Abstract
As an integral part of the Coalition for Sustainable Egg Supply (CSES) Project, this study simultaneously monitored air emissions of 3 commercially operated egg production systems at the house level and associated manure storage over 2 single-cycle flocks (18 to 78 wk of age). The 3 housing systems were 1) a conventional cage house (CC) with a 200,000-hen capacity (6 hens in a cage at a stocking density of 516 cm2/hen), 2) an enriched colony house (EC) with a 50,000-hen capacity (60 hens per colony at a stocking density of 752 cm2/hen), and 3) an aviary house (AV) with a 50,000-hen capacity (at a stocking density of 1253 to 1257 cm2/hen). The 3 hen houses were located on the same farm and were populated with Lohmann white hens of the same age. Indoor environment and house-level gaseous (ammonia [NH3] and greenhouse gasses [GHG], including carbon dioxide [CO2], methane [CH4], and nitrous oxide [N2O]) and particulate matter (PM10, PM2.5) emissions were monitored continually. Gaseous emissions from the respective manure storage of each housing system were also monitored. Emission rates (ERs) are expressed as emission quantities per hen, per animal unit (AU, 500 kg live BW), and per kilogram of egg output. House-level NH3 ER (g/hen/d) of EC (0.054) was significantly lower than that of CC (0.082) or AV (0.112) (P < 0.05). The house-level CO2 ER (g/hen/d) was lower for CC (68.3) than for EC and AV (74.4 and 74.0, respectively), and the CH4 ER (g/hen/d) was similar for all 3 houses (0.07 to 0.08). The house-level PM ER (mg/hen/d), essentially representing the farm-level PM ER, was significantly higher for AV (PM10 100.3 and PM2.5 8.8) than for CC (PM10 15.7 and PM2.5 0.9) or EC (PM10 15.6 and PM2.5 1.7) (P < 0.05). The farm-level (house plus manure storage) NH3 ER (g/hen/d) was significantly lower for EC (0.16) than for CC (0.29) or AV (0.30) (P < 0.05). As expected, the magnitudes of GHG emissions were rather small for all 3 production systems. Data from this study enable comparative assessment of conventional vs. alternative hen housing systems regarding air emissions and enhance the U.S. national air emissions inventory for farm animal operations.
Keywords: air emissions, egg production, alternative hen housing
INTRODUCTION
The U.S. egg industry may gradually transition toward alternative hen housing systems, such as enriched colony and aviary houses, to meet specific animal welfare regulations. Housing system design and management has a significant role in the environmental footprint of egg production; however, limited information is available on the environmental impact of alternative hen housing systems in the United States. Thus, quantification of aerial emissions from alternative housing systems in comparison to the conventional housing system is needed to develop baseline emission values and comparisons for inclusion in the U.S. national air emissions inventory for farm animal operations. This article results from the multidisciplinary and multiinstitutional endeavor known as the Coalition for Sustainable Egg Supply (CSES) project, which evaluated a conventional cage house (CC), an aviary house (AV), and an enriched colony house (EC) with regards to animal health and well-being, environmental impact, food safety, food affordability, and worker health (Swanson et al., 2014).
As part of the Socially Sustainable Egg Production Project (SSEP), Xin et al. (2011) reviewed the current state of science and subsequently identified specific knowledge gaps and future research needs to improve understanding of the environmental impacts of conventional and alternative laying hen production systems. Key research areas identified were: 1) quantification of indoor air quality, barn emissions, thermal conditions, and energy use in alternative hen housing systems along with conventional housing systems and 2) assessment of interactions between air quality, housing systems, worker health, and animal health and well-being. While studies on emissions of ammonia (NH3), greenhouse gases (GHGs), and particulate matter (PM) from commercial laying hen houses have been carried out on both conventional and alternative hen housing systems in Europe and the United States, studies to simultaneously compare different housing systems are difficult to accurately perform; hence, information is very limited. Indoor air quality and emissions depend on environmental conditions (weather) encountered during each study and management decisions at the farm level (e.g., stocking density and production goals and schedule, which lead to differences in flock age, feed formulation, in-house temperature, and manure management practices). Comparisons among studies with similar housing systems often yield differences that cannot be fully discerned due to the confounding effects of the environmental conditions and farm management. Laboratory and pilot-scale studies can provide insight into specific factors (e.g., stocking density, feed formulation, manure handling) affecting gaseous emissions; however, field-scale trials are often necessary to verify the impact of these factors under commercial conditions. Thus, a study that normalizes the effects of environmental factors by co-locating multiple housing systems at a common site and using similar management practices to the extent possible (system specific) provides an ideal opportunity to characterize and compare the housing systems of interest at the commercial scale.
The goal of this component of the CSES project was to assess the environmental impact of the 3 housing systems. Specific objectives were to quantify and compare 1) house-level emission rates (ERs) of NH3, GHGs (including carbon dioxide [CO2], nitrous oxide [N2O] and methane [CH4]), and particulate matter (PM10 and PM2.5); 2) gaseous ER associated with the long-term manure storage of each housing system; and 3) farm-level (i.e., house plus corresponding manure storage) gaseous emissions of each housing system. It should be noted that PM emissions from the static manure storage piles are trivial, as such PM emissions form each house were considered to represent the farm-level PM ERs. The companion paper by Zhao et al. (2014a) delineates indoor air quality, thermal environment, and building ventilation rate (VR) for each of the housing types.
MATERIALS AND METHODS
The 27 mo environmental monitoring was carried out in 3 laying hen housing systems located at the same farm in the U.S. Midwest over 2 single-cycle flocks. The housing systems included 1) a CC with a 200,000-hen capacity; 2) an EC with a 50,000-hen capacity; and 3) an AV with a 50,000-hen capacity. Lohmann LSL White laying hens of the same age were placed and managed under standard commercial practices until approx. 77 to 78 wk of age per flock with no molt. The monitoring periods were April 2011 to June 2012 for flock 1 and July 2012 to August 2013 for flock 2, with a 3 wk downtime between flocks when no monitoring was performed.
A detailed description of each housing system design and management practices is given in the companion paper by Zhao et al. (2014b). Similarly, a detailed description of installation and operation of the monitoring system for the house-level gaseous and PM concentrations and building VR is given in another companion paper by Zhao et al. (2014a). Thus, only information related to the additional monitoring system for manure storage emissions and determination of ERs is presented in this paper.
Manure Storage Monitoring System
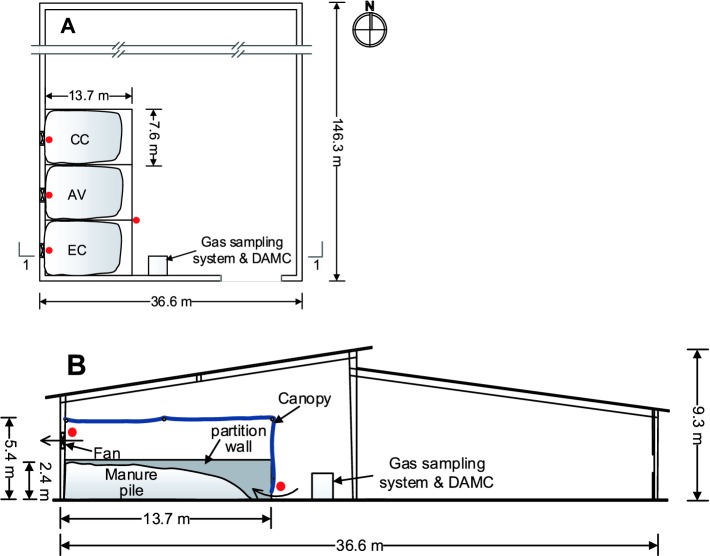
Three individual manure storage bays were constructed within the footprint of the communal manure storage to quantify the emissions of NH3, CO2, CH4, and N2O gases associated with the storage of manure generated from the 3 monitored houses. Each storage bay (13.7 by 7.6 m) was separated by 2.4 m tall concrete T-walls and enclosed with a 6 mil reinforced polyethylene canopy (DuraSkrim, Raven Industries, Sioux Falls, SD) suspended from the ceiling at a height of 5.4 m and fixed to the perimeter walls (Figure 1). The east end (entrance) of each storage bay was fitted with a sliding tarp to allow access for manure loading and unloading and provide an air inlet at the bottom when closed. Continuous mechanical ventilation of each storage bay was provided with a single-speed, 0.91 m exhaust fan installed in the west wall. The remainder of the otherwise open wall was sealed with clear plastic. The experimental manure storage bays were designed for a 12,000 bird, 6 mo storage capacity.
Figure 1.
Schematic layout (A) and 1–1 cross-section (B) view of the manure storage bay enclosures for the conventional cage house (CC), aviary house (AV), and enriched colony house (EC). Red dots represent gas sampling locations.
Prior to placement in long-term storage, manure removed from each house was weighed with a commercially operated certified grain scale located at the farm. Three continuous emission monitoring periods, each 6 mo long, were conducted during the study: November 2011 to May 2012 (flock 1), August 2012 to March 2013 (flock 2), and April 2013 to August 2013 (flock 2). During the first and second monitoring periods, the bays were loaded on a 2 wk schedule. During the first week, relatively equal amounts of manure (approx. 11 tonnes) were placed into each respective storage bay, with the remainder placed in the general storage area; during the second week, manure was weighed and placed directly into the general storage area. During the third monitoring period, the stored manure was loaded into the monitoring bays every week. At the end of each storage period, manure was removed from the monitoring bays and weighed again.
Monitoring of the manure storage system was accomplished with a self-contained air emission monitoring system housed in the manure storage shed. Similar in principle to the mobile air emissions monitoring unit (MAEMU), the self-contained system integrated a data acquisition (DAQ) system (Compact Fieldpoint, National Instruments, Austin, TX) and 4 pumps to automatically collect and analyze exhaust air samples from each bay and one sample of the intake air to the storage bays. The positive-pressure air sampling system sequentially sampled each location for 15 min (the first 7 min were for stabilization, and the last 8 min were averaged for measurement), yielding 60 min data of gaseous concentrations. Concentrations of NH3, CO2, CH4, NO2, and dew-point temperature (DP) were measured with a photoacoustic multigas analyzer (INNOVA 1412, LumaSense Technologies A/S, Ballerup, Denmark). Air temperature was measured at the air intake of the storage bays and at the exhaust fan of each bay. Manure pile temperatures were measured periodically through the storage cycles with a temperature probe attached to a portable data logger (HOBO Pro Series, Onset, Bourne, MA) at depths of 0.3 and 0.6 m below the surface. The surface temperature was measured with a thermal imaging camera (T440, FLIR Systems, Inc., Boston, MA). Each exhaust fan was calibrated in situ at the end of each storage cycle with a 1.37 m fan assessment numeration system (FANS) unit (Gates et al., 2004).
Calculation of Gaseous and Particulate Matter Emission Rates
The house-level and manure storage gaseous ERs were calculated according to Equation (1). For the house-level ERs, the gaseous concentrations from each sampling cycle were linearly interpolated to the corresponding 30 s VR data, providing a dynamic emission value that was then summed over each day to yield the daily ER. Manure storage ERs were calculated with a 1 hr integration time based on the average hourly gaseous concentration and VR. Equation (2) provides the calculation of PM ERs, which utilized the 30 s average PM10 (particulate matter with an aerodynamic diameter of 10 μm or less) and PM2.5 (particulate matter with an aerodynamic diameter of 2.5 μm or less) concentrations, measured with tapered element oscillating microbalances (TEOMs), and the corresponding house VR. Daily ERs for each system were then normalized to the units of per hen, per animal unit (AU, 500 kg live BW), and per kilogram of egg output based on the corresponding hen production and performance data.
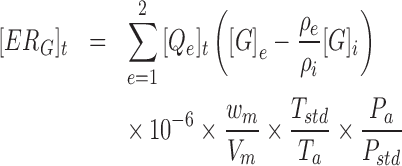 |
(1) |
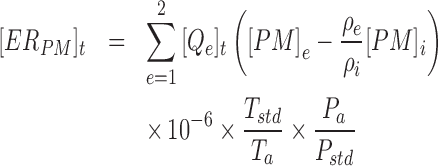 |
(2) |
Where:
[ERG]t = Gaseous emission rate of the house at sample time t (g/house/t)
[ERPM]t = PM emission rate of the house at sample time t (g/house/t)
[Qe]t = VR through location e under the field temperature and barometric pressure at sample time t (m3/house/t)
[G]i = Volumetric gaseous concentration of incoming air (ppmv)
[G]e = Volumetric gaseous concentration of the exhaust air at location e (ppmv)
[PM]i = PM concentration of incoming ventilation air (μg/m3)
[PM]e = PM concentration of exhaust ventilation air at location e (μg/m3)
wm = Molar weight of the gas under consideration (g/mole)
Vm = Molar volume of gas at standard temperature (0°C) and pressure (1 atmosphere) (STP), 0.022414 m3/mole
Tstd = Standard temperature, 273.15 K
Ta = Absolute house temperature (°C + 273.15) (K)
Pstd = Standard barometric pressure, 101.325 kPa
Pa = Atmospheric barometric pressure for the site elevation (kPa)
ρi, ρe = Air density of incoming and exhaust air (kg dry air per m3 moist air).
Statistical analysis was performed to compare the daily mean gaseous and PM ERs among the 3 housing systems using the GLIMMIX model in Statistical Analysis System version 9.3 (SAS 9.3, SAS Institute Inc., Cary, NC). Equation 3 provides the statistical model used for the analysis. Ambient temperature was divided into 5 ranges based on daily means to delineate the housing impact at different climatic conditions: <0°C, 0 to 10°C, 10 to 20°C, 20 to 25°C, and >25°C. Weekly averages of daily means were used as repeated measures in this model. The time step chosen corresponded to the weekly manure removal to reduce potential time dependence of the data. Log transformation of weekly based ERs was performed to provide even residual distribution. The effects were considered significant at a threshold probability level of 0.05. To be included in the analysis, dynamic daily data were first required to pass the quality assurance and quality control (QA/QC) checks as described by Zhao et al. (2014a). In addition, only days when all 3 houses had complete ER data sets were considered, and a minimum of 3 complete daily data sets per week were required for inclusion.
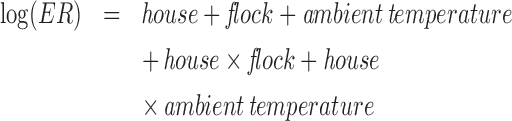 |
(3) |
RESULTS AND DISCUSSION
Over the 27 mo monitoring period, data completeness of daily house-level gaseous ERs was 64% for NH3 and CO2, and 40% for CH4. Measured concentrations of ambient and in-barn N2O concentrations were near or below the detection limit (0.2 ppm) of the INNOVA 1412 and thus were excluded from the analysis. Issues with instrument malfunction, pump failures, and on-farm instrument calibration events account for the missing days of gaseous ERs. The TEOMs operated 2 to 5 d per week with valid readings before high dust concentrations led to saturation of the filter element, providing 31% data completeness for PM10. Availability of fewer TEOM units during flock 1 and assessment of spatial variation of PM10 during flock 2 reduced the total number of days for PM2.5 measurement, leading to a lower data completeness of 17% for PM2.5. Daily manure storage ERs were determined on 329 out of 512 d during the manure loading period, giving 64% data completeness. Table 1 summarizes hen performance and production of both flocks. Table 2 summarizes the manure loading cycle for each monitoring period. It includes the total mass loaded throughout the monitoring period, total mass removed at the end of each monitoring period, the percentage of total manure production from each house placed in the monitoring bays, and the equivalent number of hens represented.
Table 1.
Two-flock summary of 20 to 78 wk production performance of Lohmann LSL white hens in the conventional cage house (CC), aviary house (AV), and enriched colony house (EC).
| Production parameter | Housing type | Reference2 | ||
|---|---|---|---|---|
| Conventional cage (CC)1 | Aviary (AV)1 | Enriched colony (EC)1 | ||
| No. of hens/house (wk 20) | 196,120 | 49,754 | 46,762 | — |
| (193,424/198,816) | (49,830/49,677) | (46,795/46,729) | ||
| Cumulative mortality (%) | 4.4 | 11.5 | 4.8 | 4 to 6 |
| (4.7/4.2) | (11.5/11.5) | (5.1/4.4) | ||
| Hen-day egg production (%) | 89.4 | 87.3 | 92.3 | 87.0 |
| (87.3/91.4) | (86.6/87.9) | (90.5/94.1) | ||
| Eggs per hen housed | 362 | 342 | 373 | 360 |
| (352/369) | (340/344) | (363/381) | ||
| Egg weight (g) | 58.8 | 58.5 | 59.1 | 66.9 |
| (58.5/59.1) | (58.4/58.6) | (59.1/59.0) | ||
| Feed use (g/hen/d) | 106 | 108 | 107 | 105 to 115 |
| (105/107) | (108/108) | (107/106) | ||
| Water use (g/hen/d) | 220 | 184 | 192 | — |
| (221/219) | (183/185) | (193/191) | ||
| Feed conversion ratio (feed:egg) | 1.99 | 2.08 | 1.97 | 2.0 to 2.1 |
| (2.02/1.96) | (2.12/2.04) | (1.99/1.94) | ||
| Average BW (kg) | 1.60 | 1.56 | 1.55 | 1.72 to 1.86 |
| (1.55/1.64) | (1.56/1.56) | (1.52/1.57) | ||
Values are the mean of 2 flocks, with values in paranthese representing flock1 and flock 2 (fock1/flock2), respectively.
Breeder company reference for Lohmann LSL white hen (20 to 78 wk) (Layer Management Guide, http://www.lskpoultry.fi/materiaalit/lsl_managementguide.pdf, accessed on December 5, 2013)
Table 2.
Summary of manure loading into and removal from the storage bays of the conventional cage (CC), aviary (AV), and enriched colony (EC) houses during the 2 flock cycles.
| Period | Manure loaded or removed* | Housing system | ||
|---|---|---|---|---|
| Conventional cage (CC) | Aviary (AV) | Enriched colony (EC) | ||
| November 2011 to May 2012 | Manure loaded (tonne) | 723 | 703 | 676 |
| Manure removed (tonne) | 531 | 509 | 498 | |
| % of total output | 6% | 32% | 26% | |
| Equivalent no. of hens | 11,600 | 14,700 | 11,700 | |
| August 2012 to March 2013 | Manure loaded (tonne) | 798 | 681 | 698 |
| Manure removed (tonne) | 548 | 502 | 504 | |
| % of total output | 7% | 28% | 26% | |
| Equivalent no. of hens | 13,600 | 13,300 | 12,200 | |
| April 2013 to August 2013 | Manure loaded (tonne) | 943 | 688 | 728 |
| Manure removed (tonne) | 730 | 504 | 583 | |
| % of total output | 15% | 54% | 54% | |
| Equivalent no. of hens | 29,700 | 24,500 | 24,500 | |
*Manure loaded is the total mass of manure placed into each storage bay over the monitoring period. Manure removed is the total mass of manure removed from each storage bay at the end of the monitoring period.
House-Level Ammonia Emission Rates
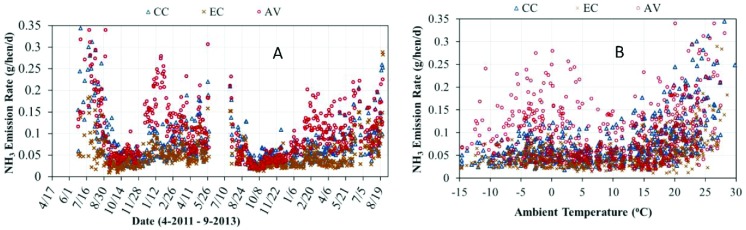
The daily mean NH3 ERs on a per hen basis and its relationship with ambient temperature observed in the study are illustrated in Figure 2 for each housing system across both flocks. The overall means and SE based on different units of per hen, per AU, and per kilogram of egg output are summarized in Table 3. The AV had the highest house-level mean ER (g/hen/d), 0.112, followed by the CC at 0.082 and the EC at 0.054 (P < 0.05). The observed NH3 ERs from this study were within literature values of 0.05 to 0.10 g/hen/d reported for conventional manure belt houses (Liang et al., 2005) and similar aviary houses operated under conditions in the U.S. Midwest: 0.13 to 0.16 g/hen/d (Hayes et al., 2013a) and 0.05 to 0.30 g/hen/d, respectively (Zhao et al., 2013).
Figure 2.
(A) Daily NH3 emission rates (ERs) (g/hen/d) and (B) their relationship to ambient temperature for the conventional cage house (CC), aviary house (AV), and enriched colony house (EC).
Table 3.
House-level daily emission rates (ERs; mean and SE) of ammonia (NH3), carbon dioxide (CO2), methane (CH4), and particulate matter (PM10 and PM2.5) for the conventional cage (CC), aviary (AV), and enriched colony (EC) houses expressed in different units for each of the 2 flocks.
| Housing system | |||||||
|---|---|---|---|---|---|---|---|
| Gas or PM | Unit | Conventional cage (CC) | Aviary (AV) | Enriched colony (EC) | |||
| Flock 1 | Flock 2 | Flock 1 | Flock 2 | Flock 1 | Flock 2 | ||
| NH3 | g/hen/d | 0.097 | 0.068 | 0.136 | 0.088 | 0.059 | 0.049 |
| SE | (0.010) | (0.004) | (0.011) | (0.006) | (0.006) | (0.004) | |
| g/AU/d | 31.2 | 20.7 | 43.4 | 28.1 | 19.3 | 15.6 | |
| SE | (3.22) | (1.22) | (3.51) | (3.22) | (1.97) | (1.27) | |
| g/(kg egg) | 1.90 | 1.26 | 2.69 | 1.71 | 1.10 | 0.88 | |
| SE | (0.20) | (0.07) | (0.22) | (0.12) | (0.11) | (0.07) | |
| CO2a | g/hen/d | 68.5 | 68.1 | 72.5 | 75.6 | 74.9 | 73.9 |
| SE | (0.85) | (0.57) | (0.93) | (1.68) | (1.19) | (1.02) | |
| g/AU/d | 22,054 | 20,750 | 23,133 | 24,169 | 24,557 | 23,550 | |
| SE | (274) | (174) | (297) | (537) | (390) | (325) | |
| g/(kg egg) | 1,341 | 1,261 | 1,434 | 1,468 | 1,400 | 1,331 | |
| SE | (17) | (11) | (18) | (33) | (22) | (18) | |
| CH4 | g/hen/d | 0.09 | 0.07 | 0.10 | 0.05 | 0.11 | 0.05 |
| SE | (0.007) | (0.004) | (0.007) | (0.003) | (0.008) | (0.005) | |
| g/AU/d | 29.0 | 21.3 | 31.9 | 16.0 | 36.1 | 15.9 | |
| SE | (2.25) | (1.22) | (2.23) | (0.96) | (2.62) | (1.59) | |
| g/(kg egg) | 1.8 | 1.3 | 2.0 | 1.0 | 2.1 | 0.9 | |
| SE | (0.14) | (0.07) | (0.14) | (0.06) | (0.15) | (0.09) | |
| PM10 | mg/hen/d | 16.9 | 14.5 | 87.6 | 113.0 | 13.9 | 17.3 |
| SE | (1.02) | (0.90) | (3.92) | (5.07) | (0.66) | (0.90) | |
| g/AU/d | 5.4 | 4.4 | 28.0 | 36.1 | 4.6 | 5.5 | |
| SE | (0.33) | (0.27) | (1.25) | (1.62) | (0.22) | (0.29) | |
| g/(kg egg) | 331 | 268 | 1,732 | 2194 | 260 | 312 | |
| SE | (20) | (17) | (78) | (98) | (12) | (16) | |
| PM2.5 | mg/hen/d | 1.0 | 0.9 | 8.6 | 9.1 | 1.5 | 1.9 |
| SE | (0.24) | (0.14) | (0.32) | (0.27) | (0.10) | (0.15) | |
| g/AU/d | 0.32 | 0.27 | 2.74 | 2.91 | 0.49 | 0.61 | |
| SE | (0.08) | (0.04) | (0.10) | (0.09) | (0.03) | (0.05) | |
| g/(kg egg) | 19.6 | 16.7 | 170 | 177 | 28.0 | 34.2 | |
| SE | (4.70) | (2.59) | (6.3) | (5.2) | (1.87) | (2.70) | |
AU = animal unit = 500 kg live body mass.
Includes CO2 contributions from animal respiration (majority) and CO2 production from manure (minor).
Ammonia ERs of the houses at different ambient temperatures are further delineated by the summary data in Table 4. For the CC and EC, NH3 ERs correlate with ambient temperature (i.e., higher ERs at higher temperatures), with temperatures above 20°C providing statistically higher ERs than lower temperatures. The AV had a different ER profile in that the lowest ERs occurred between 0 and 20°C, with higher ERs occurring at temperatures above and below this range. When comparing NH3 ERs among the 3 houses at common ambient temperatures, the CC and AV were not significantly different, but both had significantly higher ERs than the EC for ambient temperatures above 10°C. At ambient temperatures below 0°C, NH3 ERs of the CC and EC were not significantly different, but both were significantly lower than that of the AV. The higher NH3 ERs found under ambient temperatures above 20°C were partially attributed to increased water consumption by the birds (hence deposit of wetter feces on the manure belt and litter floor) and greater air velocities in the barn, both of which promote NH3 volatilization. The higher NH3 emissions of the AV at lower temperatures were attributed to extended periods of low VRs, which caused moisture to accumulate in the littered floor, thus increasing NH3 volatilization due to conditions more favorable for microbial decomposition of uric acid to NH3. The elevated NH3 ERs during the winter were the primary reason for the difference between the AV and CC. The differences in NH3 ERs between the CC and EC were likely driven by the difference in stocking density of the hens and thus manure load on the belt and effectiveness of each manure drying system. The EC had the lowest manure belt stocking density at 745 cm2/hen in comparison to the CC at 568 cm2/hen. Moisture content (MC) of the manure removed from the houses revealed that the EC had the driest manure at 45.6%, followed by the AV at 51.7% and the CC at 53.6% (unpublished data by Zhang et al., 2014, University of California–Davis). As with the littered floor, higher MC of manure on the belts makes conditions more favorable for the microbial decomposition of uric acid to NH3.
Table 4.
Summary of house-level average daily emission rates (ERs) of ammonia (NH3) and particulate matter (PM10) for the conventional cage (CC), aviary (AV), and enriched colony (EC) housing systems under different ranges of ambient temperature conditions.
| Gas or PM | Daily avg. ambient temperature range (oC) | Average daily ERs (Mean and SE) | ||
|---|---|---|---|---|
| Conventional cage (CC) | Aviary (AV) | Enriched colony (EC) | ||
| NH3 (g/hen/d) | <0 | 0.055 (0.003)c,B | 0.119 (0.010)c,A | 0.045 (0.003)c,B |
| 0 to 10 | 0.053 (0.003)c,A,B | 0.077 (0.009)d,A | 0.036 (0.002)c,B | |
| 10 to 20 | 0.075 (0.005)c,A | 0.088 (0.008)d,A | 0.048 (0.004)c,B | |
| 20 to 25 | 0.133 (0.017)b,A | 0.151 (0.019)b,A | 0.080 (0.010)b,B | |
| >25 | 0.189 (0.053)a,A | 0.197 (0.066)a,A | 0.121 (0.036)a,B | |
| PM10 (mg/hen/d) | <0 | 5.9 (0.3)b,B | 80.8 (5.5)c,A | 7.2 (0.6)c,B |
| 0 to 10 | 9.4 (0.9)b,B | 100.6 (8.0)b,A | 10.3 (0.6)b,c,B | |
| 10 to 20 | 24.5 (2.9)a,B | 138.0 (10.8)a,A | 16.5 (1.2)a,b,B | |
| 20 to 25 | 28.3 (2.0)a,B | 91.6 (17.1)b,c,A | 25.5 (1.9)a,C | |
| >25 | 37.5 (5.3)a,A,B | 65.9 (30.4)c,A | 25.9 (5.4)a,B | |
Within a housing system (column), NH3 or PM10 ER means with different lowercase superscripts are significantly different (P < 0.05). Among the housing systems (i.e., within each row), NH3 or PM10 ER means with different uppercase superscripts are significantly different (P < 0.05).
House-Level Greenhouse Gas Emission Rates
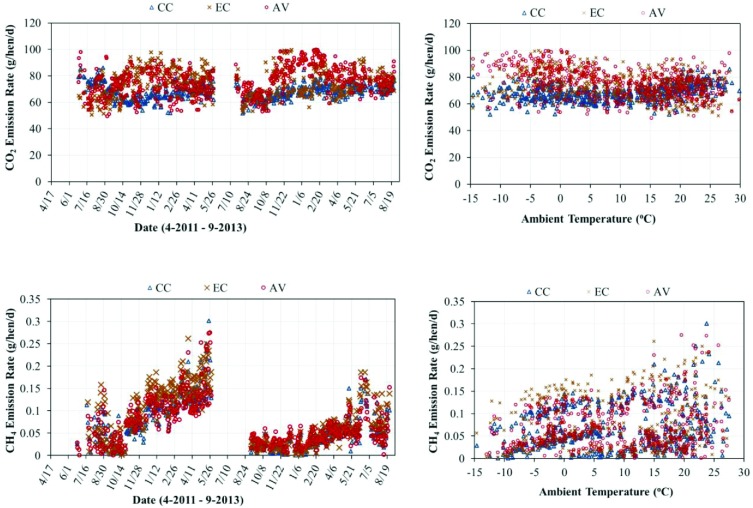
The daily mean CO2 ERs across both flocks and vs. ambient temperature are presented in Figure 3 on a per hen basis for each housing system. The overall means and SE of GHG ERs on the basis of per hen, per AU, and per kilogram of egg output are summarized in Table 3. The EC and AV had statistically higher daily CO2 ERs than the CC (74.4, 74.0, and 68.3 g/hen/d, respectively). Literature reports comparable CO2 ERs for a manure belt system of 70 to 85 g/hen/d (Liang et al., 2005; Neser et al., 1997) and 67 to 83 g/hen/d in a similar AV system housing brown birds operated in the U.S. Midwest (Hayes et al., 2013a). The relatively higher levels of CO2 emissions in the AV and EC in comparison to the CC were presumably due to increased hen activity associated with lower stocking densities. Animal activity level affects metabolic rate, and increased metabolic rate leads to a higher rate of CO2 respiration. CIGR (1999) provides guidelines for ventilation design based on the total and latent heat production rates of laying hens, which accounts for the increased activity and metabolic rates of laying hens housed in floor systems vs. cage systems. In addition to hen respiration, CO2 is generated from the decomposition of manure deposited on the manure belts in all houses and from the littered floor of the AV. Ning (2008) reported that CO2 generated from manure decomposition contributed to between 1 and 5% of the total daily CO2 emission as manure accumulation time increased from 1 to 5 d. Hayes et al. (2013b) measured and partitioned the CO2 emissions of a similar AV system and reported that the littered floor represented 3% of the house-level emissions.
Figure 3.
Daily mean CO2 and CH4 emission rates (ERs) (g/hen/d) and their relationship to ambient temperature for the conventional cage house (CC), aviary house (AV), and enriched colony house (EC).
The daily mean CH4 ERs across both flocks and vs. ambient temperature observed in the study are shown in Figure 3 on a per hen basis for each housing system. All 3 housing systems had similar average daily CH4 ERs of 0.07 to 0.08 g/hen/d. The observed CH4 ERs from this study fall within the ranges reported in literature of 0.08 to 0.13 g/hen/d in conventional manure belt systems (Fabbri et al., 2007; Groot Koerkamp et al., 1998; Monteny et al., 2001; Wathes et al., 1997) and 0.08 to 0.10 g/hen/d in U.S. AV houses with brown birds (Hayes et al., 2013a).
House-Level Particulate Matter Emission Rates
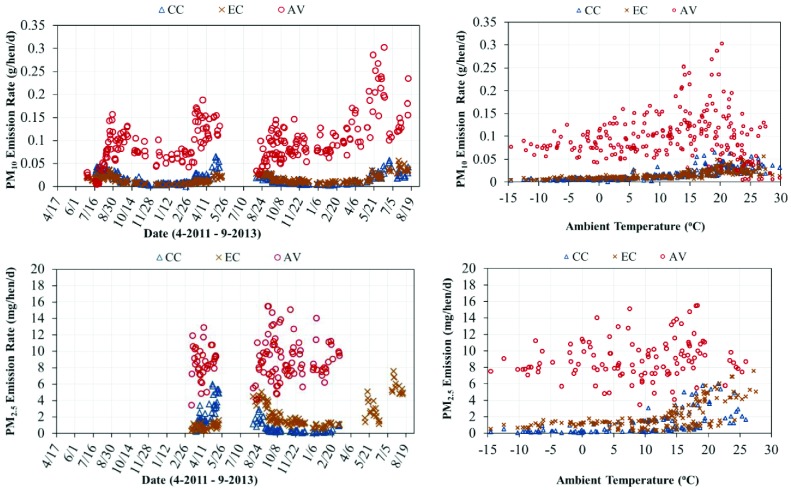
The daily mean PM10 and PM2.5 ERs across both flocks and vs. ambient temperature are shown in Figure 4 on a per hen basis for each housing system. The overall daily ER means and SE on the basis of per hen, per AU, and per kilogram of egg output are summarized in Table 3. The AV had a significantly higher average daily PM10 ER (mg/hen/d) of 100.3 than the CC at 15.7 and the EC at 15.6 (P < 0.05). The observed values of PM10 ERs of the EC and CC were in the lower end of the range reported from U.S.-based studies, i.e., 9 to 48 mg/hen/d in the CC high-rise houses (Li et al., 2011). The AV PM10 ERs were within reported values of 80 to 110 mg/hen/d for similar U.S. AV houses (Hayes et al., 2013a).
Figure 4.
Daily mean particulate matter (PM10 and PM2.5) emission rates (ERs) (g/hen/d) and (mg/hen/d), respectively and their relationship to ambient temperature for the conventional cage house (CC), aviary house (AV), and enriched colony house (EC).
The relationship between PM10 ERs and ambient temperature is further delineated by the data in Table 4. For all temperature groupings below 25°C, the AV had significantly higher PM10 ERs than the CC and EC houses (P < 0.05). For temperature ranges below 20°C, no difference in ERs was found between the CC and EC. The PM10 ERs of the CC and EC were directly related to ambient temperatures. This outcome was believed to arise from increased air velocity across the barn driven by higher VRs, allowing less PM to settle within the house. The AV showed a similar pattern to the EC and CC, but ERs at temperatures above 20°C were confounded by bird age and the absence of litter accumulation on the floor prior to 35 wk of age in both flocks. Further analysis of the PM10 data, omitting data when litter were not available or established in the AV (18 to 35 wk of age), showed no significant difference in PM10 ERs in the temperature ranges of 10 to 20°C, 20 to 25°C, and >25°C (mean ± SE of 132.5 ± 7.0, 134.5 ± 16.0, and 105.3 ± 12.5 mg/hen/d, respectively).
The AV had the highest mean daily PM2.5 ER (mg/hen/d) of 8.8, followed by the EC at 1.7 and CC at 0.9. The PM2.5 ER values observed in the CC and EC were lower than those reported for CC high-rise houses (3.6 to 14 mg/hen/d) (Li et al., 2011), whereas the AV PM2.5 ERs were comparable to ranges reported for similar U.S. AV housing (5 to 10 mg/hen/d) (Hayes et al., 2013a). The ratio of PM2.5 to PM10 found in this study, 7% (CC), 10% (AV), and 11% (EC), paralleled the PM partitioning observed in the literature (Li et al., 2011, Hayes et al., 2013a). The lower levels of both PM10 and PM2.5 found in this study for the CC and EC compared to the literature values are likely due to differences in housing/manure management and ventilation design. Specifically, the PM ER values reported by Li et al. (2011) were for high-rise houses that stored the manure in the lower level for nearly a year.
Manure Storage and Farm-Level Emission Rates
The daily mean gaseous emissions observed from the 3 manure storage monitoring periods, in g/hen/d, were NH3: 0.21 (CC), 0.18 (AV), and 0.11 (EC); CO2: 8.1 (CC), 8.0 (AV), and 7.1 (EC); CH4: 0.03 (CC), 0.03 (AV), and 0.02 (EC); and N2O: 0.03 (CC), 0.03 (AV), and 0.01 (EC). The differences in manure emissions were related to the MC of each manure source, with the EC having the driest manure at 45.6%, followed by the AV at 51.7%, and the CC at 53.6%. A lab-scale assessment of gaseous emissions from laying hen manure by Li and Xin (2010) showed a direct correlation between MC and NH3 ERs and a range of gaseous ERs (g/hen/d) of NH3: 0.06 to 0.22; CO2: 1.6 to 4.8; and CH4: 0.007 to 0.032.
Table 5 provides a summary of farm-level gaseous ERs based on per hen and per kilogram of egg output, combining the house-level and associated manure storage contributions. Farm-level ERs of NH3 (g/hen/d) were highest for the AV and CC at 0.30 and 0.29, respectively, and lowest for the EC at 0.16 (P < 0.05). The primary difference in the farm-level NH3 ERs is believed to be driven by the manure drying effectiveness in each house and the littered floor of the AV. The EC system had the lowest manure belt stocking density, followed by the AV and CC, resulting in more effective in-barn manure drying and lower house-level and farm-level NH3 emissions. The CC had the highest manure belt stocking density, manure MC, and manure storage ER, with over 70% of emissions originating from the long-term manure storage. A similar proportion (69%) of overall farm-level emissions was from the manure storage for the EC. In comparison, 60% of the farm-level emissions originated from the long-term manure storage for the AV, although the littered floor can significantly change this partitioning if moisture accumulates in the litter for an extended period. These results illustrate the impact of manure belt drying design and operation and manure/litter management on both house-level and long-term manure storage emissions.
Table 5.
Summary of house-level, manure storage, and farm-level daily emission rates of ammonia (NH3), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and particulate matter (PM10 and PM2.5) for the conventional cage (CC), aviary (AV), and enriched colony (EC) housing systems over the 27 mo monitoring period.
| Housing system | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gas or PM | Source | Conventional cage (CC) | Aviary (AV) | Enriched colony (EC) | ||||||
| g/hen/d | g/(kg egg) | % of total | g/hen/d | g/(kg egg) | % of total | g/hen/d | g/(kg egg) | % of total | ||
| NH3 | House | 0.082b | 1.62 | 28 | 0.112a | 2.19 | 40 | 0.054c | 0.99 | 31 |
| Manure storage | 0.21a | 4.00 | 72 | 0.18a | 3.52 | 60 | 0.11b | 2.02 | 69 | |
| Farm | 0.29 | 5.52 | 100 | 0.30 | 5.88 | 100 | 0.16 | 2.94 | 100 | |
| CO2 | House | 68.3b | 1,300 | 89 | 74.0a | 1,450 | 90 | 74.4a | 1,365 | 91 |
| Manure storage | 8.1 | 154 | 11 | 8.0 | 157 | 10 | 7.1 | 130 | 9 | |
| Farm | 76.4 | 1,454 | 100 | 82.0 | 1,607 | 100 | 81.5 | 1,495 | 100 | |
| CH4 | House | 0.07 | 1.33 | 70 | 0.07 | 1.37 | 70 | 0.08 | 1.47 | 80 |
| Manure storage | 0.03 | 0.57 | 30 | 0.03 | 0.59 | 30 | 0.02 | 0.37 | 20 | |
| Farm | 0.10 | 1.90 | 100 | 0.10 | 1.96 | 100 | 0.10 | 1.84 | 100 | |
| N2O | House | — | — | — | — | — | — | — | — | — |
| Manure storage | 0.03 | 0.57 | — | 0.03 | 0.59 | — | 0.01 | 0.18 | — | |
| Farm | 0.03 | 0.57 | — | 0.03 | 0.59 | — | 0.01 | 0.18 | — | |
| PM10 | House | 0.0157b | 0.299 | 100 | 0.1003a | 1. 909 | 100 | 0.0156b | 0.297 | 100 |
| Manure storage | — | — | — | — | — | — | — | — | — | |
| Farm | 0.0157 | 0.299 | 100 | 0.1003 | 1. 909 | 100 | 0.0156 | 0.297 | 100 | |
| PM2.5 | House | 0.0009b | 0.018 | 100 | 0.0088a | 0.168 | 100 | 0.0017b | 0.032 | 100 |
| Manure storage | — | — | — | — | — | — | — | — | — | |
| Farm | 0.0009 | 0.018 | 100 | 0.0088 | 0.168 | 100 | 0.0017 | 0.032 | 100 | |
Means of gaseous or particulate matter emission rates of the housing systems with different superscript letters significantly differ (P < 0.05).
Conclusions
Gaseous and particulate matter emissions from 3 commercial laying hen houses (CC, EC, and AV) and their respective manure storage were monitored over 2 single-cycle production flocks in the U.S. Midwest. The following observations and conclusions were made.
House-level NH3 emissions were highest in the AV at 0.112, followed by the CC at 0.082 and the EC at 0.054 g/hen/d (P < 0.05).
House-level CH4 emissions were similar for all houses and small (0.07 to 0.08 g/hen/d).
PM10 and PM2.5 emissions were highest for the AV at 100.3 and 8.8 mg/hen/d, respectively, resulting from hen activities on the litter floor. PM emissions of the CC and EC were similar, amounting to 16% of the AV PM10 ER and 10–20% of the AV PM2.5 ER—PM10: 15.7 (CC), 15.6 (EC); PM2.5: 0.9 (CC), 1.7 (EC) mg/hen/d (P < 0.05).
Farm-level NH3 emissions were lower for the EC (0.16 g/hen/d) than for the AV or CC (0.30 and 0.29 g/hen/d, respectively).
Ammonia emissions from the manure storage accounted for 60 to 70% of the farm-level emissions. Hence, future NH3 mitigation efforts should focus on manure storage.
Acknowledgments
Cash funding for the study was supported by the Coalition for Sustainable Egg Supply (CSES). In-kind contributions by Iowa State University and the Egg Industry Center were provided in the form of availing state-of-the-art environmental monitoring equipment (approximately $400,000 worth) to the project. We sincerely appreciate the cooperation and assistance of the egg producer in the implementation of this field study.
REFERENCES
- CIGR. CIGR Handbook of Agricultural Engineering. Vol. 2. Animal Production and Aquacultural Engineering. MI: ASAE, St. Joseph; 1999. [Google Scholar]
- Fabbri C., Valli L., Guarino M., Costa A., Mazzotta V. Ammonia, methane, nitrous oxide and particulate matter emissions from two different buildings for laying hens. Biosyst. Eng. 2007;97:441–455. [Google Scholar]
- Gates R. S., Casey K. D., Xin H., Wheeler E. F., Simmons J. D. Fans assessment numeration systems (FANS) design and calibration specifications. Trans. ASAE. 2004;47:1709–1715. [Google Scholar]
- Groot Koerkamp P. W. G., Metz J. H. M., Uenk G. H., Phillips V. R., Holden M. R., Sneath R. W., Short J. L., White R. P. P., Hartung J., Seedorf J., Schröder M., Linkert K. H., Pedersen S., Takai H., Johnsen J. O., Wathes C. M. Concentrations and emissions of ammonia in livestock buildings in northern Europe. J. Agric. Eng. Res. 1998;70:79–95. [Google Scholar]
- Hayes M. D., Xin H., Li H., Shepherd T. A., Zhao Y., Stinn J. Ammonia, greenhouse gas, and particulate matter emissions of aviary layer houses in the midwestern United States. Trans. ASAE. 2013a;56:1921–1932. [Google Scholar]
- Hayes M. D., Xin H., Li H., Shepherd T. A., Zhao Y., Stinn J. Heat and moisture production of Hy-Line brown hens in aviary houses in the midwestern U.S. Trans. ASAE. 2013b;56:753–761. [Google Scholar]
- Li H., Xin H. Lab-scale assessment of gaseous emissions from laying-hen manure storage as affected by physical and environmental factors. Trans. ASAE. 2010;53:593–604. [Google Scholar]
- Li S., Li H., Xin H., Burns R. Particulate matter concentrations and emissions of a high-rise layer house in Iowa. Trans. ASAE. 2011;54:1093–1101. [Google Scholar]
- Liang Y., Xin H., Wheeler E. F., Gates R. S., Li H., Zajaczkowski J. S., Topper P. A., Casey K. D., Behrends B. R., Burnham D. J. Ammonia emissions from U.S. laying hen houses in Iowa and Pennsylvania. Trans. ASAE. 2005;48:1927–1941. [Google Scholar]
- Monteny G. J., Groenestein C. M., Hilhorst M.A. Interactions and coupling between emissions of methane and nitrous oxide from animal husbandry. Nutr. Cycl. Agroecosys. 2001;60:123–132. [Google Scholar]
- Moody L. B., Li H., Burns R. T., Xin H., Gates R. S., Hoff S. J., Overhults D. A Quality Assurance Project Plan for Monitoring Gaseous and Particulate Matter Emissions from Broiler Housing. MI, USA: ASABE: An ASABE Special Publication. St. Joseph; 2008. [Google Scholar]
- Muhlbauer R., Shepherd T. A., Li H., Burns R. T., Xin H. Development and testing of an induction-operated current switch for monitoring fan operation. Appl. Eng. Agric. 2011;27(2):287–292. [Google Scholar]
- Neser S., Depta G., Stegbauer B., Gronauer A., Schon H. Mass balance of the compounds nitrogen and carbon in housing systems for laying hens. In: Voermans J.A.M, Monteny G., editors. Proc. Intl. Symp. on Ammonia and Odour Control from Animal Facilities. 6–10 October. Rosmalen, The Netherlands: 1997. pp. 129–137. [Google Scholar]
- Ning X. MS Thesis. Ames: Iowa State University; 2008. Feeding, defecation and gaseous emission dynamics of W-36 laying hens. [Google Scholar]
- Swanson J. C., Mench J. A., Karcher D. The Coalition for Sustainable Egg Supply: An introduction. Poul. Sci. 2014;94:473–474. doi: 10.3382/ps/peu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes C. M., Holden M. R., Sneath R. W., White R. P., Phillips V. R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997;38:14–28. doi: 10.1080/00071669708417936. [DOI] [PubMed] [Google Scholar]
- Xin H., Gates R. S., Green A. R., Mitloehner F. M., Moore P. A., Jr., Wathes C. W. Environmental impacts and sustainability of egg production systems. Poul. Sci. 2011;90:263–277. doi: 10.3382/ps.2010-00877. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Shepherd T. A., Xin H. Environmental assessment of three egg production systems. Part I. Monitoring system and indoor air quality. Poul. Sci. 2014a;94:518–533. doi: 10.3382/ps/peu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Shepherd T. A., Swanson J., Mench J. A., Karcher D. M., Xin H. Comparative evaluation of three egg production systems: Housing characteristics and management practices. Poul. Sci. 2014b;94:475–484. doi: 10.3382/ps/peu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xin H., Shepherd T. A., Hayes M., Stinn J., Li H. Thermal environment, ammonia concentrations and emissions of aviary houses with white laying hens. Trans. ASAE. 2013;56:1145–1156. [Google Scholar]