Abstract
Alternative housing systems for laying hens provide mechanical loading and help reduce bone loss. Moreover, achieving greater peak bone mass during pullet phase can be crucial to prevent fractures in the production period. The aim of this study was to determine the housing system effects on bone quality of pullets. Tibiae and humeri of White Leghorn pullets reared in conventional cages (CCs) and a cage-free aviary (AV) system were studied. At 16 wk, 120 birds at random from each housing system were euthanized. Right and left tibiae and humeri were collected and further analyzed. Cortical bone density and thickness were measured using computed tomography. Periosteal and endosteal dimensions were measured at the fracture site during mechanical testing. At 4, 8, 12, and 16 wk, serum concentrations of osteocalcin and hydroxylysyl pyridinoline were analyzed as markers of bone formation and resorption. Cortical bone density was higher (P < 0.05) in humeri of AV pullets, and tibiae were denser (P < 0.05) for AV pullets in the distal section of the bone compared to CC pullets. Ash content was higher (P < 0.05) in AV humeri with no difference in tibiae ash content. Tibiae and humeri of AV pullets had a thicker cortex than the CC pullets (P < 0.05). Additionally, the tibiae and humeri of AV pullets had greater (P < 0.05) second moment of areas than the CC pullets. While some bone material properties between groups were different (P < 0.05), the differences were so small (< 7%) that they likely have no clinical significance. Serum osteocalcin concentrations were not different between the treatments, but hydroxylsyl pyridinoline concentrations were higher in CC pullets at 12 wk compared to the AV pullets and the effect reversed at 16 wk (P < 0.05). These findings indicate that tibiae and humeri respond differently to load bearing activities during growth. The improved load bearing capability and stiffness in bones of AV pullets were related to increased cross-sectional geometry.
Keywords: pullets, aviary, bone, structure, mechanics
INTRODUCTION
Egg production systems have changed in the past 6 decades, with 90% of laying hens being kept in deep litter in 1954 (Elson, 2011) to 95% of hens in the United States being housed in conventional cages in 2008 (UEP, 2009). Meanwhile, egg production per hen has also increased mainly due to highly intensive farming systems, optimized nutrition, and enhanced genetics. Conventional cages used in intensive layer production have been associated with disuse-osteoporosis and caged layer fatigue since its introduction. Osteoporosis was first reported by Couch (1955) as a condition characterized by high bone loss and has been defined as a net decrease in the amount of mineralized structural bone that over time makes it fragile and prone to fracture (Whitehead and Fleming, 2000s).
Alternative housing systems with provision of perches and greater space are being explored to mitigate the issues of osteoporosis. In vitro and in vivo studies have established the effects of mechanical loading in bone formation and load bearing (Robling et al., 2008; Wan et al., 2013). Birds housed in alternative systems are allowed more area for load bearing exercises like wing flapping and walking or running that, in theory, alter the bone characteristics and make it stronger. On the other hand, inactivity has been reported to promote osteoporosis in birds (Nightingale et al., 1972; Whitehead and Fleming, 2000). Computed tomographic analyses suggest cortical and trabecular regions of bone from adult laying hens housed in different systems had similar bone densities despite having significant differences in cortical areas (Jendral et al., 2008; Shipov et al., 2010), indicating that loading improves bone quality in laying hens by chiefly altering its structural property rather than mineral composition. Efforts to improve bone health by loading have often been studied when birds are already into the laying stage and although loading exercise during production has helped to reduce the amount of structural bone loss, the incidences of osteoporosis and fracture in cage systems indicate that either the birds do not have enough bone mass at the time they enter into the laying cycle or the rate of resorption is too high to be compensated by loading exercises. Hence, one strategy to prevent osteoporosis can be targeted at achieving optimum bone mass before the birds enter into lay. Positive effects of exercise during growth in bone mass and mechanical properties have been reported in several human studies (Vuori, 1996; Bass et al., 2002). Prepubertal loading resulted in increased bone mineral content as well as wider cortical and periosteal area, suggesting periosteal bone formation in the humerus of female tennis players (Bass et al., 2002). Some studies have been conducted previously in experimental setting in male chicks (Biewener and Bertram, 1994; Judex and Zernicke, 2000) whereas there is gap of knowledge on response of loading conditions in pullet skeleton. Pullets housed in cages with perches had higher bone mineral content in tibia and humerus at 12 wk compared to the pullets of same age kept in cages without perches (Enneking et al., 2012), suggesting more bone formation in pullets using perches. The present study was aimed at comparing the differences in material, structural, and mechanical properties of the tibia and humerus of pullets, housed in aviary system and conventional cages in a commercial setting. The hypothesis being tested was pullets raised in an aviary system would have increased peak bone mass at the start of lay compared to pullets reared in conventional cages.
MATERIALS AND METHODS
Birds, Management, and Sampling
The experimental protocol was approved by the Michigan State University Institutional Animal Care and Use Committee. Chickens of the Lohmann White strain were raised in a commercial setting. Pullets were housed in conventional pullet cages (Univent starter, Big Dutchman, Holland, MI) and aviary system (Natura rearing, Big Dutchman). Aviary birds were moved from conventional rearing to aviary rearing at 6 wk. Fifteen pullets were kept in each conventional cage with an area of 247.74 cm2/bird. For aviary pullets, 218 birds were housed per cage with space allocation of 159.94 cm2/bird from 0 to 9 wk, after which total cage space was increased to 248.97 cm2/bird. AV pullets had floor access from 6 wk onwards, providing additional space of 100.13 cm2/bird (Jones et al., 2014). Feeding, lighting, and health management were same for both groups, and are explained in more detail by Jones et al. (2014) as the same flock was used for the current study. At 16 wk, 120 birds from each housing system were randomly sampled for bone analysis. Birds were euthanized by cervical dislocation and the right and left tibiae, humeri were excised, and samples from each bird were stored in separate plastic zip-lock bags at −20°C.
Brachial vein blood samples were collected from 30 randomly selected pullets of each housing system at 4, 8, and 12 wk, and 60 pullets were sampled in similar fashion at 16 wk from each housing system for quantification of systemic bone markers. Serum was separated and frozen at −20°C prior to analysis. Serum osteocalcin (marker of bone formation) and hydroxylysyl pyridinoline (marker of bone resorption) concentrations were quantified using commercially available ELISA tests (Quidel Corporation, San Diego, CA). Samples with values greater than the standard curve were diluted according to manufacturer's recommendations in the assay buffer for the analysis.
Computed Tomography and Bone Ash
Prior to analysis, the right tibia and humerus were thawed overnight, and a quantitative computed tomography scan of the bone along with surrounding soft tissues was taken using a BrightSpeed scanner (General Electric Healthcare, Princeton, NJ). Ends of the bone were located to obtain total bone length, which was then divided by 4 to set the location for the cross-sectional x-ray image at proximal (one-fourth), middle, and distal (three-fourths) regions. Mimics software (Materialise, Plymouth, MI) was used to measure total bone length and analyze the resulting 1 mm cross sections for cortical bone thickness, and cortical bone density at each of the 3 regions. The orientation of the cortical region in relation to the skeletal axis was identified. Cortical bone thickness and cortical bone density were measured for anterio-posterior and medio-lateral planes. Density of bone cortex was measured as an average density within a 10 × 20 mm region at each of anterior, posterior, medial, and lateral region, and a further average of whole cross-sectional slice was measured. Profile line feature of the software was used to calculate appropriate threshold density to mask only the cortical region for measurements. The Haugh units values obtained from the quantitative computed tomography scans were converted to milligrams per cubic centimeter in reference to the standard phantoms that were scanned along with the bones.
Each sample, after quantitative computed tomography scans, was further analyzed for ash content. The bones were cleaned of surrounding muscles and soft tissues. Tibia was separated from fibula, and both humerus and tibia were cut into pieces to fit into a soxhlet for ether extraction. Ether extracted bone pieces were dried and weighed and placed in crucibles, and further dried at 105°C for 24 h in a DN-81 constant-temperature oven (American Scientific, Portland, OR). Finally bones were placed in an ash oven (Thermolyne 30400, Barnstead International, Dubuque, IA) at 600°C for 6 h and the ash percentage was determined.
Mechanical Testing
Two days prior to mechanical testing, the left legs and wings were thawed at room temperature. The tibia and humerus were harvested and cleaned of all soft tissue. The bones were wrapped in saline soaked gauze and kept moist throughout all preparations and testing procedures. A uniform middiaphysis section, 20 mm long for the humerus and 30 mm for the tibia, was identified for testing and the remaining ends were potted in cups filled with polyester resin (Martin Senour Fiber Strand Plus 6371, Sherwin-Williams, Cleveland, OH). A custom rig secured the bones in alignment with the cups while the resin cured. After potting, anterior-posterior and medial-lateral outer dimensions of the bones were measured at the ends and center with digital calipers.
The potted specimens were installed in a 4-point bending fixture mounted on a servohydraulic testing machine (model 1331, Instron, Norwood, MA). Freely pivoting cups secured the potted ends and a crossbar resting on pins attached to the cups transferred the linear displacement of the testing machine actuator to rotation of the cups. This setup applied an equal bending moment to each end of the specimen, uniformly loading the test section in pure bending. An actuator preload of 2 N was applied to eliminate residual system compliance before bone failure was induced with a 1 Hz, 10 mm haversine displacement. Load and displacement output of the actuator were recorded at 5,000 Hz with a 100 lb load cell (model 1500ASK-100, Interface, Scottsdale, AZ) and a 6 in linear variable differential transformer mounted on the actuator (model HR 3000, Measurement Specialties, Hampton, VA). The tibiae were oriented with the lateral surface loaded in tension and humeri with the posterior surface loaded in tension. The orientation was selected based on the assumption that the tibiae were likely to break when landing with the distal end medial of the proximal, putting the lateral side in tension. For the humeri, the orientation was selected based on the supposition that wing flapping, namely, adduction was the action most likely to result in a fracture.
After fracture, the bone fragments were reassembled in order to measure anterior, posterior, medial, and lateral cortical thicknesses at the fracture site. Outer dimensions and diaphysis thicknesses were used to approximate the cortical cross-section as a hollow ellipse.
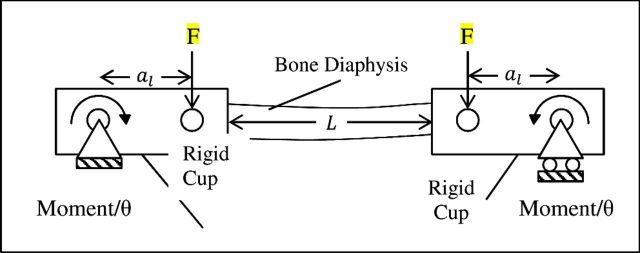
The material properties of the bones were determined based on classical beam theory with the exposed bone test section modeled as a uniform beam with a moment applied to each end. The computations required the bone's geometrical resistance to bending, called the second moment of area I, to be computed using the expression
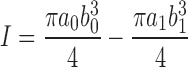 |
(1) |
where α and b were the radii parallel and perpendicular to the neutral axis of the bone, and subscripts 0 and 1 denoted outer and inner dimensions of the bone, respectively. The applied bending moment to each end of the specimen was calculated from the actuator force using the expression
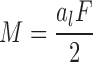 |
(2) |
where F was the actuator force applied to both cups, and al was the distance between the pivot and load application points on the cups (Figure 1). Bone-end deflection angles θ were calculated using the expression
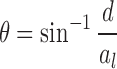 |
(3) |
where d was the actuator displacement. Whole-bone bending stiffness, K, was determined by the slope of a line fit to the initial, linear portion of the moment-bone rotation plot. This mechanical stiffness and bone geometry were substituted into classical beam equations to compute material stiffness, known as Young's modulus E, using the equation
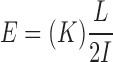 |
(4) |
where L was the length of the test section (Figure 1). The material strength of each bone was determined based on a computation of the maximum (failure) bending stress (σf) in the bone using the expression
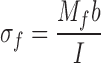 |
(5) |
where Mf was the maximum bending moment exerted on each rigid cup at the ends of the specimen at failure (Figure 1).
Figure 1.
Four-point bending mechanical test setup.
Statistical Analysis
Data were analyzed by using the multivariate PROC MIXED analysis of SAS Version 9.3 (SAS Institute, Cary, NC). Repeated measures statement with the model including fixed effect of housing and section of bone, the interaction between housing and section, and the residual error was used to analyze all data other than length. Differences between means were tested using Fisher's least-square mean with significance accepted at P < 0.05. Data are presented as least-square means with their respective standard errors (least-square mean ± standard error of the mean). Mechanical data for tibia and humerus were analyzed using the Student's t-test.
RESULTS
Bone Geometrical and Compositional Properties
Tibiae and humeri were longer in conventional-cage (CC) pullets compared to aviary (AV) system pullets (P < 0.05; Figure 2A). However, cortical thickness of both tibiae and humeri were wider in AV pullets, compared to CC pullets in proximal, middle, and distal sections, along all anatomical planes except the antero-posterior plane in proximal tibia (P < 0.05; Table 1). There was neither a difference in the medio-lateral nor antero-posterior outer dimensions of the tibia between housing systems (Table 4). Cortical thickness measured manually at the fracture site corroborated with quantitative computed tomography measurements for both bones with tibial and humeral cortex wider in AV birds compared to the CC birds (P < 0.01; Table 3). Unlike the tibia, medio-lateral and antero-posterior outer dimensions of the humerus were higher in AV birds compared to the CC birds (P < 0.01; Table 3). Cross-sectional areas of tibiae and humeri were greater in AV birds than those of CC birds, which eventually translated into higher values of second moments of area in bones of AV birds compared to CC birds (P < 0.01; Table 5). The difference in second moments of inertia between the housing conditions was more pronounced in the humerus than in the tibia.
Figure 2.
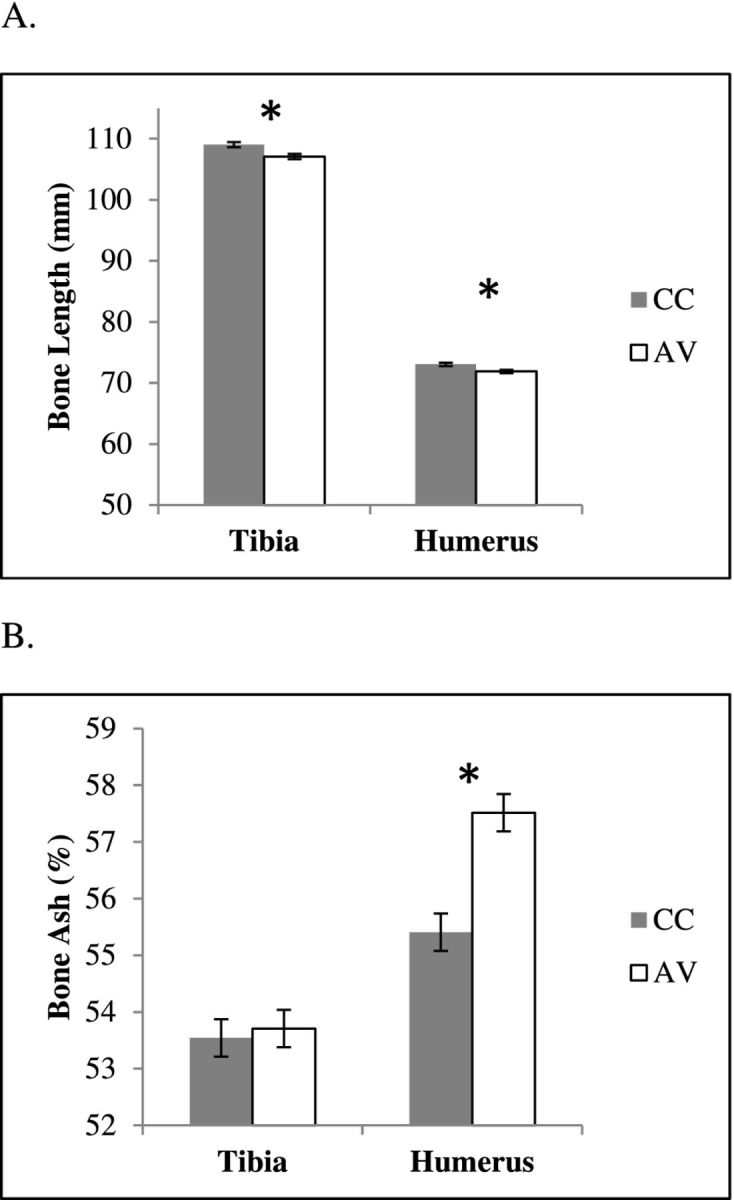
A = Mean total length; B = percentage ash content of tibia and humerus, with respective standard error of the mean of 16 wk Lohmann White pullets housed in AV system and CCs.
Table 1.
Humerus and tibia cortical thickness (millimeters) of 16 wk White Leghorn pullets housed in AV system and CCs.1
| Planar orientation of bone | ||||
|---|---|---|---|---|
| Bone type and housing | Medial2 | Lateral | Anterior | Posterior |
| Humerus | ||||
| Proximal | ||||
| AV | 1.02 ± 0.02 | 1.02 ± 0.02 | 1.18 ± 0.02 | 1.29 ± 0.02 |
| CC | 0.86 ± 0.02 | 0.88 ± 0.02 | 0.91 ± 0.02 | 1 ± 0.02 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Mid | ||||
| AV | 1.26 ± 0.02 | 1.21 ± 0.02 | 1.39 ± 0.02 | 1.45 ± 0.02 |
| CC | 1.03 ± 0.02 | 1.02 ± 0.02 | 1.07 ± 0.02 | 1.16 ± 0.02 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Distal | ||||
| AV | 1.24 ± 0.02 | 1.40 ± 0.03 | 1.32 ± 0.02 | 1.37 ± 0.02 |
| CC | 1.03 ± 0.02 | 1.12 ± 0.03 | 1.06 ± 0.03 | 1.08 ± 0.02 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Tibia | ||||
| Proximal | ||||
| AV | 1.52 ± 0.03 | 1.70 ± 0.03 | 1.52 ± 0.02 | 1.32 ± 0.02 |
| CC | 1.44 ± 0.03 | 1.57 ± 0.03 | 1.49 ± 0.02 | 1.36 ± 0.02 |
| P value | 0.0261 | 0.0007 | 0.4318 | 0.1334 |
| Mid | ||||
| AV | 1.59 ± 0.03 | 1.65 ± 0.03 | 1.54 ± 0.02 | 1.48 ± 0.02 |
| CC | 1.47 ± 0.03 | 1.55 ± 0.03 | 1.42 ± 0.02 | 1.39 ± 0.02 |
| P value | 0.0015 | 0.0124 | <.0001 | 0.0032 |
| Distal | ||||
| AV | 1.53 ± 0.02 | 1.48 ± 0.02 | 1.23 ± 0.02 | 1.38 ± 0.02 |
| CC | 1.34 ± 0.02 | 1.36 ± 0.02 | 1.13 ± 0.02 | 1.25 ± 0.02 |
| P value | <.0001 | 0.0002 | <.0001 | <.0001 |
AV = aviary system; CC = conventional cages.
Values are presented as least-squares mean ± standard error of the mean.
Table 4.
Geometrical properties of tibia of 16 wk pullets housed in AV system and CCs.1
| Dependent variable | Housing | ||
|---|---|---|---|
| AV | CC | P value | |
| Geometrical properties | |||
| Area (mm2) | 14.16 ± 0.112 | 12.52 ± 0.12 | <.0001 |
| Medial thickness (mm) | 0.90 ± 0.01 | 0.78 ± 0.01 | <.0001 |
| Lateral thickness (mm) | 0.88 ± 0.01 | 0.78 ± 0.01 | <.0001 |
| Anterior thickness (mm) | 0.80 ± 0.01 | 0.70 ± 0.01 | <.0001 |
| Posterior thickness (mm) | 0.80 ± 0.01 | 0.70 ± 0.01 | <.0001 |
| Average medio-lateral thickness (mm) | 0.89 ± 0.01 | 0.78 ± 0.01 | <.0001 |
| Average antero-posterior thickness (mm) | 0.80 ± 0.01 | 0.70 ± 0.01 | <.0001 |
| Average thickness (mm) | 0.84 ± 0.01 | 0.74 ± 0.01 | <.0001 |
| Proximal medio-lateral diameter (mm) | 6.73 ± 0.03 | 6.69 ± 0.03 | 0.3825 |
| Middle medio-lateral diameter (mm) | 6.44 ± 0.03 | 6.37 ± 0.03 | 0.1121 |
| Distal medio-lateral diameter (mm) | 6.81 ± 0.03 | 6.73 ± 0.03 | 0.0912 |
| Prox antero-posterior diameter (mm) | 6.07 ± 0.03 | 5.94 ± 0.03 | 0.0029 |
| Middle antero-posterior diameter (mm) | 5.57 ± 0.02 | 5.51 ± 0.03 | 0.1252 |
| Distal antero-posterior diameter (mm) | 5.67 ± 0.03 | 5.67 ± 0.03 | 0.9947 |
| Average medio-lateral diameter (mm) | 6.66 ± 0.03 | 6.60 ± 0.03 | 0.1125 |
| Average antero-posterior diameter (mm) | 5.77 ± 0.02 | 5.71 ± 0.02 | 0.0831 |
| Average diameter (mm) | 6.21 ± 0.02 | 6.15 ± 0.02 | 0.0585 |
AV = aviary system; CC = conventional cages.
Values are presented as least-squares mean ± standard error of the mean.
Table 3.
Geometrical properties of humerus of 16 wk pullets housed in AV system and CCs.1
| Housing | |||
|---|---|---|---|
| Dependent variable | AV | CC | P value |
| Geometrical properties | |||
| Area (mm2) | 12.92 ± 0.122 | 9.42 ± 0.13 | <.0001 |
| Medial thickness (mm) | 0.74 ± 0.01 | 0.54 ± 0.01 | <.0001 |
| Lateral thickness (mm) | 0.75 ± 0.01 | 0.55 ± 0.01 | <.0001 |
| Anterior thickness (mm) | 0.68 ± 0.01 | 0.52 ± 0.01 | <.0001 |
| Posterior thickness (mm) | 0.70 ± 0.01 | 0.52 ± 0.01 | <.0001 |
| Average medio-lateral thickness (mm) | 0.74 ± 0.01 | 0.55 ± 0.01 | <.0001 |
| Average antero-posterior thickness (mm) | 0.69 ± 0.01 | 0.52 ± 0.01 | <.0001 |
| Average thickness (mm) | 0.72 ± 0.01 | 0.53 ± 0.01 | <.0001 |
| Proximal medio-lateral diameter (mm) | 7.52 ± 0.03 | 7.17 ± 0.03 | <.0001 |
| Mid medio-lateral diameter (mm) | 6.82 ± 0.03 | 6.39 ± 0.03 | <.0001 |
| Distal medio-lateral diameter (mm) | 6.88 ± 0.03 | 6.46 ± 0.04 | <.0001 |
| Prox antero-posterior diameter (mm) | 5.98 ± 0.02 | 5.74 ± 0.02 | <.0001 |
| Mid antero-posterior diameter (mm) | 5.79 ± 0.02 | 5.59 ± 0.02 | <.0001 |
| Distal antero-posterior diameter (mm) | 5.92 ± 0.02 | 5.73 ± 0.02 | <.0001 |
| Average medio-lateral diameter (mm) | 7.07 ± 0.03 | 6.68 ± 0.03 | <.0001 |
| Average antero-posterior diameter (mm) | 5.90 ± 0.02 | 5.69 ± 0.02 | <.0001 |
| Average diameter (mm) | 6.49 ± 0.02 | 6.18 ± 0.02 | <.0001 |
AV = aviary system; CC = conventional cages.
Values are presented as least-squares mean ± standard error of the mean.
Table 5.
Mechanical and geometrical properties of tibia and humerus of 16 wk pullets housed in AV system and CCs.1
| Dependent variable | Housing | ||
|---|---|---|---|
| AV | CC | P value | |
| Tibia | |||
| Mechanical properties | |||
| Failure moment (Nm) | 5.08 ± 0.482 | 4.53 ± 0.46 | <0.001 |
| Failure rotation (degree) | 8.90 ± 1.15 | 8.57 ± 1.27 | 0.042 |
| Stiffness (Nm/degree) | 0.94 ± 0.10 | 0.85 ± 0.11 | <0.001 |
| Failure stress (MPa) | 312.40 ± 31.50 | 301.80 ± 32.70 | 0.012 |
| Young's modulus (GPa) | 13.65 ± 1.20 | 13.74 ± 1.42 | 0.627 |
| Second moment of inertia (mm4) | 59.91 ± 8.53 | 53.55 ± 8.02 | <0.001 |
| Humerus | |||
| Mechanical properties | |||
| Failure moment (Nm) | 3.62 ± 0.43 | 2.51 ± 0.39 | <0.001 |
| Failure rotation (degree) | 6.97 ± 1.05 | 6.49 ± 1.01 | 0.001 |
| Stiffness (Nm/degree) | 0.82 ± 0.11 | 0.61 ± 0.09 | <0.001 |
| Failure stress (MPa) | 242.90 ± 28.80 | 229.80 ± 34.30 | 0.002 |
| Young's modulus (GPa) | 10.25 ± 1.22 | 10.77 ± 1.30 | 0.002 |
| Second moment of inertia (mm4) | 46.25 ± 7.13 | 32.91 ± 5.64 | <0.001 |
AV = aviary system; CC = conventional cages.
Values are presented as least-squares mean ± standard error of the mean.
The changes in geometrical properties of the humerus of AV birds compared to the humerus of CC birds were accompanied by changes in compositional parameters. AV birds had humeri with denser cortex compared to CC birds in all planes in proximal, middle, and distal sections (P < 0.01; Table 2). Bone mineral content as measured by ash percentage of humerus was also higher in AV birds compared to the CC birds (P < 0.05; Figure 2B). Average cortical bone density of tibia was not different between the housing systems, except for distal tibia where AV birds had a denser cortex compared to CC birds (Table 2).
Table 2.
Humerus and tibia cortical density (milligrams per cubic centimeter) of 16 wk White Leghorn pullets housed in AV system and CCs.1
| Planar orientation of bone | |||||
|---|---|---|---|---|---|
| Bone type and housing | Medial2 | Lateral | Anterior | Posterior | Average |
| Humerus | |||||
| Proximal | |||||
| AV | 247.18 ± 7.09 | 259.25 ± 7.65 | 296.10 ± 8.47 | 280.36 ± 8.12 | 275.58 ± 7.08 |
| CC | 203.28 ± 7.37 | 219.36 ± 7.94 | 210.42 ± 8.80 | 217.62 ± 8.43 | 218.73 ± 7.28 |
| P value | <.0001 | 0.0004 | <.0001 | <.0001 | <.0001 |
| Mid | |||||
| AV | 376.93 ± 10.25 | 378.94 ± 10.17 | 422.61 ± 11.06 | 420.14 ± 10.79 | 391.72 ± 9.85 |
| CC | 300.48 ± 10.64 | 295.44 ± 10.55 | 318.91 ± 11.48 | 322.48 ± 11.20 | 304.60 ± 10.13 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Distal | |||||
| AV | 344.30 ± 9.20 | 422.85 ± 10.51 | 385.22 ± 10.10 | 395.52 ± 10.57 | 385.93 ± 9.47 |
| CC | 284.31 ± 9.46 | 331.42 ± 10.81 | 282.11 ± 10.38 | 289.55 ± 10.87 | 298.90 ± 9.74 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Tibia | |||||
| Proximal | |||||
| AV | 567.69 ± 31.23 | 589.16 ± 10.05 | 601.41 ± 10.85 | 557.46 ± 11.35 | 575.90 ± 9.99 |
| CC | 594.41 ± 31.23 | 571.07 ± 10.05 | 591.11 ± 10.85 | 569.89 ± 11.35 | 571.22 ± 10.03 |
| P value | 0.5449 | 0.2037 | 0.5021 | 0.4385 | 0.7409 |
| Mid | |||||
| AV | 639.40 ± 12.80 | 655.86 ± 12.83 | 663.15 ± 33.76 | 632.14 ± 12.36 | 643.22 ± 12.19 |
| CC | 605.09 ± 12.80 | 617.84 ± 2.83 | 657.69 ± 33.76 | 602.55 ± 12.36 | 609.45 ± 12.30 |
| P value | 0.0594 | 0.0373 | 0.909 | 0.0919 | 0.0524 |
| Distal | |||||
| AV | 668.92 ± 13.29 | 605.15 ± 12.54 | 559.69 ± 12.02 | 590.98 ± 11.77 | 612.06 ± 11.90 |
| CC | 615.33 ± 13.29 | 574.85 ± 12.54 | 519.21 ± 12.02 | 545.93 ± 11.77 | 571.64 ± 11.96 |
| P value | 0.0048 | 0.0889 | 0.0181 | 0.0073 | 0.0174 |
AV = aviary system; CC = conventional cages.
Values are presented as least-squares mean ± standard error of the mean.
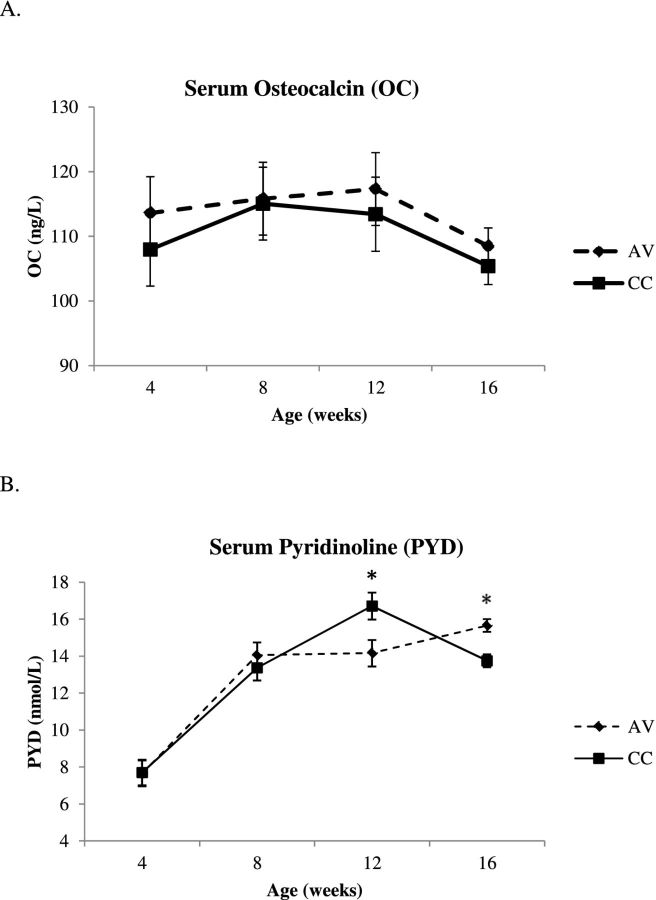
Serum Bone Markers
No effect of age or housing condition was observed for mean serum OC levels in the pullets (Figure 3A). Serum hydroxylysyl pyridinoline level increased from 4 to 8 weeks of age with no effect of housing system observed until 12 wk (Figure 3B). The hydroxylysyl pyridinoline concentration was 15.2% higher in caged pullets at 12 wk and the opposite was observed by 16 wk when the hydroxylysyl pyridinoline level was 12.2% higher in aviary pullets than caged pullets (P < 0.05; Figure 3B).
Figure 3.
Effect of age and housing systems in systemic markers of bone formation and resorption in Lohmann White pullets housed in AV system and CCs: A = serum osteocalcin concentration; B = serum pyridinoline concentration.
Bone Mechanical Properties
An overlay of representative moment-rotation data of a bone from each housing condition illustrates the general differences in bone mechanics up to failure (Figure 4). The failure moment, Mf, was greater for AV tibia and humerus than that of the CC birds (P < 0.001; Figure 5A). As a result, aviary birds had stiffer tibiae and humeri compared to the caged birds, as represented by the slope of the curve (Figure 4).
Figure 4.
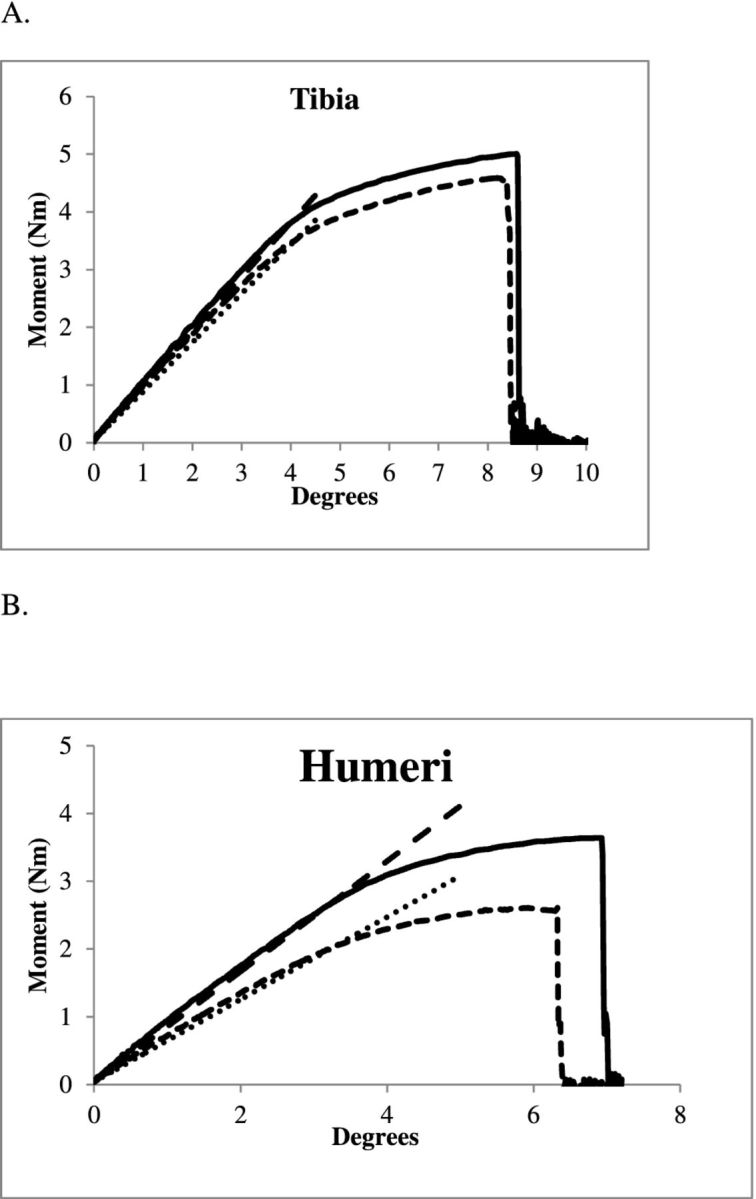
Representative moment-rotation curves showing the mechanical behavior in bending up to failure of 16 wk Lohmann White pullets housed in AV system and CCs: A = behavior of the tibia; B = behavior of the humerus; stiffness was determined from the slope of the initial, linear portion of the curves.
Figure 5.
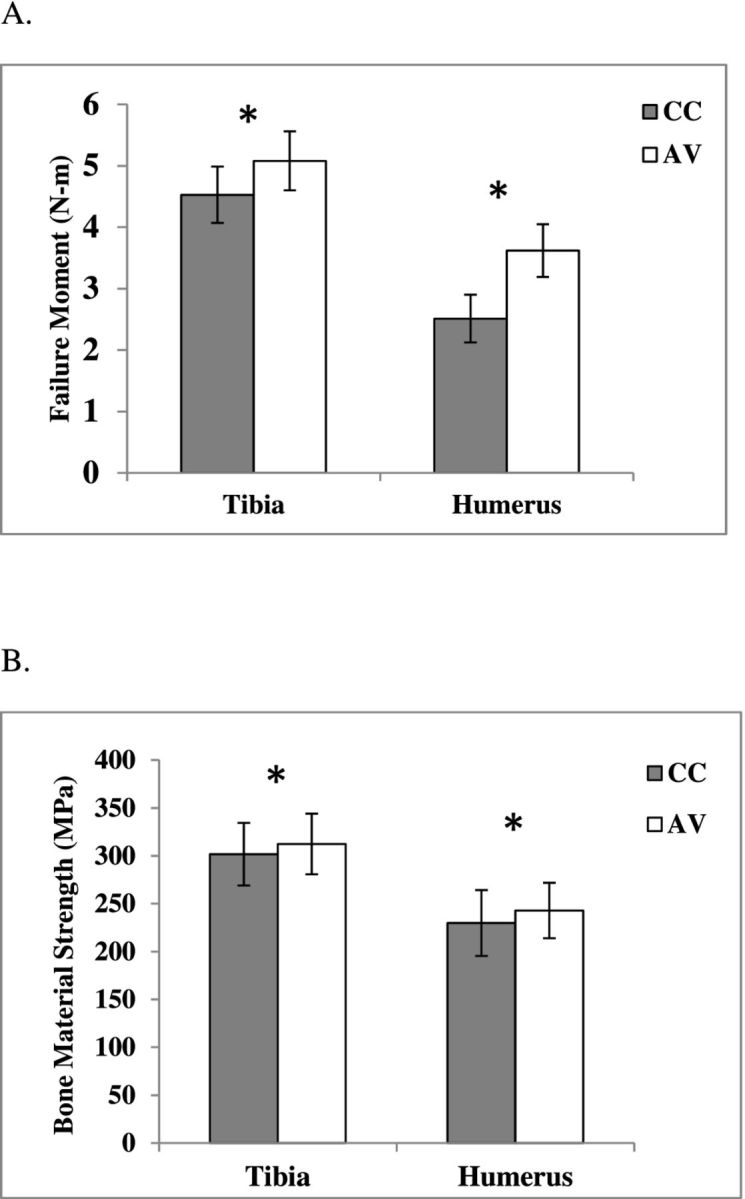
A = Ability of tibia and humerus to withstand bending moments; B = breaking strength (least-squares mean ± standard error of the mean) of tibia and humerus in 16 wk Lohmann White pullets housed in AV system and CCs.
Aviary birds also had stronger bones with tibia material strength, σf, 3.7% greater than that of the CC tibia (P = 0.012) and humerus strength 6.3% greater than that of the CC humerus (P = 0.002; Figure 5B). There was no difference in Young's modulus, E, of the tibia between housing conditions (P = 0.4889; Table 5). In contrast, E of CC humeri were greater than that of AV humeri (P < 0.05; Table 5).
DISCUSSION
Previous research exploring bone qualities in laying hens has often done so when the birds are in the laying stage. This study examines the effect of loading provided by difference in housing system in the tibia and humerus of growing pullets. The aviary (AV) system provides birds with more opportunity of dynamic loading exercises like running and flying which are not possible in cages. Early mechanical loading has been reported to result in narrower growth plates and shorter bones in broiler chicks (Reich et al., 2005). The common response of bones undergoing loading in compression is shortening in length and widening in cross section (Seeman and Delmas, 2006) which was also the case in this study, even though the percentage change in length was very small. Conventionally housed pullets had longer tibiae and humeri at the end of the pullet phase compared to those kept in an AV system, whereas AV birds had greater bone width and cortical thickness. Measurements of bone thickness from quantitative computed tomography scans as well as the measurements taken after fracture found that bones from birds reared in AV systems developed a thicker cortex than birds from conventional rearing systems.
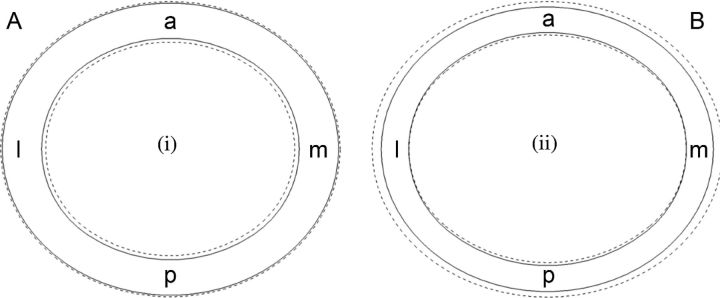
In the tibiae, there was no difference in the outer (periosteal) dimensions between housing conditions. Thus, the increased cross-sectional area of AV tibiae was due to a narrowed medullary canal [Figure 6 (i)]. Contrary to the results of this study, increase in cortical area in 8 wk male White Leghorn chicks under controlled exercise regimen has been reported to be a result of periosteal deposition rather than endosteal apposition (Biewener and Bertram, 1994). In another study, the effect of high-impact exercise like jumping was limited to periosteal surface until 16 wk in the tarso-metatarsus of male Leghorn chicks; however, after that age, growth was more pronounced at the endocortical surface (Judex and Zernicke, 2000). The varied response of growing leg bone in White Leghorns to mechanical stimuli is likely to be the result of differences in age, sex, and the mechanical environment in which the birds are reared. Whereas such inward growth (as observed in present study) increases the second moment of area, the addition of bone more proximal to the neutral axis means that second moment of area differences were not as pronounced as cross-sectional area differences. The primary function of endosteal growth is to increase a bone's axial rigidity as has been observed in response to in vivo dynamic longitudinal compressive loading (Robling et al., 2002). Thus, the cross-sectional geometry differences observed suggests that the additional loading on AV system pullet tibiae may have been primarily along the axis of the bone.
Figure 6.
Diagrammatic representation of bone cross-sections reconstructed using average measurements of 16 wk White Leghorn pullets housed in AV system (dashed line) and CCs (solid line): A = measurements for tibiae; B = measurements foe humeri.
The increased cross-sectional area in the humerus of AV birds was due to increased periosteal diameters with the endosteal dimensions remaining largely unchanged [Figure 6 (ii)]. In addition to increasing axial rigidity, outward expansion of the cortex greatly increases the second moment of area. The humeri's second moment of area increased more dramatically than the cross-sectional area in AV housing conditions. An increased second moment of area is a characteristic response in bone that has been subjected to additional bending or torsional loads (Bass et al., 2002; Ducher et al., 2009) such as wing flapping which was possible in the AV systems. These findings suggest that humerus loading might be different to tibia, resulting in distinct growth patterns in each bone. The study results corroborate the findings that torsional resistance is the principal component to drive humerus structural design, while axial bending drives the structure of tibiotarsus in birds (de Margerie et al., 2005).
Structural improvement in AV pullets’ humeri was accompanied by an increase in volumetric bone density and bone ash. The effect in cortical density of tibia was limited to the distal section and no difference was observed in the ash content. Concentration of hydroxylysyl pyridinoline decreased in the pullets switched to AV until 12 wk but then increased to even higher levels compared to CC pullets at 16 wk. Although net bone resorption is decreased in birds undergoing exercise (Fleming et al., 2006), why the level went up after 12 wk was unclear. Unlike deoxypyridinoline which is often used as bone specific marker of collagen turnover, hydroxylysyl pyridinoline used in this study is not bone-specific and the ambiguous result might be due to collagen turnover in muscles and other organs of the growing pullets. Bone strength and modulus were calculated from the geometry and moment-bone rotation data to assess the material properties of the bones. Although the differences between groups for some of these quantities were statistically significant, the percentage changes were small. The statistically significant material differences were likely a product of the large sample sizes that boosted the sensitivity or due to the differences in structure and properties of organic matrix of the bone, which was not studied in this experiment. The changes in bone structure and density were not highly reflected by the mechanical testing when young bull calves were subjected to exercise (Hiney et al., 2004). The researchers suggested that physical measurements may provide more reliable assessment of bone mineral content than quantitative computed tomography, especially with smaller bones. Similar results were observed in mature White Leghorn roosters, where cortical areas and load bearing capacity were improved with exercise but the Young's modulus was not (Loitz and Zernicke, 1992). Laying hens housed in aviary houses have been reported to have significantly wider tibio-tarsal cortical area along with heavier bone mass, and denser tibia and humeri, compared to conventionally caged birds (Fleming et al., 2006). Whereas more recent quantitative computed tomography studies of bones in laying hens have demonstrated no changes in volumetric density of cortical and trabecular tissues between the housing systems despite having significant differences in structure (Jendral et al., 2008; Shipov et al., 2010), which suggests that increased bone mineral content was a response to increased bone quantity and not a result of improved bone mineral density. Enneking et al. (2012) reported no difference in pooled data of various bones and ages for areal density, bone length, and width in pullets housed in cages with perches compared to pullets in cages without perches. However, the bone mineral content of tibia and humerus were significantly different at 12 wk between the groups.
This study indicated that skeletal loading provided by activities within pullet AV housing resulted in structural and material changes that improved the load-bearing capability and stiffness to the tibia and humerus. Providing greater access to activities including flying, perching, and running during pullet phase can be crucial to the increased bone quantity that might help prevent fractures due to osteoporosis in cage birds, and impact injuries during the production phase in the extensive systems.
Acknowledgments
The authors thank Coalition for Sustainable Egg Supply for funding the project. The authors also appreciate the contributions Clifford Beckett from the orthopedic and biomechanical laboratory at Michigan State University for collection and analysis of the data. We appreciate Natalie Smith, Kailynn Vandewater, Emily Hayes, Lisa Kitto, and Natalie McKeon for sample collection and analysis.
Footnotes
Research support provided in part by a grant from the Coalition for a Sustainable Egg Supply (Kansas City, MO).
REFERENCES
- Bass S., Saxon L., Daly R., Turner C., Robling A., Seeman E., Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J. Bone Miner. Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Bertram J. E. Structural response of growing bone to exercise and disuse. J. Appl. Physiol. 1994;76:946–955. doi: 10.1152/jappl.1994.76.2.946. [DOI] [PubMed] [Google Scholar]
- Couch J. R. Cage layer fatigue. Feed Age. 1955;5:55–57. [Google Scholar]
- de Margerie E., Sanchez S., Cubo J., Castanet J. Torsional resistance as a principal component of the structural design of long bones: Comparative multivariate evidence in birds. Anat. Rec. 2005;282A:49–66. doi: 10.1002/ar.a.20141. [DOI] [PubMed] [Google Scholar]
- Ducher G., Daly R. M., Bass S. L. Effects of repetitive loading on bone mass and geometry in young male tennis players: A quantitative study using MRI. J. Bone Miner. Res. 2009;24:1686–1692. doi: 10.1359/jbmr.090415. [DOI] [PubMed] [Google Scholar]
- Elson H. A. Housing and husbandry of laying hens: Past, present and future. Lohmann Inform. 2011;46:16–24. [Google Scholar]
- Enneking S. A., Cheng H. W., Jefferson-Moore K. Y., Einstein M. E., Rubin D. A., Hester P. Y. Early access to perches in caged White Leghorn pullets. Poult. Sci. 2012;91:2114–2120. doi: 10.3382/ps.2012-02328. [DOI] [PubMed] [Google Scholar]
- Fleming R. H., McCormack H. A., McTeir L., Whitehead C. C. Relationships between genetic, environmental and nutritional factors influencing osteoporosis in laying hens. Br. Poult. Sci. 2006;47:742–755. doi: 10.1080/00071660601077949. [DOI] [PubMed] [Google Scholar]
- Hiney K. M., Nielsen B. D., Rosenstein D., Orth M. W., Marks B. P. High-intensity exercise of short duration alters bovine bone density and shape. J. Anim. Sci. 2004;82:1612–1620. doi: 10.2527/2004.8261612x. [DOI] [PubMed] [Google Scholar]
- VA USA: Promar International; 2009. Impacts of banning cage egg production in the United States: A report prepared for United Egg Producers. [Google Scholar]
- Jendral M. J., Korver D. R., Church J. S., Feddes J. J. R. Bone mineral density and breaking strength of White Leghorns housed in conventional, modified, and commercially available colony battery cages. Poult. Sci. 2008;87:828–837. doi: 10.3382/ps.2007-00192. [DOI] [PubMed] [Google Scholar]
- Jones D. R., Karcher D. M., Abdo Z. Effect of commercial housing system on egg quality during extended storage. Poult. Sci. 2014;93:1282–1288. doi: 10.3382/ps.2013-03631. [DOI] [PubMed] [Google Scholar]
- Judex S., Zernicke R. F. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J. Appl. Physiol. 2000;88:2183–2191. doi: 10.1152/jappl.2000.88.6.2183. [DOI] [PubMed] [Google Scholar]
- Loitz B. J., Zernicke R. F. Strenuous exercise-induced remodeling of mature bone: Relationships between in vivo strains and bone mechanics. J. Exp. Biol. 1992;170:1–18. doi: 10.1242/jeb.170.1.1. [DOI] [PubMed] [Google Scholar]
- Nightingale T. E., Littlefield L. H., Merkley L. W. Osteoporosis induced by unilateral wing immobilization. Poult. Sci. 1972;51:1844–1845. [Google Scholar]
- Reich A., Jaffe N., Tong A., Lavelin I., Genina O., Pines M., Sklan D., Nussinovitch A., Monsonego-Ornan E. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J. Appl. Physiol. 2005;98:2381–2389. doi: 10.1152/japplphysiol.01073.2004. [DOI] [PubMed] [Google Scholar]
- Robling A. G., Hinant F. M., Burr D. B., Turner C. H. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 2002;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- Robling A. G., Niziolek P. J., Baldridge L. A., Condon K. W., Allen M. R., Alam I., Mantila S. M., Gluhak-Heinrich J., Bellido T. M., Harris S. E., Turner C. H. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J. Biol. Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Seeman E., Delmas P. D. Bone quality–The material and structural basis of bone strength and fragility. New England J. Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- Shipov A., Sharir A., Zelzer E., Milgram J., Monsonego-Ornan E., Shahar R. The influence of severe prolonged exercise restriction on the mechanical and structural properties of bone in an avian model. Vet. J. 2010;183:153–160. doi: 10.1016/j.tvjl.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Vuori I. Peak bone mass and physical activity: A short review. Nutr. Rev. 1996;54:S11–S14. doi: 10.1111/j.1753-4887.1996.tb03892.x. [DOI] [PubMed] [Google Scholar]
- Wan Z. M., Li J. Y., Li R. X., Li H., Guo Y., Liu L., Zhang X. C., Zhang X. Z. Bone formation in rabbit cancellous bone explant culture model is enhanced by mechanical load. Biomed. Eng. Online. 2013;12:35. doi: 10.1186/1475-925X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C. C., Fleming R. H. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]