 0.710a,A ± 0.116 (40) | 0.072a,B ± 0.013 (67) | |
| 0 to 10 | 0.040a,b,B ± 0.014 (39) | 0.510b,A ± 0.122 (46) | 0.062a,B ± 0.021 (51) | |
| 10 to 20 | 0.032c,B ± 0.012 (70) | 0.263c,A ± 0.132 (73) | 0.048b,B ± 0.021 (109) | |
| 20 to 25 | 0.030c,A ± 0.010 (16) | 0.066d,A ± 0.029 (17) | 0.044b,A ± 0.015 (41) | |
| >25 | 0.036c,A ± 0.006b (7) | 0.053e,A ± 0.013 (7) | 0.050b,A ± 0.019 (15) | |
Note: Values outside parentheses are mean±SD for concentrations. Values inside parentheses are the number of data. For each gas or PM, within a housing system (i.e., within each column), means with different lower case superscripts are significantly different (P < 0.05). Among the housing systems (i.e., within each row), means with different upper case superscripts are significantly different (P < 0.05).
As shown by the data in Appendix 1, deep-pit/high-rise and aviary housing systems have the highest indoor NH3 concentrations due to the long-term manure storage in the houses. In comparison, EC houses have the lowest NH3 concentration likely because of low stocking density, better manure-drying efficiency, and regular manure removal. The results of the current study, while falling in the range of the literature data, are at the lower end of the range, which is probably due to better manure management (i.e., frequent manure removal and continuous drying of manure on the belt). Moreover, instead of full-day litter access in aviary systems as practiced in European countries, the AV system involved in the CSES study and other U.S. operations allowed part-time litter access. This management reduced the amount of manure deposited/accumulated on the floor, thus, less of a nutrient source for NH3 generation from the litter.
Table 6 shows the spatial variation of NH3 concentrations at specific ambient temperature ranges for each house. The CC house sampling locations noted as ‘East’ and ‘West’ represent stage-1 ventilation fans at the respective house ends (Figure 1), while ‘Hen’ represents bird-level sampling locations at the middle of the house. Sampling locations within the AV and EC noted as ‘Mid’ represent the exhaust air at stage-1 ventilation fans located in the middle of the houses; ‘End’ represents the exhaust air at the stage-2 ventilation fans located at the ends of each house; and ‘Hen’ represents bird-level sampling locations in the middle of each house. Considerable spatial variations in indoor NH3 concentration were observed. The spatial variations primarily stemmed from non-uniform VR distribution in the hen houses, with higher NH3 level locations corresponding to lower VR. The NH3 concentrations at the hen-level locations were typically lower than those near the primary exhaust fans, as the middle locations of each house received fresher air. The overall coefficient of variation (COV), representing the extent of spatial variation in NH3 concentration within a house, was 27% for the CC house, 16% for the AV house, and 13% for the EC house.
Table 6.
Spatial distribution of ammonia (NH3) in the conventional cage (CC), aviary (AV), and enriched colony (EC) houses under different ranges of ambient temperature conditions.
| CC | AV | EC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amb. Temp (°C) | NH3 concentration (ppm) | COV (%) | NH3 concentration (ppm) | COV (%) | NH3 concentration (ppm) | COV (%) | ||||||
| East | West | Hen | Mid | End | Hen | Mid | End | Hen | ||||
| <−10 | 5.0 ± 1.9 | 8.9 ± 4.0 | 5.0 ± 2.5 | 36 ± 7 | 13.6 ± 5.0 | 16.6 ± 7.1 | 12.8 ± 3.8 | 13 ± 7 | 4.9 ± 1.3 | 4.9 ± 1.5 | 4.6 ± 1.2 | 6 ± 3 |
| −10 to 0 | 5.0 ± 2.0 | 8.5 ± 3.6 | 5.0 ± 2.4 | 34 ± 9 | 11.7 ± 6.0 | 15.1 ± 7.8 | 11.3 ± 5.4 | 17 ± 10 | 4.6 ± 1.7 | 4.6 ± 1.9 | 4.3 ± 2.0 | 9 ± 8 |
| 0 to 10 | 3.4 ± 1.6 | 5.2 ± 2.7 | 3.7 ± 1.8 | 27 ± 13 | 7.0 ± 4.9 | 8.5 ± 7.0 | 6.6 ± 4.5 | 13 ± 12 | 2.9 ± 1.3 | 2.9 ± 1.4 | 2.6 ± 1.3 | 8 ± 5 |
| 10 to 20 | 2.6 ± 1.4 | 3.2 ± 1.7 | 2.4 ± 1.4 | 22 ± 9 | 3.6 ± 1.9 | 3.7 ± 2.0 | 3.1 ± 1.8 | 14 ± 7 | 1.8 ± 1.0 | 1.9 ± 0.9 | 1.8 ± 1.0 | 13 ± 10 |
| 20 to 25 | 2.5 ± 1.2 | 2.8 ± 1.3 | 2.0v1.1 | 22 ± 10 | 3.0 ± 1.7 | 3.1 ± 1.7 | 2.3 ± 1.5 | 20 ± 9 | 1.7 ± 1.1 | 2.1 ± 1.2 | 1.8 ± 1.0 | 18 ± 10 |
| >25 | 3.3 ± 1.5 | 3.4 ± 1.6 | 2.4 ± 1.5 | 27 ± 11 | 2.5 ± 1.1 | 3.2 ± 2.0 | 1.9 ± 1.0 | 28 ± 11 | 1.9 ± 1.4 | 3.4 ± 2.0 | 1.9 ± 1.0 | 37 ± 13 |
| Overall | 3.5 ± 1.9 | 5.1 ± 3.4 | 3.3 ± 2.1 | 27 ± 11 | 6.5 ± 5.4 | 7.8 ± 7.3 | 6.0 ± 5.2 | 16 ± 10 | 2.8 ± 1.8 | 3.0 ± 1.8 | 2.7 ± 1.7 | 13 ± 11 |
Note: COV is the coefficient of variation, representing the spatial deviation of NH3 concentration within a hen house. Numerically, COV equals the standard deviation of daily mean NH3 concentrations from three sampling locations in a hen house divided by the overall house-level daily mean NH3 concentration.
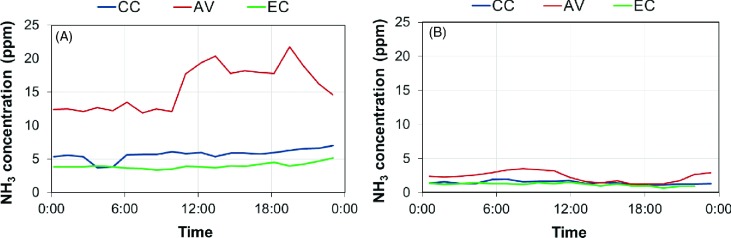
The diurnal NH3 concentrations for each house on a cold day (February 13, 2013) and a warm day (July 24, 2013) are delineated in Figure 8. The VR of all three houses was relatively constant on both days, at the minimum on the cold day and the maximum on the warm day. As a result, the NH3 concentrations in the CC and EC houses were quite stable. However, noticeable variation in diurnal NH3 concentration existed in the AV house, especially on the cold day. The elevation of the NH3 level occurred during the period when the birds became active on the litter floor. The diurnal and spatial variations of NH3 concentration illustrate the importance of continuous (throughout a day) and multi-location sampling, specific to the ventilation design of each house, to obtain representative samples for assessment of indoor air-quality and gaseous emissions.
Figure 8.
Diurnal ammonia (NH3) concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses on two example days. (A) Cold day: February 13, 2013; (B) Warm day: July 24, 2013.
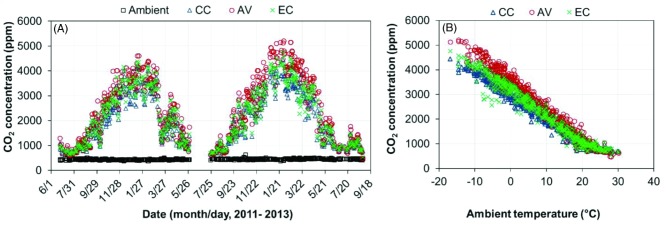
The overall daily mean CO2 concentrations were 2084 ppm for the CC house (95% C.I. of 1997 to 2170 ppm), 2475 ppm for the AV house (95% C.I. of 2367 to 2582 ppm), and 2216 ppm for the EC house (95% C.I. of 2124 to 2309 ppm) (Table 4). The daily mean CO2 concentration was consistently below 5,000 ppm (Permissible Exposure Limit set by Occupational Safety & Health Administration, OSHA) in the CC and EC houses while it slightly exceeded this level in the AV house on the six coldest days encountered during the study (average ambient temperatures below -12.5°C).
It is well known that indoor CO2 concentration is closely related to ambient temperature and VR. Our results show the CO2 concentration almost linearly decreases with increasing ambient temperature (and VR) until VR reaches its maximal value at ∼25°C ambient temperature (Figure 9). Table 5 showed that the CO2 concentration under most ambient temperature conditions tended to be higher in the AV house than in the EC and CC houses. The numerically higher CO2 concentrations in the AV house were presumably due to the combination of higher hen activity levels, thus, more CO2 respiration, lower VR for the AV house (1.9, 1.9 and 2.2 mg3/h/hen in the CC, AV and EC houses, respectively), and some contribution from the floor litter (Zhao et al., 2013c).
Figure 9.
Daily mean carbon dioxide (CO2) concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses. (A) Daily mean CO2 concentration; (B) Daily mean CO2 concentration vs. ambient temperature.
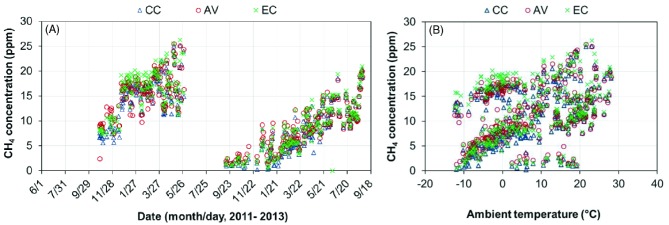
Although the low level of CH4 is not a relevant indicator of air quality in terms of hen or human health, it is one of the most important greenhouse gases responsible for global warming, thus, its inclusion in this environmental impact monitoring. The indoor CH4 concentration tended to be correlated with ambient temperature (Figure 10); however, the relationship could be confounded by other factors such as the amount of manure accumulation and moisture content (i.e., anaerobic condition). The overall daily mean CH4 concentrations were similar among the three houses: 10.9 ppm for the CC house (95% C.I. of 10.4 to 11.6 ppm), 11.7 ppm for the AV house (95% C.I. of 11.1 to 12.3 ppm), and 11.9 ppm for the EC (95% C.I. of 11.3 to 12.6 ppm) (Table 4). The CH4 concentrations observed in this study were comparable to those measured in other Midwest U.S. aviary houses (Hayes et al., 2013), but was about 2.5 times higher than those reported for European aviary houses (Wathes et al., 1997).
Figure 10.
Daily mean methane (CH4) concentrations of the conventional cage (CC), aviary (AV) ,and enriched colony (EC) houses. (A) Daily mean CH4 concentration; (B) Daily mean CH4 concentration vs. ambient temperature.
Ambient and indoor N2O concentrations in all houses were very low and constantly below the detection limit (0.2 ppm) of the instrument. Therefore, the data were excluded from presentation.
Particulate Matter (PM) Concentrations
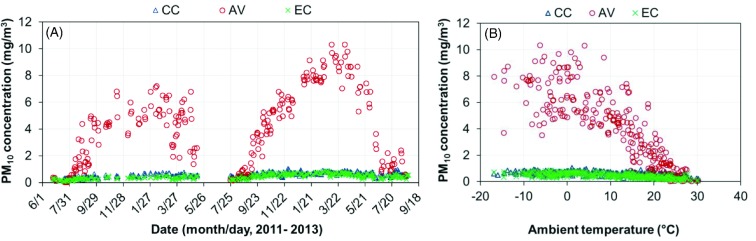
The PM10 concentrations were found to be significantly higher in the AV house than in the CC and EC houses (Table 4, Figure 11). The overall daily mean PM10 concentrations were 0.59 mg/m3 for the CC house (95% C.I. of 0.57 to 0.61 mg/m3), 3.95 mg/m3 for the AV house (95% C.I. of 3.61 to 4.29 mg m−3), and 0.44 mg/m3 for the EC house (95% C.I. of 0.42 to 0.46 mg/m3) (Table 4). Based on the review of previous PM monitoring in laying-hen houses, AV housing systems have much higher PM concentrations than cage housing systems (Appendix 2). It is well known that PM levels are closely related to animal activities in livestock and poultry houses (Takai et al., 1998; Zhao et al., 2014b). When floor bedding or litter is provided in housing systems (such as AV housing) to accommodate animal natural behaviors (e.g., dust-bathing and foraging for laying hens), PM generation can be higher by a pronounced amount. Figure 12 shows the diurnal variation of PM10 concentrations on an example day. It is apparent that spikes of PM10 concentrations coincided with the light-on time when hens woke up and started the first feeding. The PM10 concentration in the AV house further increased during litter access period, and sometimes exceeded the upper limit (20 mg/m3) of the TEOM measurement. Eventually, PM10 returned to lower levels after the lights were turned off.
Figure 11.
Daily mean PM10 concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses. (A) Daily mean PM10 concentration; (B) Daily mean PM10 concentration vs. ambient temperature.
Figure 12.
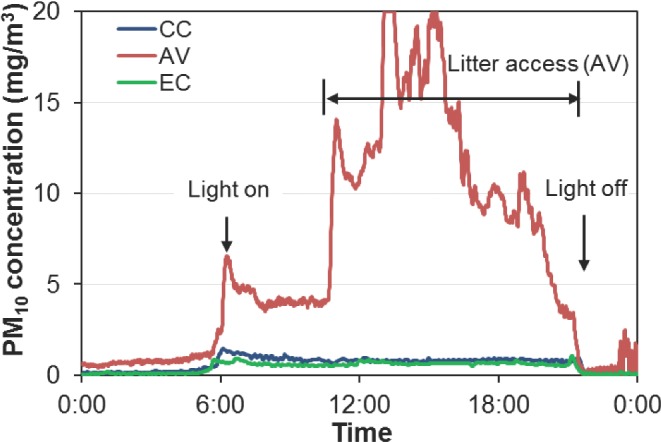
An example of diurnal PM10 concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses.
Table 5 shows that PM10 concentrations were much higher in the AV house than those in the other two houses at ambient temperature <25°C. However, this housing effect diminished when VR reached the maximum, in both quantity and dilution effect. Table 5 also shows the seasonal variations in indoor PM10 concentration in the three houses, being higher under cold weather and lower under warm weather.
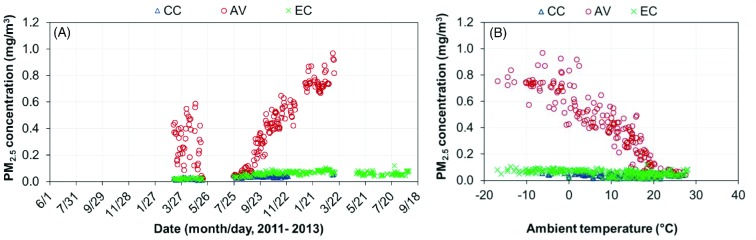
Similar to PM10, PM2.5 concentrations were higher in the AV house than in the CC and EC houses (Figure 13). In fact, it has been reported that PM2.5 accounts for a relatively stable portion (5% to 13%) of PM10 in hen houses. In this study, the portion of PM2.5 relative to PM10 was found to be 5.9% in the CC house, 10.4% in the AV house, and 12.6% in the EC house. Compared to PM10, PM2.5 concentration was less influenced by ambient temperature and, thus, VR.
Figure 13.
Daily mean PM2.5 concentrations of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses. (A) Daily mean PM2.5 concentration; (B) Daily mean PM2.5 concentration vs. ambient temperature.
CONCLUSIONS
This paper describes the environmental monitoring system and the results of thermal environment (temperature and RH), indoor air quality (gaseous and particulate matter concentrations), and building ventilation rate (VR) of three laying-hen housing systems, i.e., conventional cage (CC), enriched colony (EC), and aviary (AV) for the Coalition for Sustainable Egg Supply (CSES) project. The monitoring was performed over a 27-month period covering two single-cycle flocks. The following observations and conclusions were made.
All three houses had similar thermal environment conditions throughout the two-flock periods.
Indoor air quality of the CC and EC houses were comparable, which was better than that of the AV house that had higher ammonia (occasionally exceeding 25 ppm) and PM concentrations, especially at ambient temperature <10°C.
Overall, ammonia concentrations in all three houses were at the lower end of the range observed in previous studies (involving both high-rise and manure-belt hen houses).
Gaseous and PM concentrations were inversely related to ambient temperature or VR.
Spatial variations in the aerial constituents can exist in hen houses due to differences in ventilation air distribution and localized generation of the constituents. This characteristic points out the importance of multi-location sampling when assessing indoor air quality and aerial emissions.
Mitigation practices for litter-floored AV houses should be explored to safeguard animal and human health and to reduce the environmental impact.
Acknowledgments
Cash funding for the study was supported by the Coalition for Sustainable Egg Supply (CSES). In-kind contributions by Iowa State University and the Egg Industry Center were provided in the form of availing state-of-the-art environmental monitoring equipment (approximately $400,000 worth) to the project. We sincerely appreciate the cooperation and assistance of the egg producer in the implementation of this field study.
APPENDIX
Table A1.
Summary of published data on ammonia (NH3) concentrations in different laying-hen housing systems.
| NH3 Concentration | Housing | Manure | Manure Removal | Country/Region | Measurement | Measurement | Measurement | Reference |
|---|---|---|---|---|---|---|---|---|
| (ppm) | System1 | System3 | Frequency | Technique4 | Duration | Frequency | ||
| 2.8–5.4 | CC | MB | Daily / Semi-weekly | United States | Dräeger | 1 year | Two days every 1 or 3 weeks | Liang et al. (2005) |
| 12.9–13.3 | CC | MB | Once every 3 days | United States | Innova 1412 | 2 years | Continuous | Ni et al. (2012) |
| 4.0 | CC | MB | Twice per week | United States | Innova 1412 | 27 months | Continuous | This study |
| 2.3–6.8 | CC | MC | Daily | China | Innova 1312 | 1 year | 5 consecutive days per season | Zhu et al. (2011) |
| 3–5 | CC | - | - | Switzerland | - | - | 4 days | Aggrey et al. (1990) |
| 2.7 | CC | - | - | Germany | NH3 Analyzer | - | - | Seedorf and Hartung (1999) |
| 3.5–7.0 | CC | - | - | Taiwan | Portable gas monitor and NH3 tubes | 1 year | 4 (summer) or 2 (winter) times | Cheng et al. (2011) |
| 11.9 | CC | MB or PT | - | United Kingdom | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 5.9 | CC | MB or PT | - | The Netherlands | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 6.1 | CC | MB or PT | - | Denmark | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 1.6 | CC | MB or PT | - | Germany | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 13.5 | CC | PT | - | United Kingdom | NH3 Analyzer | 1 year | 1 day (at least) in summer and winter | Wathes et al. (1997) |
| 23 | CC | HR | - | United States | NH3 Analyzer | 0.5 year (Dec–Jun) | Continuous | Lim et al. (2003a) |
| 35.9–44.8 | CC | HR | Annually | United States | Dräeger | 1 year | Two days every 2 or 3 weeks | Liang et al. (2005) |
| 48.9–51.9 | CC | HR | Annually or less | United States | Innova 1412 | 2 years | Continuous | Ni et al. (2012) |
| 20.7–22.9 | CC | HR | Annually | United States | Innova 1412 | 2 years | Continuous | Wang et al. (2012) |
| 2.5–5.2 | EC | MB | Semi-weekly / Every 5 days | Sweden | Kitagawa, Dräeger | 0.4 year (Jan–Apr) | - | Nimmermark et al. (2009) |
| 0.4–4.2 | EC | MB | Weekly | Germany | Innova 1302 | 2 years | 1 day (2 hours at noon) per month | Hinz et al. (2010) |
| 2.8 | EC | MB | Twice per week | United States | Innova 1412 | 27 months | Continuous | This study |
| 5–35 | AV | PT and L | - | Switzerland | - | - | 4 days | Aggrey et al. (1990) |
| 12.3 | AV | PT | - | United Kingdom | NH3 Analyzer | 1 year | 1 day (at least) in summer and winter | Wathes et al. (1997) |
| 11.1–16.0 | AV | MB and L | Once per 0.5–5 days | The Netherlands | NH3 Analyzer | - | 5 consecutive 3-week periods | Groot Koerkamp and Bleijenberg (1998) |
| 8.3 | AV | L | - | United Kingdom | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 29.6 | AV | L | - | The Netherlands | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 25.2 | AV | L | - | Denmark | NH3 Analyzer | 1 year | 4 days in summer and winter | Groot Koerkamp et al. (1998) |
| 6.8–11.9 | AV | L | - | Italy | Bruel&Kjiaer | 0.3 year (Jul–Oct) | 4 days | da Borso et al. (2004) |
| 8–282 | AV | MB and L | Once per 8 days or more | Sweden | Infrared spectrophotometer | 1.25 years | Continuous | Gustafsson and von Wachenfelt (2005) |
| 32–38 | AV | MB and L | Weekly (MB); end of flock (L) | Sweden | Kitagawa, Dräeger | 0.4 year (Jan–Apr) | - | Nimmermark et al. (2009) |
| 57–85 | AV | L | End of flock (L) | Sweden | Kitagawa, Dräeger | 0.4 year (Jan–Apr) | - | Nimmermark et al. (2009) |
| 2.2–18.5 | AV | MB and L | Weekly (MB); end of flock (L) | Germany | Innova 1302 | 2 years | 1 day (2 hours at noon) per month | Hinz et al. (2010) |
| 9.2–47.4 | AV | L | End of flock (L) | Germany | Innova 1302 | 2 years | 1 day (2 hours at noon) per month | Hinz et al. (2010) |
| 0.4–12.8 | AV | MB and L | 1/2 or 1/3 manure were removed daily (MB); end of flock (L) | United States | Dräeger | 1 year | Two days every 2 weeks | Zhao et al. (2013a) |
| 8.7 | AV | MB and L | 1/3 or 1/7 manure were removed daily (MB); end of flock (L) | United States | Innova 1412 | 1.75 years | Continuous | Hayes et al. (2013) |
| 6.7 | AV | MB and L | Twice per week | United States | Innova 1412 | 27 months | Continuous | This study |
| 1.9–33.6 | FR | PT | End of flock (L) | Germany | Innova 1302 | 2 years | 1 day (2 hours at noon) per month | Hinz et al. (2010) |
| 12.7–15.5 | FR | MB and L | Semi-weekly / Weekly | The Netherlands | Impinger | 1.4 years | 2 consecutive days per season | Dekker et al. (2011) |
CC = conventional cage; EC = enriched colony; AV = aviary; and FR = free range.
Estimated values from figure.
MB = manure belt; MC = manure channel (shallow manure pit scrapped regularly); PT = deep pit; HR = high-rise; L = litter.
Manufactory information: Dräeger (Dräeger Safety, Inc., Pittsburgh, PA), Innova (LumaSense Technologies, Ballerup, Denmark), NH3 analyzer (Thermo Environmental Instruments, USA), Portable gas monitor (VRAE Hand-Held 5-Gas Surveyor; RAE Systems, San Jose, CA), NH3 tubes (No. 3L; Gastec Corp., Ayase, Japan), Kitagawa (105SC, 105SD, 126SF, Komyo Rikagako Kogyo K.K., Kanagawa, Japan), infrared spectrophotometer (Miran 203, Foxboro Analytical, UK), Bruel&Kjiaer (Model 1302, Brüel & Kjær Sound & Vibration Measurement A/S, Nærum, Denmark).
Note: References are searched in Google Scholar and Academic Search Premier (EBSCO) using keywords ‘ammonia’, ‘concentration’, and ‘hen’.
Table A2.
Summary of published data on particulate matter (PM) concentrations in different laying-hen housing systems.
| PM10 Concentration (mg/m3) | PM2.5 Concentration (mg/m3) | Housing System1 | Manure System2 | Country/Region | Measurement Technique3 | Measurement Duration | Measurement Frequency | Reference |
|---|---|---|---|---|---|---|---|---|
| 0.094 | - | CC | MC | Italy | Photometer | 1 year | Continuous | Costa and Guarino (2009) |
| 0.215 | - | CC | L | Italy | Photometer | 1 year | Continuous | Costa and Guarino (2009) |
| 0.108 | - | CC | MB | Italy | Photometer | 1 year | Continuous | Costa and Guarino (2009) |
| 0.381–0.11 | 0.032–0.113 | CC | PT | Italy | Photometer | 0.5 year (Jun–Dec) | 4 one-week periods | Fabbri et al. (2007) |
| 0.074–0.42 | 0.021–0.13 | CC | MB | Italy | Photometer | 0.5 year (Jun–Dec) | 4 one-week periods | Fabbri et al. (2007) |
| CC | ||||||||
| 0.553 | 0.033 | CC | HR | United States | TEOM | - | 5 days for PM10, 1 day for PM2.5 | Heber et al. (2006) |
| 0.393 | 0.044 | CC | HR | United States | TEOM | 1.5 years | Continuous | Li et al. (2011) |
| 0.518 | 0.039 | CC | HR | United States | TEOM | 6 days (Jun) | Continuous | Lim et al. (2003b) |
| 0.224–1.16 | - | CC | HR | United States | TEOM | 0.5 year (Aug–Jan) | Continuous | Lim et al. (2007) |
| 0.2–0.38 | 0.06–0.104 | CC | PT | Italy | Photometer | 0.3 year (Aug–Nov) | 34 consecutive days in Aug–Sep, and 34 consecutive days in Oct–Nov | Guarino et al. (2002) |
| 0.085–0.48 | 0.027–0.11 | CC | MB | Italy | Photometer | 0.3 year (Aug–Nov) | 34 consecutive days in Aug to Sep, and 34 consecutive days in Oct to Nov | Guarino et al. (2002) |
| 0.540–0.552 | - | CC | HR | United States | TEOM | 2 years | Continuous | Ni et al. (2012) |
| 0.415–0.761 | - | CC | MB | United States | TEOM | 2 years | Continuous | Ni et al. (2012) |
| 0.265 | - | CC | MB | United States | TEOM | 0.5 year (Aug–Feb) | Continuous | Zhao et al. (2005) |
| 0.59 | 0.035 | CC | MB | United States | TEOM | 27 month | Continuous | This study |
| 0.44 | 0.056 | EC | MB | United States | TEOM | 27 month | Continuous | This study |
| 2.403 | 0.103 | AV | L | The Netherlands | Cyclone | Mar - Apr | 2 days | Zhao et al. (2009) |
| 2.3 | 0.25 | AV | MB and L | United States | TEOM | 1.75 years | Continuous | Hayes et al. (2013) |
| 3.95 | 0.41 | AV | MB and L | United States | TEOM | 27 month | Continuous | This study |
CC = conventional cage; EC = enriched colony; and AV = aviary.
MB = manure belt; MC = manure channel (shallow manure pit scrapped regularly); PT = deep pit; HR = high-rise; L = litter.
Manufactory information: Photometer (EPAM 5000, HAZ-Dust; Environmental Devices Corporation, Plaistow, NH), TEOM (Model 1400a, Thermo Fisher Scientific Inc., Waltham, MA), Cyclone (URG corp., USA).
Note: References are searched in Google Scholar and Academic Search Premier (EBSCO) using keywords ‘particulate matter’, ‘concentration’, and ‘hen’.
REFERENCES
- Aggrey S. E., Kroetzl H., Foelsch D. W. Behaviour of laying hens during induced moulting in three different production systems. Appl. Anim. Behav. Sci. 1990;25(1):97–105. [Google Scholar]
- Cheng W. H., Chou M. S., Tung S. C. Gaseous ammonia emission from poultry facilities in Taiwan. Environ. Eng. Sci. 2011;28(4):283–289. [Google Scholar]
- Chepete H. J., Xin H. Ventilation rates of a laying hen house based on new vs. old heat and moisture production data. Appl. Eng. Agric. 2004;20(6):835–842. [Google Scholar]
- Costa A., Guarino M. Particulate matter concentration and emission factor in three different laying hen housing systems. J. Agr. Eng. 2009;40(3):15–24. [Google Scholar]
- da Borso F., Chiumenti A., Rodar T. Gaseous emissions from alternative housing systems for laying hens; Proc. Proceedings of the 11th International Conference of RAMIRAN: Sustainable Organic Waste Management for Environmental Protection and Food Safety; 2004. [Google Scholar]
- Dekker S. E. M., Aarnink A. J. A., de Boer I. J. M., Groot Koerkamp P. W. G. Emissions of ammonia, nitrous oxide, and methane from aviaries with organic laying hen husbandry. Biosyst. Eng. 2011;110(2):123–133. [Google Scholar]
- Fabbri C., Valli L., Guarino M., Costa A., Mazzotta V. Ammonia, methane, nitrous oxide and particulate matter emissions from two different buildings for laying hens. Biosyst. Eng. 2007;97(4):441–455. [Google Scholar]
- Gates R. S., Casey K. D., Xin H., Wheeler E. F., Simmons J. D. Fan assessment numeration system (FANS) design and calibration specifications. Trans. ASABE. 2004;47(5):1709–1715. [Google Scholar]
- Groot Koerkamp P. W. G., Metz J. H. M., Uenk G. H., Phillips V. R., Holden M. R., Sneath R. W., Short J. L., White R. P. P., Hartung J., Seedorf J., Schröder M., Linkert K. H., Pedersen S., Takai H., Johnsen J. O., Wathes C. M. Concentrations and emissions of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998;70(1):79–95. [Google Scholar]
- Groot Koerkamp P. W. G., Bleijenberg R. Effect of type of aviary, manure and litter handling on the emission kinetics of ammonia from layer houses. Br. Poult. Sci. 1998;39(3):379–392. doi: 10.1080/00071669888935. [DOI] [PubMed] [Google Scholar]
- Guarino M., Navarotto P., Valli L., Sonzogni A. Proc. Particulate Matter in and from Agriculture. Germany: Braunschweig; 2002. Particulate matter concentrations in two different buildings for laying hens: a first note. [Google Scholar]
- Gustafsson G., von Wachenfelt E. Measures against ammonia release in a floor housing system for laying hens. Agricultural Engineering International: The CIGR EJournal. 2005:71–11. [Google Scholar]
- Hayes M. D., Xin H., Li H., Shepherd T. A., Zhao Y., Stinn J. Ammonia, greenhouse gas, and particulate matter emissions of aviary layer houses in the midwestern United State. Trans. ASABE. 2013;56(5):1921–1932. [Google Scholar]
- Heber A. J., Lim T., Ni J. Q., Tao P., Schmidt C. A. M., Koziel J. A., Hoff S. J., Jacobson L. D., Zhang Y., Baughman G. B. Quality-assured measurements of animal building emissions: particulate matter concentrations. J. Air Waste Manage. 2006;56(12):1642–1648. doi: 10.1080/10473289.2006.10464569. [DOI] [PubMed] [Google Scholar]
- Hinz T., Winter T., Linke S. Luftfremde Stoffe in und aus verschiedenen Haltungssystemen für Legehennen–Teil 1: Ammoniak [Air pollutants concentrations and emissions of different systems for laying hens–Part1: Ammonia] Landbauforsch. 2010;60(3):139–150. [Google Scholar]
- Li S., Li H., Xin H., Burns R. Particulate matter concentrations and emissions of a high-rise layer house in Iowa. Trans. ASABE. 2011;54(3):1093–1101. [Google Scholar]
- Liang Y., Xin H., Wheeler E. F., Gates R. S., Li H., Zajaczkowski J. S., Topper P. A., Casey K. D., Behrends B. R., Burnham D. J. Ammonia emissions from U. S. laying hen houses in Iowa and Pennsylvania. Trans. ASAE. 2005;48(5):1927–1941. [Google Scholar]
- Lim T. T., Heber A. J., Ni J. Q. Proc. American Society of Agricultural and Biological Engineers Conference. 2003a. Air quality measurements at a laying hen house: Ammonia concentrations and emissions. [Google Scholar]
- Lim T. T., Heber A. J., Ni J. Q., Gallien J. X., Xin H. Proc. Air Pollution from Agricultural Operations III. NC, USA: Research Triangle Park; 2003b. Air quality measurements at a laying hen house: Particulate matter concentrations and emissions. [Google Scholar]
- Lim T. T., Sun H., Ni J. Q., Zhao L., Diehl C. A., Heber A. J., Hanni S. M. Field tests of a particulate impaction curtain on emissions from a high-rise layer barn. Trans. ASABE. 2007;50(5):1795–1805. [Google Scholar]
- Moody L. B., Li H., Burns R. T., Xin H., Gates R. S., Hoff S. J., Overhults D. A quality assurance project plan for monitoring gaseous and particulate matter emissions from broiler housing. MI, USA: ASABE St. Joseph; 2008. [Google Scholar]
- Muhlbauer R., Shepherd T. A., Li H., Burns R. T., Xin H. Development and testing of an induction-operated current switch for monitoring fan operation. Appl. Eng. Agric. 2011;27(2):287–292. [Google Scholar]
- Ni J. Q., Chai L. L., Chen L. D., Bogan B. W., Wang K. Y., Cortus E. L., Heber A. J., Lim T. T., Diehl C. A. Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses. Atmos. Environ. 2012. pp. 57165–174.
- Nimmermark S., Lund V., Gustafsson G., Eduard W. Ammonia, dust and bacteria in welfare-oriented systems for laying hens. Ann. Agr. Env. Med. 2009;16(1):103–113. [PubMed] [Google Scholar]
- Seedorf J., Hartung J. Survey of ammonia concentrations in livestock buildings. J. Agric. Sci. 1999;133(4):433–437. [Google Scholar]
- Shepherd T., Zhao Y., Xin H. Environmental assessment of three laying-hen housing systems–Part II: Air emissions. Poul. Sci. 2014;94(3):534–543. doi: 10.3382/ps/peu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H., Pedersen S., Johnsen J. O., Metz J. H. M., Koerkamp P., Uenk G. H., Phillips V. R., Holden M. R., Sneath R. W., Short J. L., White R. P., Hartung J., Seedorf J., Schroder M., Linkert K. H., Wathes C. M. Concentrations and emissions of airborne dust in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998;70(1):59–77. [Google Scholar]
- UEP. Animal husbandry guidelines for U.S. egg laying flocks (2014 Edition) 2014. United Egg Producers ed. ( http://www.unitedegg.org/information/pdf/UEP-Animal-Welfare-Guidelines-2014.pdf10.3382/ps/peu076.html, accessed on August 31, 2014)
- Wang-Li L., Li Q., Wang K., Bogan B. W., Ni J., Cortus E. L., Heber A. J. National air emisison monitoring study—Southeast layer site: Part III—Ammonia concentrations and emissions. Trans. ASABE. 2012;56(3):1185–1197. [Google Scholar]
- Wathes C. M., Holden M. R., Sneath R. W., White R. P., Phillips V. R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997;38(1):14–28. doi: 10.1080/00071669708417936. [DOI] [PubMed] [Google Scholar]
- Zhao L. Y., Lim T. T., Heber A. J., Sun H. W., Diehl C. A., Ni J. Q., Tao P., Hanni S. M. Proc. 2005 ASAE Annual International Meeting. Tampa, Florida: 2005. Particulate matter emissions from a Ohio belt-battery laying barn. [Google Scholar]
- Zhao Y., Aarnink A. J. A., Hofschreuder P., Groot Koerkamp P. W. G. Evaluation of an impaction and a cyclone pre-separator for sampling high PM10 and PM2.5 concentrations in livestock houses. J. Aerosol Sci. 2009;40(10):868–878. [Google Scholar]
- Zhao Y., Xin H., Shepherd T. A., Hayes M. D., Stinn J. P. Modelling ventilation rate, balance temperature and supplemental heat need in alternative vs. conventional laying-hen housing systems. Biosyst. Eng. 2013a;115(3):311–323. [Google Scholar]
- Zhao Y., Xin H., Shepherd T. A., Hayes M. D., Stinn J. P., Li H. Thermal environment, ammonia concentrations and emissions of aviary houses with white laying hens. Trans. ASABE. 2013b;56(3):1145–1156. [Google Scholar]
- Zhao Y., Zhao D., Wang W., Xin H. Proc. ASABE Annual International Meeting. Kansas City, Missouri: 2013c. Characterizing manure and litter properties and their carbon dioxide production in an aviary laying-hen housing system. [Google Scholar]
- Zhao Y., Shepherd T. A., Swanson J., Mench J. A., Karcher D. M., Xin H. Comparative evaluation of three egg production systems: Housing characteristics and management practices. Poul. Sci. 2014a;94(3):475–484. doi: 10.3382/ps/peu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A. J. A., de Jong M. C. M., Groot Koerkamp P. W. G. Airborne microorganisms from livestock production systems and their relation to dust. Crit. Rev. Env. Sci. Tec. 2014b;44(10):1071–1128. doi: 10.1080/10643389.2012.746064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Dong H., Zhou Z., Xin H., Chen Y. Ammonia and greenhouse gases concentrations and emissions of a naturally ventilated laying hen house in Northeast China. Trans. ASABE. 2011;54(3):1085–1091. [Google Scholar]