Abstract
Study Objectives:
Suvorexant is an orexin receptor antagonist approved for treating insomnia at a maximum dose of 20 mg. Phase-3 trials evaluated two age-adjusted (non-elderly/elderly) dose-regimes of 40/30 mg and 20/15 mg with the primary focus on 40/30 mg. We report here results from pooled analyses of the 20/15 mg dose-regime, which was evaluated as a secondary objective in the trials.
Methods:
Prespecified analysis of pooled data from two identical randomized, double-blind, placebo-controlled, parallel-group, 3-month trials in non-elderly (18–64 years) and elderly (≥ 65 years) patients with insomnia. Patients were randomized to suvorexant 20/15 mg (non-elderly/elderly), suvorexant 40/30 mg (non-elderly/elderly), or placebo; by design, fewer patients were randomized to 20/15 mg. Efficacy was assessed by self-reported and polysomnography (PSG; subset of patients) sleep maintenance and onset endpoints.
Results:
Suvorexant 20/15 mg (N = 493 treated) was effective compared to placebo (N = 767 treated) on patient-reported and PSG sleep maintenance and onset endpoints at Night-1 (PSG endpoints) / Week-1 (subjective endpoints), Month-1 and Month-3, except for effects on PSG sleep onset at Month-3. Suvorexant 20/15 mg was generally well tolerated, with 3% of patients discontinuing due to adverse events over 3 months vs. 5.2% on placebo. Somnolence was the most common adverse event (6.7% vs. 3.3% for placebo). There was no systematic evidence of rebound or withdrawal signs or symptoms when suvorexant was discontinued after 3 months of nightly use.
Conclusions:
Suvorexant 20/15 mg improved sleep onset and maintenance over 3 months of nightly treatment and was generally safe and well tolerated.
Clinical Trial Registration:
ClinicalTrials.gov trial registration numbers: NCT01097616, NCT01097629.
Citation:
Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, Matzura-Wolfe D, Benca RM, Krystal AD, Walsh JK, Lines C, Roth T, Michelson D. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med 2016;12(9):1215–1225.
Keywords: insomnia, sleep, orexin, suvorexant, randomized controlled trial, pharmacotherapy
INTRODUCTION
In 1998, the gene encoding the hypothalamic orexin neuropeptides OX-1 and OX-2 was described.1,2 Sixteen years later, in 2014, suvorexant became the first agent specifically targeting the orexin system (via orexin receptor antagonism) to be approved as a therapeutic agent, for the treatment of insomnia.3–8 This relatively rapid progress reflects advances in understanding orexin biology. The orexin signaling system comprises of a restricted number (50,000–80,000) of orexin neurons which originate from the lateral hypothalamus and project widely throughout the central nervous system.9 A role for orexin in regulating wakefulness was suggested from studies which showed that orexin neuron loss was associated with narcolepsy in mice, dogs, and humans.10–13 Further research has shown diurnal variation of orexin activity in normal animals, with increased activity during wakefulness and reduced activity during sleep.14,15 Elucidation of orexin's role in regulating wakefulness suggested that orexin receptor antagonists could provide a new approach to treating insomnia by blocking orexin-mediated wake signaling.16 This approach is distinct from current insomnia drugs, most of which promote sleep by enhancing sleep signaling via gamma-aminobutyric acid (GABA) inhibitory effects.17
BRIEF SUMMARY
Current Knowledge/Study Rationale: Suvorexant is a first-in-class orexin receptor antagonist approved for treating insomnia at a maximum dose of 20 mg. We performed a pooled analysis of suvorexant 20/15 mg, which was evaluated as a secondary objective in Phase-3 clinical trials.
Study Impact: The results of the pooled analysis showed that suvorexant 20/15 mg improved sleep onset and maintenance over 3 months of nightly treatment and was generally safe and well-tolerated. Our analysis validates orexin receptor antagonism as a novel therapeutic approach for treating insomnia.
The suvorexant phase-3 development program in insomnia patients was comprised of two 3-month pivotal trials, each of which evaluated two age-adjusted (non-elderly/elderly) dose regimes of 40/30 mg and 20/15 mg,8 and a 1-year trial of 40/30 mg.7 The primary objective of the trials was to establish the efficacy, safety, and tolerability of the 40/30 mg dose. By design, fewer patients were assigned to 20/15 mg than 40/30 mg and each of the pivotal phase-3 trials were individually not adequately powered with regard to 20/15 mg effects on all efficacy endpoints. The two pivotal efficacy studies were identical in design, and pooled analyses of the results were prespecified in the statistical analysis plan. Here we report the results of these pooled analyses for the Food and Drug Administration (FDA) approved 20/15 mg doses. We also report on additional analyses of suvorexant effects on maintaining sleep hourly over the course of a night, efficacy and tolerability in subgroups such as those defined by age and gender, and efficacy as assessed by responder analyses.
METHODS
Overview
The pooled efficacy analyses included data from two phase-3 randomized, double-blind, placebo-controlled, parallel-group, 3-month efficacy and safety trials in non-elderly (18–64 years) and elderly (≥ 65 years) patients with insomnia (protocol 028 [P028] and protocol 029 [P029]).8 P028 also included an optional randomized, double-blind, placebo-controlled, 3-month extension. Suvorexant doses of 40/30 mg (non-elderly/elderly) and 20/15 mg (non-elderly/elderly) were evaluated, with fewer patients randomized to 20/15 mg than 40/30 mg or placebo. Each trial incorporated a 1-week, randomized, double-blind run-out after double-blind treatment (3 months in P029, 3 or 6 months in P028) to assess withdrawal and rebound insomnia.
Patients
Non-elderly (18–64) and elderly (≥ 65) patients who met Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria for primary insomnia18 and were otherwise in good physical and mental health were enrolled. All patients provided subjective sleep estimates using an electronic sleep diary/questionnaire. Approximately 75% of the patients also underwent polysomnography (PSG) over 8 hours. Patients who received only self-report assessments are referred to as the questionnaire (Q)-cohort; those who received self-report and PSG assessments are referred to as the PSG+questionnaire (PQ)-cohort. To enter the Q-cohort, patients had to report a total sleep time (sTST) < 6.5 h and time to sleep onset (sTSO) ≥ 30 min, both on ≥ 4 of 7 nights during the last week of a 2-week placebo run-in before randomization. For the PQ-cohort, patients had to meet the following PSG criteria for screening and baseline PSG nights: latency to onset of persistent sleep (LPS; the time from the beginning of lights off to the onset of 10 consecutive minutes of sleep) > 20 min, and mean (across screening and baseline) wakefulness after persistent sleep onset (WASO) ≥ 60 min with neither night ≤ 45 min. They were not required to meet the Q-cohort entry criteria. All patients (PQ+Q cohorts) also had to report ≥ 1 h of wakefulness after sleep onset (sWASO) on ≥ 3 of 7 nights each week during the 4 weeks prior to the initial screening visit.
Design and Procedure
Patients were discontinued from existing hypnotic medications prior to entering each trial. Patients were randomized to treatment with suvorexant 40/30 mg, suvorexant 20/15mg, or placebo in a 3:2:3 ratio in P028, and a 1:1:1 ratio (Q-cohort) or a 2:1:2 ratio (PQ-cohort) in P029. Doses differed by age to adjust for previously-observed plasma exposure differences (< 65: 40 mg or 20 mg; ≥ 65: 30 mg or 15 mg). Randomization was stratified by age category (non-elderly vs. elderly) in all trials and also by cohort (Q vs. PQ) in P028 and P029. For the run-out phase, patients initially assigned to suvorexant were randomized to either continue on the same dose of suvorexant (suvorexant→suvorexant) or to switch to placebo (suvorexant→placebo) in a 1:1 ratio, while patients initially assigned to placebo continued to receive placebo (placebo→placebo).
Patients were blindly assigned to treatment according to a randomization schedule generated by a Merck statistician using a computerized allocation schedule system. Treatment assignment was implemented through an interactive voice response system. Study investigators, site staff, patients, PSG scorers, and Merck monitoring staff remained blinded to treatment allocation throughout the study. Patients were instructed to take medication orally once daily immediately prior to bedtime (within 5 to 10 minutes).
The trials were conducted in accordance with principles of Good Clinical Practice and were approved by the appropriate institutional review boards and regulatory agencies for each site. Informed consent was obtained from all patients. The trials were registered at ClinicalTrials.gov (NCT01097616, NCT01097629).
Assessments
Patients used an electronic diary each morning to report sTST (min), sTSO (min), sWASO (min), number of awakenings (sNAW), quality of sleep (sQUAL, 4-point scale), and refreshed feeling on waking (sFRESH, 5-point scale). The Insomnia-Severity-Index (ISI)19 was completed at Months 1 and 3, and clinician and patient ratings of global severity (CGI-S [7-point scale], PGI-S [6-point scale]) and global improvement (CGI-I, PGI-I [7-point scales]) were recorded at Week 2 and Months 1, 2, and 3. PSG measures included LPS, WASO, and total sleep time (TST) assessed at Night 1, Month 1, and Month 3.
Safety assessments included open-ended questioning for adverse events (AEs), the Columbia Suicidality Severity Rating Scale,20 and periodic physical, chemistry, hematology, and electrocardiogram assessments. A Motor-Vehicle-Accidents-and-Violations questionnaire was administered during clinic visits or phone calls to assess the occurrence of traffic or motor vehicle accidents (and related injuries) or citations since the last visit when the patient was the driver.
A program-wide guidance document containing definitions of prespecified events of clinical interest (ECIs) was provided to investigators. A blinded independent committee comprised of 3 experts in neurology, psychiatry, and sleep, respectively, adjudicated all ECIs potentially suggestive of intrusion of rapid eye movement (REM) sleep into wakefulness (cataplexy) or at initiation of sleep (sleep-onset paralysis). Falls were also adjudicated to ascertain whether they were due to a possible episode of cataplexy.
Residual effects were assessed in the PQ-cohort by the Digit Symbol Substitution Test (DSST) within 0.5–1 h following lights-on on the morning after PSG.
Potential withdrawal symptoms were assessed after completion of the double-blind treatment period by the Tyrer Withdrawal Symptom Questionnaire21 administered prior to dosing on 3 consecutive evenings at the start of the run-out.
Efficacy Endpoints
The primary efficacy endpoints were change from baseline in sleep diary and PSG measures of sleep maintenance (sTST, WASO) and sleep onset (sTSO, LPS). Monthly values for the self-report endpoints were the mean of the daily values for the last week (Week 1, Month 1) or 2 weeks (Month 3) of the month. Changes from baseline on other diary endpoints (sWASO, sNAW, sQUAL, sFRESH), PSG endpoints (TST), and rating scale endpoints (ISI, CGI-S, PGI-S, CGI-I, PGI-I) were also assessed. PSG sleep efficiency (SE [%]), defined as TST/time in bed (fixed at 8 h for PSG recording) × 100, was assessed post hoc. SE provides an additional parameter of interest to practitioners but is effectively double reporting of TST results in situations where time in bed is fixed, as in the present studies. Subjective sleep efficiency could not be calculated because time in bed was not captured on the patient diaries. In addition, we assessed: WASO by hour of night; the percentage of responders using various definitions (ISI ≥ 6 point improvement from baseline22; sTSO and sTST ≥ 15% improvement from baseline, with the cutoff corresponding to the degree of change likely to exceed the mean placebo change from baseline based on historical data from similarly designed clinical trials conducted by Merck); and efficacy in subgroups defined by age, gender, race, region, baseline severity (above or below the median for the corresponding baseline values of the endpoint), cohort (Q or PQ), and, for the PQ-cohort only, by Q-cohort entry criteria (those who met Q-cohort entry criteria versus those who did not).
Statistical Analysis
A pooled analysis of phase-3 efficacy and safety data was prespecified, to allow a more robust evaluation of the 20/15 mg dose given that each individual trial was underpowered for this dose. It should be noted that for some analyses (of AEs, measures of rebound and withdrawal) the analysis plan prespecified that the placebo arm for the 20/15mg comparison would also include 0- to 3-month placebo data from the P009 1-year trial of 40/30 mg.7 However, given that 20/15 mg was not included in P009 and because 20 mg is the maximum FDA approved dose, we decided it would be most valid to restrict the placebo dataset to P028+P029 for the 20/15 mg comparison.
The efficacy analyses included all patients who took ≥ 1 dose of treatment, had ≥ 1 post-treatment efficacy measure, and had baseline data (for analyses requiring baseline data). Efficacy endpoints (i.e., change from baseline in sTST, WASO, sTSO, LPS) were assessed using a longitudinal data analysis model with terms for baseline value, age group (non-elderly vs. elderly), region, gender, treatment, trial, time, and interaction of treatment-by-time; cohort was also included in the models for sTST and sTSO and other subjective endpoints. Analysis of responders was based on a generalized linear mixed model using the same terms.
No formal multiplicity strategy was employed for these pooled analyses since the primary purpose was to provide improved precision in the estimates of the treatment group comparisons; note that nominal p values were computed as a measure of strength of effect rather than a formal test of hypothesis.
The safety analysis included all patients who took ≥ 1 dose of treatment. The percentages of patients with AEs, including prespecified ECIs, were calculated. Summary statistics were calculated for predefined limits of change in laboratory, vital signs, or ECG measures.
Rebound insomnia on diary endpoints was assessed in patients who entered the 1-week run-out at the end of treatment (3 months or 6 months). The proportion of patients in each treatment group with lower sTST (or greater sTSO) relative to pretreatment baseline, regardless of the magnitude of the difference, was calculated for each of the first 3 nights of run-out as well as on any of the 3 nights. For PSG parameters (WASO and LPS), values during the first night of the run-out at the end of 3 months of treatment were compared to those obtained at pretreatment baseline. The primary comparisons were between the suvorexant→placebo groups and the placebo→placebo group. Additionally, rebound effects were assessed by between group comparisons of the mean changes from pretreatment baseline (in minutes) during the run-out for sTST, sTSO, WASO, and LPS.
To assess withdrawal symptoms, the proportion of patients with ≥ 3 newly emergent or worsened (compared to the last treatment measurement) symptoms on the 20-item Tyrer Withdrawal Symptom Questionnaire for each of the first 3 nights of run-out as well as across all 3 nights was calculated. The primary comparisons were between the suvorexant→suvorexant groups and the suvorexant→placebo groups.
Next-morning residual effects were assessed by the number of attempts and number of correct items on the DSST.
Power
Based upon pooled sample sizes of 1,260 (493 for suvorexant 20/15 mg, 767 for placebo) for self-report efficacy endpoints and 930 (343 for suvorexant 20/15 mg, 587 for placebo) for PSG efficacy endpoints, and assuming an overall dropout rate of approximately 1% at Night 1 (for PSG endpoints), 5% at Week 1 (for self-report endpoints), 10% at Month 1, and 20% at Month 3, the marginal power for the comparison of suvorexant 20/15 mg versus placebo was > 99% to detect standardized effect sizes ranging from 33% to 76% for the maintenance endpoints (sTST, WASO) and > 93% to detect standardized effect sizes ranging from 29% to 50% for the onset endpoints (sTSO, LPS). The primary purpose of the subgroup analyses was to evaluate the consistency of the treatment effect across various subgroups; therefore, no power calculations were made for the subgroup analyses.
RESULTS
Patient Accounting
Patient accounting for the 3-month treatment periods of the pooled data sets are shown in Figure S1 in the supplemental material. Completion rates for 3 months were high (85–89%) and similar among treatment groups.
Patient Characteristics
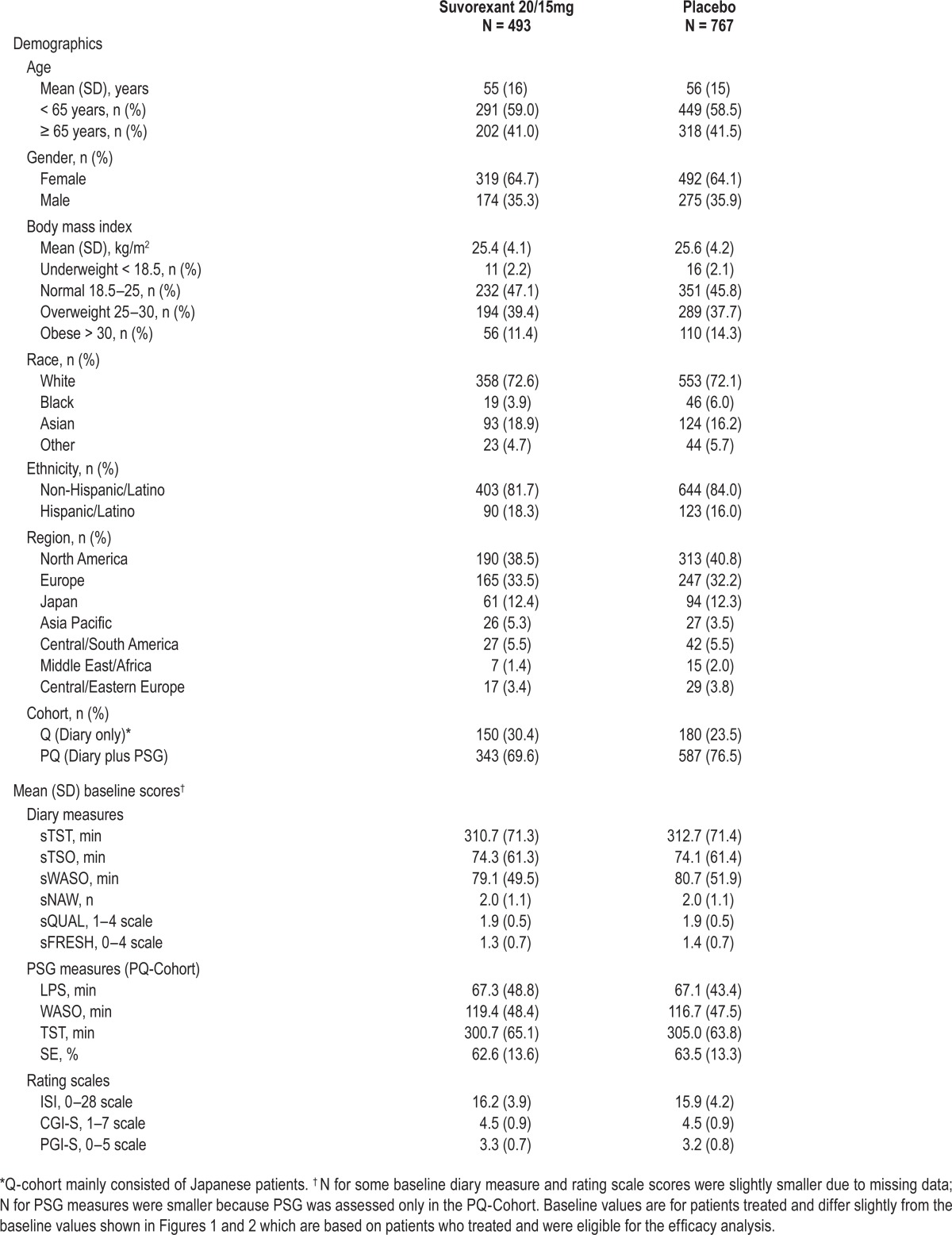
Patient characteristics and baseline symptom severity were generally similar among treatment groups and are summarized in Table 1. At baseline, patients reported a mean sTST of ∼5 h and a mean sTSO of ∼1.25 hours.
Table 1.
Baseline characteristics of treated patients for P028+P029.
Efficacy
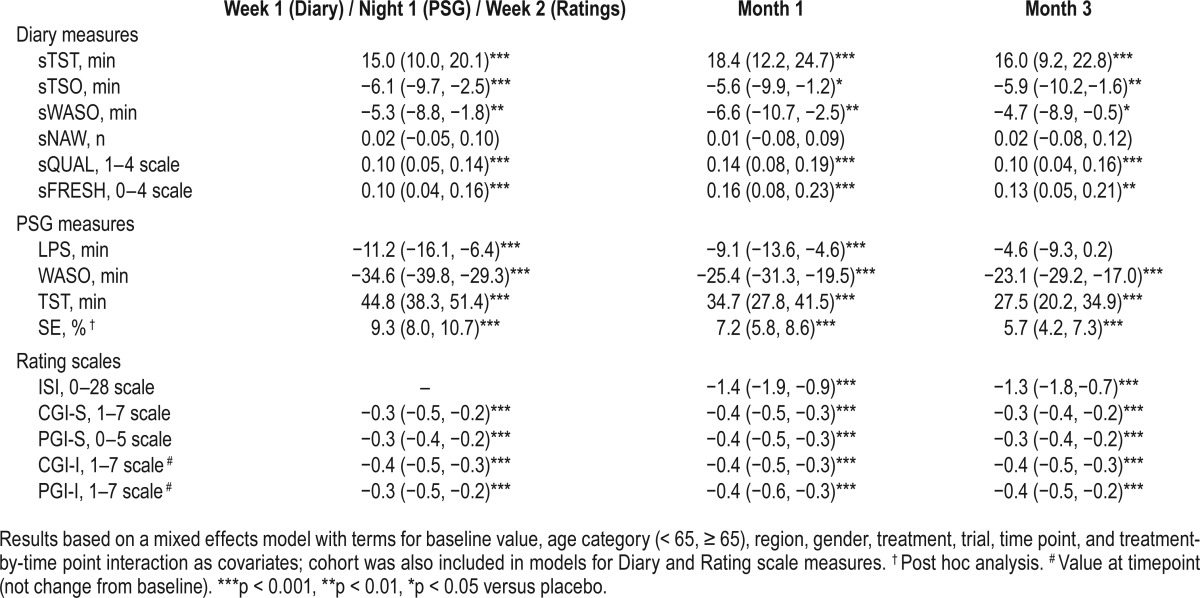
Mean changes-from-baseline in each treatment group for diary sleep measures, PSG sleep measures, and global rating scales are shown in Table S1 in the supplemental material. Suvorexant 20/15 mg differences from placebo are summarized in Table 2. Suvorexant 20/15 mg provided improvement compared to placebo for all endpoints except sNAW, and LPS at Month-3.
Table 2.
Summary of efficacy for suvorexant 20/15 mg over 3 months for P028+P029: difference (95% CI) between suvorexant and placebo in least squares mean change from baseline.
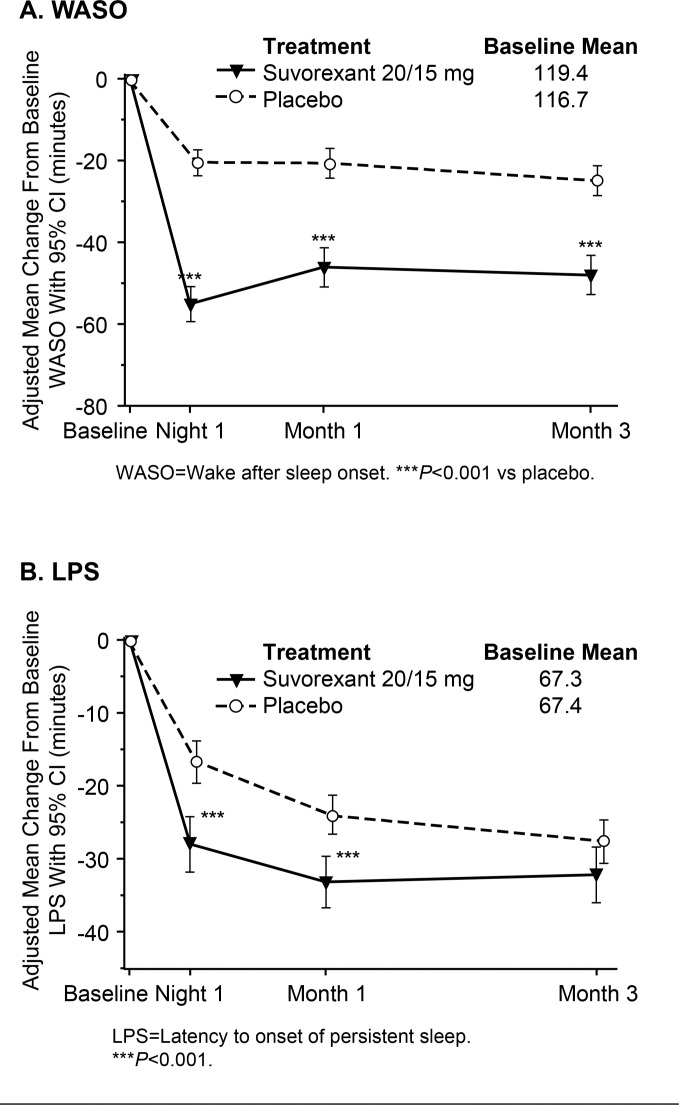
Effects of suvorexant 20/15 mg on sleep maintenance and sleep onset over time are illustrated graphically in Figure 1 (self-report measures) and Figure 2 (PSG measures). It can be seen that suvorexant effects were apparent on the first PSG night or first week of diary data and were maintained throughout 3 months of nightly use (except for LPS at Month 3). Standardized effect sizes for suvorexant 20/15 mg on self-report measures at Week 1 were 34% for sTST and 20% for sTSO; standardized effect sizes for PSG measures at Night-1 were 89% for WASO and 31% for LPS. At Month 3, standardized effect sizes for suvorexant 20/15 mg on self-report measures were 29% for sTST and 17% for sTSO, and standardized effect sizes for PSG measures were 54% for WASO and 14% for LPS.
Figure 1. Adjusted means and 95% CIs for change from baseline in self-report endpoints for P028+P029.
Figure 2. Adjusted means and 95% CIs for change from baseline in PSG endpoints for P028+P029.
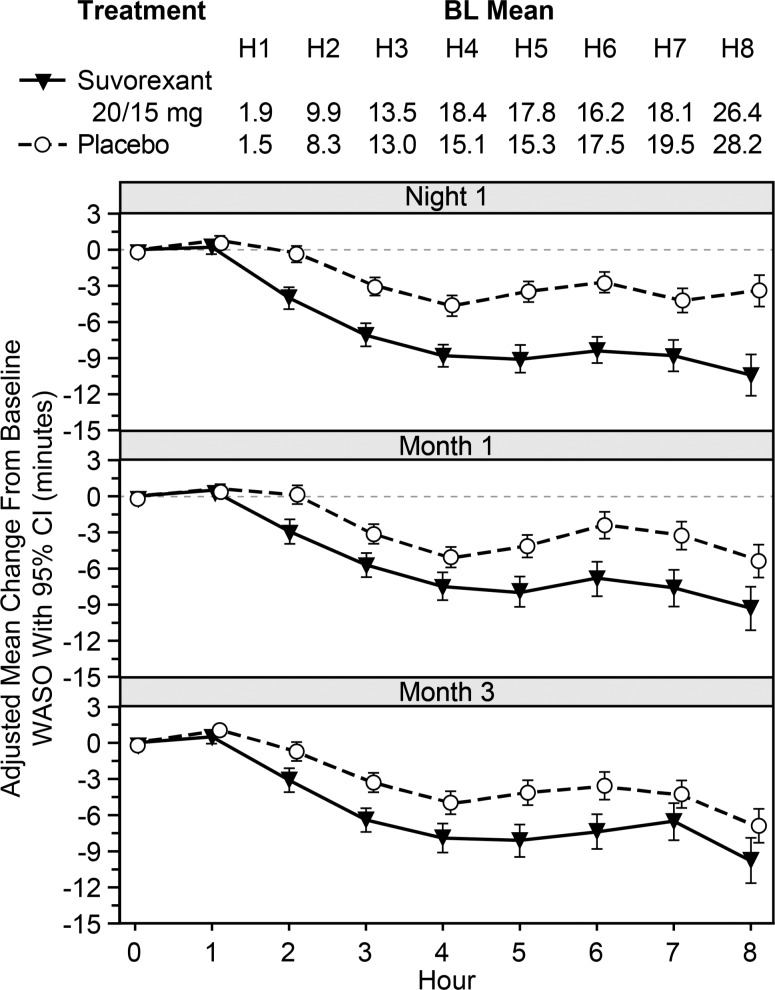
Effects of suvorexant 20/15 mg on WASO by hour of the night are shown in Figure 3. Suvorexant generally improved WASO from 2–8 h, except for hours 7 and 8 at Month 3.
Figure 3. Adjusted means and 95% CIs for change from baseline (BL) in WASO by hour of the night for P028+P029.
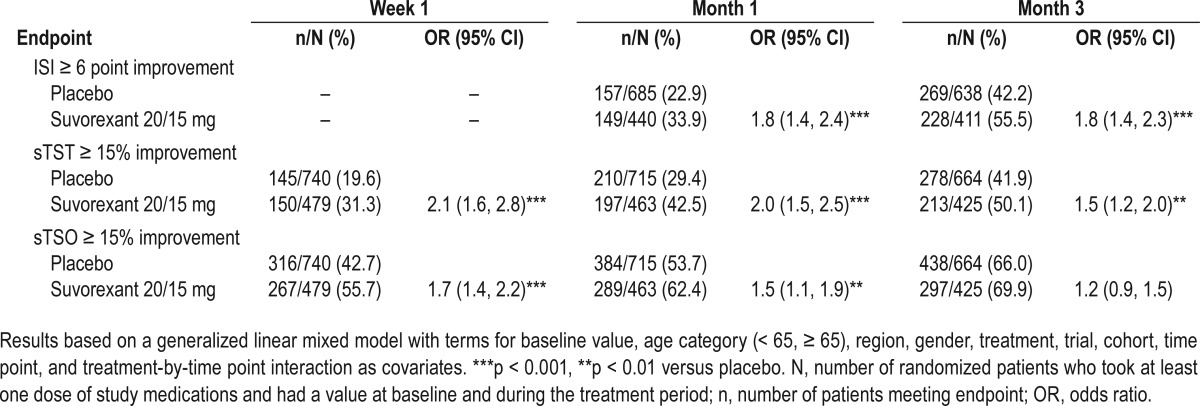
The analyses of the percentages of responders according to the various definitions are shown in Table 3. A greater odds of responding was evident for suvorexant 20/15 mg versus placebo, except for the “sTSO ≥ 15% improvement” definition at Month 3.
Table 3.
Summary of responder analyses for suvorexant 20/15 mg over 3 months for P028+P029: number (%) of responders and odds ratio (95% CI) for the difference between suvorexant and placebo.
Suvorexant 20/15 mg differences versus placebo for sleep maintenance and sleep onset endpoints in subgroups defined by age, gender, race, region, baseline severity, cohort, and entry criteria are shown in Figure S2 (sleep maintenance) and Figure S3 (sleep onset) in the supplemental material. The findings were generally consistent across the subgroups (95% CIs overlapped) and mirrored those for the overall population.
Safety
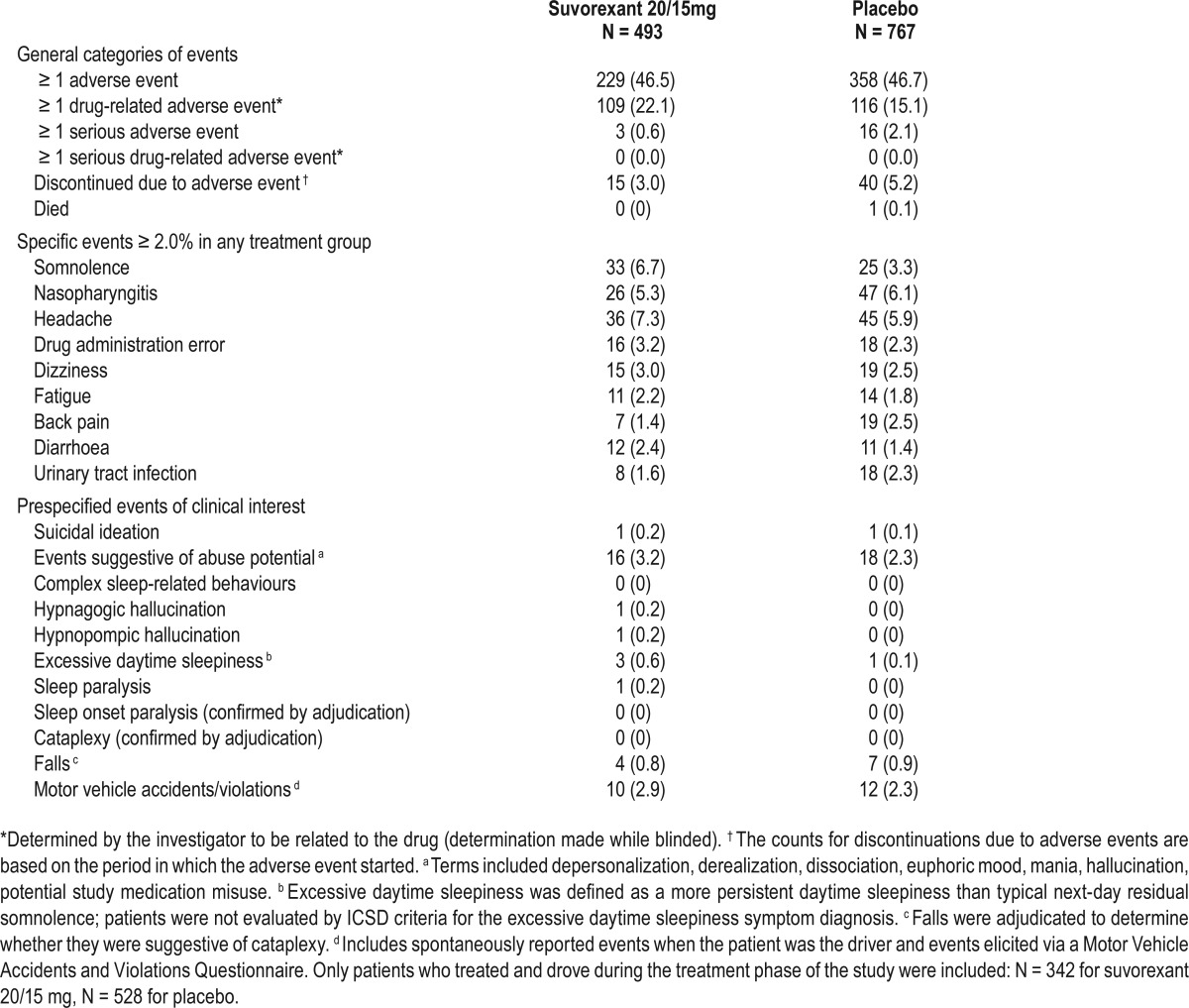
AEs over 3 months are summarized in Table 4. Patients treated with suvorexant 20/15mg had generally similar incidences of any AEs or discontinuations due to AEs compared with placebo. The proportion of patients with serious adverse events was similar among the treatment groups and there was no important difference between treatment groups in the specific types of serious adverse events that were reported. The proportion of patients that had drug-related AEs was somewhat higher with suvorexant, but none of the drug-related AEs were serious. The most common AE that was increased for suvorexant versus placebo was next-day somnolence (6.7% versus 3.3%). As shown in Table 5, somnolence rarely resulted in discontinuation and was mostly mild or moderate in severity. Among patients assigned to suvorexant who reported somnolence within 3 months, 62% experienced it within the first week and 93% experienced it within the first month (Table 5). Of the 33 patients on suvorexant 20/15 mg who initially reported somnolence within 3 months, 2 had a subsequent report.
Table 4.
Summary of adverse events over 3 months for P028+P029: number (%) of patients by treatment.
Table 5.
Summary of somnolence adverse events over 3 months for P028+P029: number (%) of patients by treatment except where noted.
The incidence of predefined ECIs over 3 months is shown in Table 4. The most common category for both suvorexant and placebo was events that could potentially indicate abuse, but most appeared to be drug administration errors without evidence of intentional misuse (investigators were instructed to report potential study medication misuse when a patient returned less study medication than expected and denied taking extra study medication; review of these cases suggested that the majority of events were due to accidental loss of study medication). Other ECIs reported in ≥ 1 patient on suvorexant but no patients on placebo included hypnagogic hallucination (n = 1), hypnopompic hallucination (n = 1), and sleep paralysis (n = 1). The proportions of patients reporting excessive daytime sleepiness (defined as more persistent daytime sleepiness than typical next-day residual somnolence and associated with impairment) were low in all groups but higher for suvorexant (0.6%, n = 3) versus placebo (0.1%, n = 1). There were no reports of cataplexy confirmed by adjudication in either group. The proportions of patients with falls and motor vehicle accident or violation events were similar in both treatment groups.
Over 3 months, based on clinician's assessments of adverse events and patient responses to the Columbia Suicide Severity Rating Scale, suicidal ideation was reported by 1 patient on suvorexant and 1 patient on placebo. Both reports were associated with clearly identified precipitating stressful life events. No suicidal behavior was reported.
The number of patients with ≥ 1 AE and with somnolence by age and gender subgroups for each treatment group are summarized in Table S2 in the supplemental material. Across all treatment groups, the elderly tended to have more AEs than the non-elderly and women tended to have more AEs than men. The pattern of findings for suvorexant versus placebo in age and gender subgroups generally mirrored those for the overall population, except that men receiving suvorexant did not show an increase in somnolence versus placebo whereas women did.
No clear trends were seen between treatment groups with regard to the percentages of patients who met predetermined criteria on vital signs or ECG measures (Table S3 in the supplemental material), or with regard to changes in laboratory measures.
Rebound Insomnia
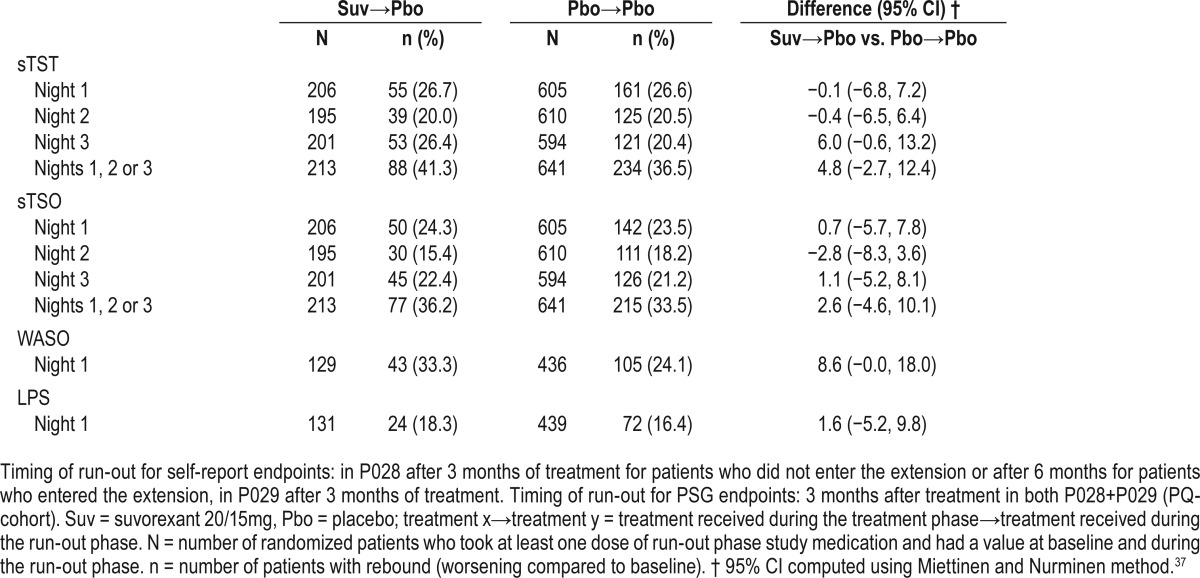
Analyses of rebound insomnia for self-report endpoints during the first 3 nights of the run-out, and PSG endpoints on the first night, are summarized in Table 6. There were no statistically significant differences between the suvorexant→placebo group and the placebo→placebo group on any measure on any night. The supportive approach looking at mean changes from baseline in minutes also showed that in both groups (suvorexant→placebo and placebo→placebo) self-report and PSG endpoints either continued to be improved from baseline during the run-out nights or were comparable to baseline (in the case of suvorexant→placebo for sTSO on Night 1), i.e., the mean values did not worsen relative to baseline (Table S4 in the supplemental material).
Table 6.
Number and percentage of patients meeting criteria for rebound insomnia on self-report endpoints during the first 3 nights of the run-out phase, and meeting criteria for rebound insomnia on PSG endpoints during the first night of the run-out for P028+P029.
Withdrawal
Analyses of change in Tyrer Withdrawal Symptom Questionnaire scores during the first 3 nights of the run-out are summarized in Table S5 in the supplemental material. There were no significant differences in the numbers of patients meeting the prespecified withdrawal criteria for the comparisons of the suvorexant→suvorexant group versus the suvorexant→placebo group.
Residual Effects
No statistically significant differences were observed between suvorexant and placebo in terms of baseline-adjusted number of correct responses or attempted responses on the DSST (Table S6 in the supplemental material).
DISCUSSION
These prespecified analyses of pooled data from phase-3 trials8 showed that 20/15 mg doses of suvorexant improved self-report and PSG measures of sleep onset and sleep maintenance at the initiation of treatment and over 3 months of nightly use. Generally, effects were greater on objective than subjective measures. Suvorexant 20/15 mg also improved patients' ratings of sleep quality and feeling refreshed in the morning. The global benefits of these improvements in sleep were reflected in patient and clinician global assessments of insomnia severity and improvement, and in a variety of responder analyses including changes in ISI. The efficacy of suvorexant 20/15 mg was consistent across subgroups including those defined by age and gender.
Of particular interest was the observation that the PSG measure of hour-by-hour wake time during the night generally showed improvement for suvorexant 20/15 mg versus placebo from 2 through 8 hours, although this effect was somewhat diminished at Month 3. This pattern of findings differs from that reported for extended release zolpidem which improves PSG sleep maintenance during the first 4 to 5 hours of the night.23 However, this difference has not been examined in a direct head-to-head study. Importantly, for most patients the greatest amount of wake time is in the last 3 hours of the night, as is evidenced in the placebo group. This is probably due to the circadian timing of histamine and orexin release and the dissipation of homeostatic sleep drive.24
This pooled analysis also showed that suvorexant 20/15 mg was generally well tolerated over 3 months, and most patients completed the planned 3 months of treatment. The most common AE associated with suvorexant was somnolence which mostly occurred early during treatment (within the first week to month), was generally mild-to-moderate in severity, and rarely resulted in discontinuation. Severe and impairing daytime somnolence, prospectively defined as the ECI of “excessive daytime sleepiness,” occurred in more patients on suvorexant 20/15 mg than placebo but was infrequent (0.6%). DSST scores suggested that suvorexant 20/15 mg did not impair next morning psychomotor function. There was no apparent difference between suvorexant and placebo with regard to motor vehicle accidents or violations, but due to the relative infrequency of such events and limitations of sample size the possibility of an increase cannot be excluded. Dedicated on-the-road driving studies in healthy non-elderly and elderly men and women suggest that suvorexant 20/15 mg does not produce a clinically meaningful impairment in next-morning driving performance as assessed by overall group mean changes in deviation from lane position.25,26 However, the possibility of individual variation in response is suggested by some individuals who had increases in deviation from lane position and prematurely stopped tests due to somnolence.26
The suvorexant phase-3 program included assessment of AEs that could be mechanism-based. Narcolepsy has been shown to be associated with a progressive degeneration of orexin neurons12,13,27 and consequently it has been suggested that antagonism of orexin receptors could mimic signs or symptoms of narcolepsy, particularly cataplexy (sudden intrusion of REM into the waking state associated with sudden loss of muscle tone). No events, including falls, were confirmed by adjudication as cataplexy in the phase-3 trials. However, patients known to have narcolepsy were excluded from the phase-3 trials, and to date there are no data on suvorexant effects in these patients.
Sleep-specific AEs were also assessed in the phase-3 trials. Suvorexant 20/15 mg was associated with a small number of reports of sleep-related hallucinations (2/493) and sleep paralysis (1/493) whereas there were no such reports for placebo. Given that both sleep-related hallucinations and sleep paralysis occur spontaneously in the general population it is unknown whether the observed events are specifically related to antagonism of orexin receptors.28,29 There were no reports of complex sleep behaviors for suvorexant 20/15 mg.
Possible effects on mood and suicidality are a concern for all centrally acting drugs. In the pooled analysis suicidal ideation was infrequent: 1/493 (0.2%) on suvorexant 20/15mg and 1/767 (0.1%) on placebo. Patients with major depression or other major psychiatric disorders were excluded from the trials in the pooled analysis. Insomnia and mood disorders have a high comorbidity, however. Consequently, as with other hypnotics, clinicians should be aware of the possibility that worsening of depression or suicidal thinking may occur.
The pooled analysis evaluated abrupt suvorexant 20/15 mg discontinuation during a run-out which occurred after chronic use for periods ranging from 3–6 months. No evidence of rebound insomnia (worsening of insomnia relative to baseline) or withdrawal symptoms was observed. Thus, these results suggest that abrupt termination of suvorexant 20/15 mg after nightly use for 3–6 months is generally well tolerated in most patients.
The clinical profile of suvorexant 20/15 mg in subgroups defined by age and gender was of particular interest given that the elderly and women are generally thought to have an increased prevalence of insomnia, increased risk of adverse events, and possibly different responsiveness to insomnia treatment.30–34 The subgroup analyses of pooled data showed that the efficacy of suvorexant did not vary as a function of age or gender. With regard to safety, there was no evidence to suggest that any difference in AEs (total number and somnolence) between suvorexant and placebo was more marked in elderly than non-elderly patients. It should be noted that elderly patients included in our trials were usually in good general health and the results may differ in the overall elderly population which includes frail elderly. In the gender subgroup AE analysis, men taking suvorexant 20/15 mg did not show an increase in somnolence versus placebo whereas women did. This could be a chance finding given the small sizes of the subgroups with somnolence, or could indicate that women taking suvorexant 20/15 mg may be more prone to next-day somnolence than men.
Several factors limit the interpretation of our data. The trials were conducted in patients with DSM-IV criteria primary insomnia, and results could differ in patients with comorbidi-ties. Many patients take hypnotics for longer than 3 months and results could differ over time, although the efficacy and safety of suvorexant 40/30 mg over 3 months in P028 and P029 was similar to that over 12 months in P009.7 Finally, no active comparator was included, and therefore we cannot make direct inferences about suvorexant relative to other insomnia medications. However, it is interesting to note that in the present analysis changes in suvorexant versus placebo sleep endpoints tended to be smaller for subjective than objective measures whereas the opposite finding (greater subjective than objective effects) has been reported for benzodiazepine receptor agonists.35,36 We speculate that possible amnestic properties and euphoric effects of benzodiazepine receptor agonists might conflate patient recall of subjective efficacy causing patients to subjectively overestimate sleep effects relative to objective measures. Based on the present results, suvorexant appears to be effective without inflating subjective report relative to objective assessment. Direct head-to-head comparative studies would be required to confirm possible differences in clinical profiles between suvorexant and other insomnia medications.
DISCLOSURE STATEMENT
These studies were funded by Merck & Co., Inc., Kenilworth, NJ, USA. The funding organization was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Dr. Herring, Dr. Connor, Dr. Snyder, Mr. Snavely, Dr. Zhang, Ms. Hutzelmann, Ms. Matzura-Wolfe, Dr. Lines, and Dr. Michelson are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and own or owned stock/stock options in Merck. Dr. Benca has served as a consultant to and receives research support from Merck, and has served as a consultant to Janssen and Jazz. Dr. Krystal has received grants/research support from NIH, Teva, Sunovion, Astellas, Abbott, Neosync, Brainsway, Janssen, ANS St. Jude, and Novartis. He has served as a consultant to: Abbott, Astellas, AstraZeneca, Attentiv, BMS, Teva, Eisai, Eli Lilly, GlaxoSmithKline, Jazz, Janssen, Merck, Neurocrine, Novartis, Otsuka, Lundbeck, Roche, Sanofi-Aventis, Somnus, Sunovion, Somaxon, Takeda, Transcept, and Vantia. Dr. Walsh has received research support from Apnex, Merck, Novo Nordisk, Respironics, and Vanda, and has provided consulting services to Merck, Somnus, Transcept, Vanda, Ventus, and Vivus. Dr. Roth has received grants/research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth and Xenoport; has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter & Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport; and has participated in speaking engagements supported by Cephalon, Sanofi, and Takeda.
ACKNOWLEDGMENTS
Mingqui Wu from Merck & Co., Inc., contributed to the statistical analysis. Sheila Erespe from Merck & Co., Inc. assisted with submission.
ABBREVIATIONS
- AE(s)
adverse event(s)
- CGI-I
clinician global impression of improvement
- CGI-S
clinician global impression of severity
- CI
confidence interval
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DSST
digit symbol substitution test
- ECG
electrocardiogram
- ECI(s)
event(s) of clinical interest
- FDA
Food and Drug Administration
- ISI
Insomnia Severity Index
- GABA
gamma-aminobutyric acid
- LPS
latency to onset of persistent sleep (assessed by polysomonography)
- P009
protocol (study) 009
- P028
protocol (study) 028
- P029
protocol (study) 029
- PGI-I
patient global impression of improvement
- PGI-S
patient global impression of severity
- PQ-cohort
polysomonography plus questionnaire cohort
- PSG
polysomonography
- Q-cohort
questionnaire only cohort
- REM
rapid eye movement sleep
- SD
standard deviation
- SE
sleep efficiency (assessed by polysomonography)
- sFRESH
self-reported refreshed feeling on waking
- sQUAL
self-reported sleep quality
- sTSO
self-reported time to sleep onset
- sTST
self-reported total sleep time
- sWASO
self-reported wakefulness after sleep onset
- TST
total sleep time (assessed by polysomonography)
- WASO
wakefulness after persistent sleep onset (assessed by polysomonography)
REFERENCES
- 1.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamusspecific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist[(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–32. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 4.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by MK-4305 - a novel dual orexin receptor antagonist. J Neurogenetics. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 5.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–74. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–67. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant, an orexin receptor antagonist, during 1-year treatment of insomnia followed by abrupt discontinuation of treatment: a randomized, double-blind, placebo-controlled clinical trial. Lancet Neurol. 2014;13:461–71. doi: 10.1016/S1474-4422(14)70053-5. [DOI] [PubMed] [Google Scholar]
- 8.Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79:136–48. doi: 10.1016/j.biopsych.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 12.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 13.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 14.Taheri S, Sunter D, Dakin C, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett. 2000;279:109–12. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 15.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status. CNS Drugs. 2013;27:83–90. doi: 10.1007/s40263-012-0036-8. [DOI] [PubMed] [Google Scholar]
- 17.Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9:728–38. doi: 10.1007/s13311-012-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Text Revision. 4th ed. Washington, D.C.: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19:53–6. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25:2487–94. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 23.Roth T, Soubrane C, Titeux L, Walsh JK Zoladult Study Group. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Shan L, Dauvilliers Y, Siegel JM. Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol. 2015;11:401–13. doi: 10.1038/nrneurol.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeeren A, Vets E, Vuurman EF, et al. On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology (Berl) 2016 Jul 16; doi: 10.1007/s00213-016-4375-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeeren A, Sun H, Vuurman EF, et al. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep. 2015;38:1803–13. doi: 10.5665/sleep.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14:318–28. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohayon MM, Zulley J, Guilleminault C, Smirne S. Prevalence and pathologic associations of sleep paralysis in the general population. Neurology. 1999;52:1194–200. doi: 10.1212/wnl.52.6.1194. [DOI] [PubMed] [Google Scholar]
- 29.Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97:153–64. doi: 10.1016/s0165-1781(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 30.Toner LC, Tsambiras BM, Catalano G, Catalano MC, Cooper DS. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23:54–8. doi: 10.1097/00002826-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64:999–1004. doi: 10.1007/s00228-008-0494-6. [DOI] [PubMed] [Google Scholar]
- 32.Huedo-Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corsonello A, Onder G, Maggio M, Corica F, Lattanzio F. Medications affecting functional status in older persons. Curr Pharm Des. 2014;20:3256–63. doi: 10.2174/13816128113196660695. [DOI] [PubMed] [Google Scholar]
- 34.Levy HB. Non-benzodiazepine hypnotics and older adults: what are we learning about zolpidem? Expert Rev Clin Pharmacol. 2014;7:5–8. doi: 10.1586/17512433.2014.864949. [DOI] [PubMed] [Google Scholar]
- 35.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 36.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miettinen OS, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.