Abstract
The aim of the present experiment was to examine the effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. The experimental design was a 2 × 2 factorial arrangement of treatments evaluating two levels of betaine supplementation (0 and 960 g betaine/t of feed) without or with coccidia challenge. Each treatment was fed to 8 cages of 8 male broilers (Ross 308) for 1 to 21d. On d 14, birds in the 2 challenged groups received mixed inocula of Eimeria species from a recent field isolate, containing approximately 180,000 E. acervulina, 6,000 E. maxima, and 18,000 E. tenella oocysts. At 21d, digesta from the terminal ileum was collected for the determination of dry matter, energy, nitrogen, amino acids, starch, fat, and ash digestibilities. Lesion scores in the different segments of the small intestine were also measured on d 21. Performance and nutrient digestibility data were analyzed by two-way ANOVA. Lesion score data were analyzed using Pearson chi-square test to identify significant differences between treatments. Orthogonal polynomial contrasts were used to assess the significance of linear or quadratic models to describe the response in the dependent variable to total lesion scores. Coccidia challenge reduced (P < 0.0001) the weight gain and feed intake, and increased (P < 0.0001) the feed conversion ratio. Betaine supplementation had no effect (P > 0.05) on the weight gain or feed intake, but lowered (P < 0.05) the feed conversion ratio. No interaction (P > 0.05) between coccidia challenge and betaine supplementation was observed for performance parameters. Betaine supplementation increased (P < 0.05) the digestibility of dry matter, nitrogen, energy, fat, and amino acids only in birds challenged with coccidia as indicated by the significant interaction (P < 0.0001) between betaine supplementation and coccidia challenge. The main effect of coccidia challenge reduced (P < 0.05) starch digestibility. Betaine supplementation improved (P < 0.05) starch digestibility regardless of the coccidia challenge. For each unit increase in the total lesion score, there was a linear (P < 0.001) decrease in digestibility of mean amino acids, starch, and fat by 3.8, 3.4 and 16%, respectively. Increasing total lesion scores resulted in a quadratic (P < 0.05) decrease in dry matter digestibility and ileal digestible energy. No lesions were found in the intestine or ceca of the unchallenged treatments. In the challenged treatments, betaine supplementation reduced (P < 0.01) the lesion scores at the duodenum, lower jejunum, and total lesion scores compared to the treatment without supplements. In conclusion, coccidia challenge lowered the digestibility of energy and nutrients and increased the feed conversion ratio of broilers. However, betaine supplementation reduced the impact of coccidia challenge and positively affected nutrient digestibility and the feed conversion ratio.
Keywords: broilers, coccidia challenge, betaine, nutrient digestibility, lesion scoring
INTRODUCTION
Coccidiosis is an expensive disease with an estimated cost to the world's poultry industry of 3.2 billion USD per year (Dalloul and Lillehoj, 2006; De Gussem, 2007). The major part of these costs, around 80%, is due to losses in performance, and the rest is due to the costs of prophylaxis and treatment (Vermeulen et al., 2001). Price (2012) categorized avian coccidiosis into three forms of infection severity, namely clinical coccidiosis, subclinical coccidiosis, and coccidiasis (a mild interaction between host and parasite with no detectable adverse effects). Subclinical coccidiosis, which does not immediately demonstrate clinical signs, is the major cause for the economical losses due to the difficulty of diagnosis but at the same time adversely influencing the performance of birds (Williams, 1999; De Gussem 2007). Coccidial infection in broilers results in damage to epithelial cells, diarrhea, osmotic stress in the intestine, (Kettunen et al., 2001; Perez-Carbajal et al., 2010) and, consequently, malabsorption of nutrients (Persia et al., 2006; Metzler-Zebeli et al., 2009).
Concerns over the future use of anticoccidials have increased the need to better understand the effects of coccidiosis infection on nutrient utilization and response to feed additives (Persia et al., 2006). Previous studies have reported a beneficial effect of betaine (trimethyglycine) on reducing performance losses of broilers exposed to coccidiosis (Augustine et al. 1997; Kettunen et al., 2001). However, there is a lack of recent studies examining the effect of coccidiosis and betaine supplementation on nutrient digestibility response of broilers, given the continuous genetic selection for fast growing strains altering the immune system (Korver, 2012). A recent meta-analysis has shown that the severity of coccidiosis is influenced by the genetic line of birds (Kipper et al., 2013).
The hypothesis for this study was that coccidia challenge would cause intestinal damage and reduce the nutrient digestibility and that betaine supplementation would partially recover the reductions in nutrient digestibility in growing broilers. The aim of the present experiment was to examine the effect of natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal levels of dietary methionine and exposed to experimental coccidia challenge.
MATERIALS AND METHODS
Birds and Housing
Experimental procedures were conducted in accordance with the Massey University Animal Ethics Committee guidelines. Day-old male broilers (Ross 308) were obtained from a commercial hatchery, individually weighed, and assigned to 32 cages (8 birds per cage) in electrically heated battery brooders so that the average bird weight was similar for each cage. The birds were transferred to grower cages on d 12. The battery brooders and grower cages were housed in an environmentally controlled room with 20 h of fluorescent illumination daily. The temperature was maintained at 31ºC on d 1 and gradually reduced to 22ºC by 21 d of age. Body weight and feed intake (FI) were recorded by cage at 21 d of age. Mortality was recorded daily. Any bird that died was weighed and feed conversion ratio (FCR) values were calculated by dividing total feed intake by weight gain (WG) of live plus dead birds.
Diets and Treatments
The experimental diets were offered from 1 to 21 days of age and were based on wheat and soybean meal (Table 1). Diets were formulated to meet Ross 308 strain nutrient recommendations for broiler starters (Ross, 2007), except for methionine. The methionine concentrations were adjusted considering the methionine sparing value of betaine, as calculated by the Betacheck® software (Danisco Animal Nutrition, Marlborough, UK). Betacheck® software is a model that was developed to calculate the amount of methionine and/or choline that can be replaced by betaine in broiler diets. A safety margin is also included for methionine and sulphur amino acids, to account for variation in feed ingredient quality. The basal diet was either not supplemented or supplemented with natural betaine (Betafin® S1, 96% natural betaine, Danisco Animal Nutrition, Marlborough, UK) to supply 960g betaine/ton of feed. Both diets were fed to two experimental groups (without or with coccidia challenge). Each treatment was randomly assigned to 8 cages. The diets contained titanium dioxide (0.3%) as an inert marker and were pelleted at 70°C. The diets were offered ad libitum and water was available at all times throughout the trial.
Table 1.
Composition and calculated analysis (g/ 100 g as fed) of the basal diet1.
| Ingredient | Amount |
|---|---|
| Wheat | 60.01 |
| Soybean meal, 48%CP | 33.99 |
| Soybean oil | 2.74 |
| L-Lysine HCl | 0.14 |
| DL-methionine | 0.12 |
| L-threonine | 0.05 |
| Salt | 0.29 |
| Limestone | 1.17 |
| Dicalcium phosphate | 0.86 |
| Vitamin and trace mineral premix1 | 0.30 |
| Titanium dioxide | 0.30 |
| Phytase2 | + |
| Calculated analysis | |
| Crude protein | 23.34 |
| Metabolizable energy, kcal/kg | 2950 |
| Calcium3 | 0.88 |
| Total phosphorus | 0.56 |
| Non-phytate phosphorus3 | 0.40 |
| Digestible lysine | 1.15 |
| Digestible methionine | 0.41 |
| Digestible methionine + cysteine | 0.74 |
| Choline (mg/kg) | 1453 |
1Supplied, per kilogram of diet: antioxidant, 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 60 μg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; trans-retinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; DL- α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 200 μg; Zn, 60 mg.
2Phyzyme XP 10000 TPT, Danisco Animal Nutrition, Marlborough, UK. The enzyme was included at a rate of 50 g/t to supply a guaranteed minimum of 500 FTU/kg of feed.
3Includes the contribution from phytase of 0.11% Ca and 0.12% digestible P.
Coccidia Challenge
On d 14, birds in the two challenged groups were orally gavaged directly into the crop with doses of 2 and 1.5 ml at two-hour intervals (3.5 ml dose of inoculum containing 180,000, 6,000 and 18,000 sporulated oocysts of E. acervulina, E. maxima, and E. tenella, respectively). Birds in the unchallenged groups were gavaged with the same volume of sterile distilled water. After inoculation, birds were observed four times daily.
Lesion Scoring
On d 7 post-challenge (d 21post-hatch), two birds were randomly selected from each replicate cage, killed by cervical dislocation, and lesion scoring was undertaken by an experienced avian veterinarian who was blind to treatment allocations. The intestines were opened and scored for lesions of coccidiosis at three sites: the duodenum (from the pyloric junction to the most distal point of insertion of the duodenal mesentery), upper half of the jejunum, and lower half of the jejunum on a scale of 0 to 4, according the method of Johnson and Reid (1970). The jejunum was defined from the most distal point of insertion of the duodenal mesentery to the junction with Meckel's diverticulum. A score of zero represented absence of gross lesions and 4 represented extensive haemorrhage or lesions (depending on the Eimeria spp.). The same chickens were also used for lesion scoring in the ceca. The lesion scores were then recorded as the average across the two birds at each segment. Total lesion score was calculated as the sum of lesion scores in the three intestinal segments (duodenum, upper jejunum, and lower jejunum).
Measurements
On d 21, all remaining birds were euthanized by an intracardial injection of sodium pentobarbitone solution. The ileum was immediately excised and divided into two parts, which were the anterior and posterior ileum. The ileum was defined as the portion of the small intestine extending from Meckel's diverticulum to a point 40 mm proximal to the ileo-cecal junction. Contents of the posterior ileum were collected by gently flushing with distilled water into plastic containers. Digesta were pooled within a cage, lyophilized, ground to pass through a 0.5-mm screen size, and stored at −20°C until analyzed for dry matter (DM), gross energy (GE), nitrogen (N), amino acids (AA) starch, fat, and ash.
Chemical Analysis
The DM, crude fat, and ash contents were analyzed according to the procedures of the Association of Official Analytical Chemists (AOAC, 2005). Nitrogen was determined using an FP-428 nitrogen determinator (LECO Corporation, St Joseph, MI). Gross energy was determined using an adiabatic oxygen calorimeter (Gallenkamp Autobomb, London, UK) standardized with benzoic acid. Starch was measured using an assay kit (Megazyme, Boronia, Victoria, Australia) based on the thermostable α-amylase and amyloglucosidase (McCleary et al., 1997). Amino acid concentration was determined by HPLC as described by Ravindran et al. (2009). Cysteine and methionine were analyzed as cysteic acid and methionine sulphone by oxidation with performic acid for 16 h at 0ºC and neutralisation with hydrobromic acid before hydrolysis. Tryptophan was not determined. Titanium (Ti) was determined on a UV spectrophotometer following the method of Short et al. (1996).
Calculations
The apparent ileal digestibility of DM, GE, N, AA, starch, fat, and ash was calculated by the following formula using the Ti marker ratios in the diet and ileal digesta (Ravindran et al., 2009).
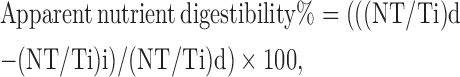 |
where (NT/Ti)d = ratio of nutrient and Ti in diet and (NT/Ti)i = ratio of nutrient and Ti in ileal digesta.
Apparent ileal digestible energy (AIDE) was calculated by multiplying the diet GE content by the apparent ileal energy digestibility.
The apparent ileal undigested AA was calculated as shown below (Cowieson, 2010).
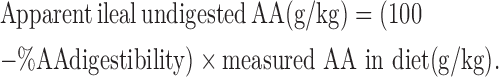 |
Data Analysis
Performance and nutrient digestibility data were analyzed by two-way ANOVA to determine the main effects (coccidia challenge and supplemental betaine) and their interaction using the generalized linear model (GLM) procedure of SAS (2004). Cage means were considered as experimental units. A probability value of P < 0.05 was considered to be statistically significant. Paired t tests were conducted to compare the challenged and the unchallenged groups in the not supplemented diets and the supplemented and the not supplemented diets in the challenged groups for ileal undigested AA fractions. Mortality and lesion scores were analyzed using Pearson chi-square test to identify significant differences between treatments. Orthogonal polynomial contrasts were used to assess the significance of linear or quadratic models to describe the response in the dependent variable to total lesion scoring using the Proc Mixed procedure of SAS (SAS, 2004).
RESULTS
Bird Performance
The effects of coccidia challenge and betaine supplementation on WG, FI, FCR, and mortality rate are summarized in Table 2. Although mortality was numerically high in the challenged, not supplemented treatment, the Pearson chi-square test showed no difference (P > 0.05) in the mortality rate between dietary treatments. Coccidia challenge reduced (P < 0.05) the WG and FI, and increased (P < 0.05) the FCR compared to the unchallenged groups. Betaine supplementation had no effect (P > 0.05) on the WG and FI, but lowered (P < 0.05) the FCR compared to the not supplemented groups. There was no interaction (P > 0.05) between coccidia challenge and betaine supplementation on performance parameters.
Table 2.
Effect of coccidia challenge and natural betaine supplementation on the weight gain (g/bird), feed intake (g/bird), feed conversion ratio (FCR, g/g) and mortality (%) in broilers fed a wheat-soy based diet (1 to 21d post- hatch)1.
| Coccidia challenge | Betaine supplementation | Weight gain | Feed intake | FCR | Mortality |
|---|---|---|---|---|---|
| No | - | 933 | 1228 | 1.325 | 3.1 |
| No | + | 977 | 1252 | 1.287 | 1.6 |
| Yes | - | 789 | 1152 | 1.500 | 9.4 |
| Yes | + | 780 | 1119 | 1.440 | 1.6 |
| SEM2 | 6.8 | 10.1 | 0.006 | - | |
| Main Effect | |||||
| Coccidia | |||||
| No | 955a | 1240a | 1.306b | - | |
| Yes | 785b | 1135b | 1.470a | - | |
| Betaine | |||||
| − | 861 | 1190 | 1.412a | - | |
| + | 879 | 1185 | 1.363b | - | |
| P ≤ | |||||
| Coccidia challenge (CC) | <0.0001 | <0.0001 | <0.0001 | - | |
| Betaine supplementation (BS) | 0.20 | 0.83 | 0.0003 | - | |
| CC × BS | 0.06 | 0.17 | 0.38 | - | |
a–eMeans in a column not sharing a common superscript are different (P < 0.05).
1Each value represents the mean of 8 replicates (8 birds per replicate).
2Pooled standard error of the mean.
Energy and Nutrient Digestibility
The effects of coccidia challenge and betaine supplementation on energy, DM, N, starch, fat, and ash digestibility are summarized in Table 3. There was a significant interaction between coccidia challenge and betaine supplementation on energy, DM, N, and fat. Betaine supplementation improved (P < 0.05) digestibility of these nutrients only in birds challenged with coccidia. Coccidia challenge reduced (P < 0.05) starch digestibility, but had no effect (P > 0.05) on ash digestibility. Betaine supplementation improved (P < 0.05) the digestibility of starch and ash.
Table 3.
Effect of coccidia challenge and natural betaine supplementation on the digestibility (%) of dry matter (DM), nitrogen (N), starch, fat, ash, and ileal digestible energy (IDE) of broilers fed a wheat-soy based diet1.
| Coccidia challenge | Betaine supplementation | DM | N | Starch | Fat | Ash | IDE (kcal/kg DM) |
|---|---|---|---|---|---|---|---|
| No | - | 68.1a | 81.4a | 94.3 | 86.2a | 50.6 | 3198ab |
| No | + | 69.8a | 81.2a | 95.0 | 86.3a | 55.9 | 3234a |
| Yes | - | 48.0b | 61.3c | 76.6 | 3.3c | 45.7 | 2129c |
| Yes | + | 66.5a | 75.3b | 86.2 | 48.1b | 60.7 | 2966b |
| SEM2 | 2.5 | 1.0 | 2.2 | 2.7 | 2.5 | 67 | |
| Main Effect | |||||||
| Coccidia | |||||||
| No | 68.9 | 81.3 | 94.7a | 86.2 | 53.2 | 3217 | |
| Yes | 57.3 | 68.3 | 81.4b | 25.7 | 53.2 | 2548 | |
| Betaine | |||||||
| − | 58.1 | 71.3 | 85.5b | 44.8 | 48.1b | 2662 | |
| + | 68.2 | 78.6 | 90.6a | 67.2 | 58.3a | 3100 | |
| P-value | |||||||
| Coccidia challenge (CC) | .0001 | .0001 | .0001 | .0001 | 0.99 | .0001 | |
| Betaine supplementation (BS) | .0001 | .0001 | 0.026 | .0001 | .0004 | .0001 | |
| CC × BS | .0001 | .0001 | .057 | .0001 | .065 | .0001 | |
a–cMeans in a column not sharing a common superscript are different (P < 0.05).
1Each value represents the mean of 8 replicates (8 birds per replicate).
2Pooled standard error of the mean.
The effects of coccidia challenge and betaine supplementation on AA digestibility are summarized in Table 4. There was a significant (P < 0.001 to 0.0001) interaction between coccidia challenge and betaine supplementation for the digestibility of all AA, with supplemental betaine improving AA digestibility only in birds challenged with coccidia.
Table 4.
Effect of coccidia challenge and natural betaine supplementation on apparent ileal amino acid (AA) digestibility (%) of broilers fed a wheat/soy based diet1.
| Coccidia challenge | Betaine | His | Ser | Arg | Gly | Asp | Glu | Thr | Ala | Pro | Lys | Tyr | Met | Val | Iso | Leu | Phe | Cys | Mean AA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | - | 81.5a | 82.4a | 86.0ab | 78.2a | 80.3a | 88.3a | 77.8a | 81.4a | 85.5a | 89.9a | 84.7a | 85.8a | 82.3a | 84.0a | 84.5a | 85.6a | 69.9ab | 82.8a |
| No | + | 81.3a | 82.3a | 86.9a | 77.8a | 80.4a | 88.2a | 77.0a | 80.7a | 85.4a | 90.3a | 84.0a | 86.5a | 81.7a | 83.3a | 83.8a | 85.3a | 71.8a | 82.7a |
| Yes | - | 61.5c | 61.7c | 71.4c | 56.0c | 59.6c | 73.7c | 54.1c | 55.6c | 68.4c | 74.5b | 63.2c | 66.2c | 58.9c | 61.0c | 63.2c | 66.4c | 45.8c | 62.4c |
| Yes | + | 75.8b | 75.9b | 81.8b | 72.0b | 73.9b | 83.6b | 70.6b | 72.0b | 80.7b | 85.4a | 77.0b | 79.0b | 74.3b | 75.6b | 76.9b | 79.1b | 65.0b | 76.4b |
| SEM2 | 1.3 | 1.1 | 1.2 | 1.4 | 1.1 | 0.9 | 1.2 | 1.2 | 1.1 | 1.3 | 1.1 | 1.5 | 1.1 | 1.1 | 1.0 | 0.9 | 1.6 | 1.1 | |
| Main Effect | |||||||||||||||||||
| Coccidia | |||||||||||||||||||
| No | 81.4 | 82.4 | 86.4 | 78.0 | 80.4 | 88.2 | 77.4 | 81.1 | 85.4 | 90.1 | 84.3 | 86.2 | 82.0 | 83.6 | 84.1 | 85.4 | 70.8 | 82.8 | |
| Yes | 68.6 | 68.8 | 76.6 | 64.0 | 66.7 | 78.6 | 62.3 | 63.8 | 74.6 | 79.9 | 70.1 | 72.6 | 66.6 | 68.3 | 70.0 | 72.8 | 55.4 | 69.4 | |
| Betaine | |||||||||||||||||||
| − | 71.5 | 72.0 | 78.7 | 67.1 | 69.9 | 81.0 | 65.9 | 68.5 | 77.0 | 82.2 | 73.9 | 76.0 | 70.6 | 72.5 | 73.8 | 76.0 | 57.8 | 72.6 | |
| + | 78.5 | 79.1 | 84.3 | 74.9 | 77.2 | 85.9 | 73.8 | 76.3 | 83.0 | 87.8 | 80.5 | 82.8 | 78.0 | 79.4 | 80.4 | 82.2 | 68.4 | 79.6 | |
| P-value | |||||||||||||||||||
| Coccidia challenge (CC) | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | |
| Betaine supplementation (BS) | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0003 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | |
| CC × BS | .0001 | .0001 | .0005 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0006 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | |
a–cMeans in a column not sharing a common superscript are different (P < 0.05).
1Each value represents the mean of 8 replicates (8 birds per replicate).
2Pooled standard error of the mean.
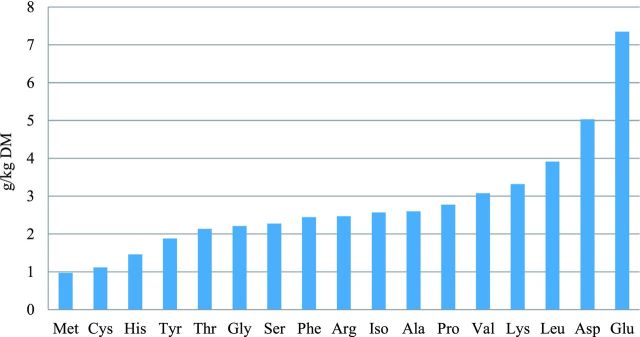
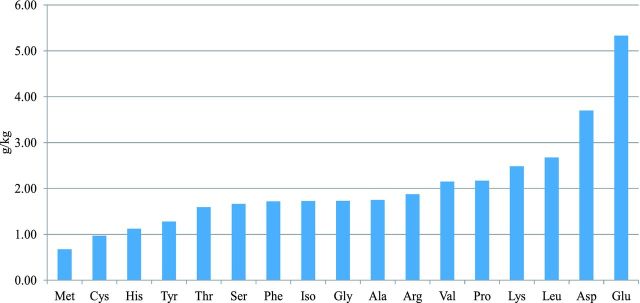
The effects of coccidia challenge and betaine supplementation on ileal undigested AA fraction showed a similar pattern as AA digestibility (data not shown). Significant differences were observed between the supplemented and the not supplemented diets in the challenged groups for ileal undigested AA fractions. The differences between the challenged and the unchallenged groups in the not supplemented diets for the ileal undigested AA fraction are shown in Figure 1. The effect of betaine on the uplift of the undigested fraction of AA (g/kg) in the coccidia challenged groups is shown in Figure 2.
Figure 1.
Difference in apparent ileal undigested amino acids (g/kg) as a result of the coccidia challenge in birds fed unsupplemented diets.
Figure 2.
Effect of betaine on improvements in digestion of individual amino acids (g/kg) in the undigested fraction in the coccidia challenged birds
Lesion Scores
No lesions were found in the intestine or ceca of the unchallenged treatments. In the challenged treatments, betaine supplementation reduced (P < 0.01) the lesion scores at duodenum and lower jejunum and total lesion scores compared to the not supplemented treatment (Table 5). No effect (P > 0.05) of betaine was observed at the upper jejunum or the ceca. Regressions for the relationship between total intestinal lesion scores and digestibility of DM, mean AA, starch, fat, and IDE are shown on Table 6. Higher total lesion scores led to linear (P < 0.0001) decreases in the digestibility of mean AA, starch and fat, and quadratic (P < 0.05) decreases in dry matter digestibility and IDE. Ash digestibility was unaffected (P > 0.05) by total lesion scores.
Table 5.
Influence of betaine supplementation on lesion scores of broilers challenged with coccidia1,2,3,4.
| Betaine | Duodenum (D) | Upper jejunum (UJ) | Lower jejunum (LJ) | Total (D+UJ+LJ) | Ceca |
|---|---|---|---|---|---|
| No | 2.63 | 1.56 | 0.63 | 4.82 | 1.25 |
| Yes | 1.56 | 1.25 | 0.13 | 2.94 | 1.81 |
| Probability, P = | 0.01 | 0.67 | 0.02 | 0.04 | 0.39 |
a,bMeans in the same column followed by different superscripts differ (P < 0.05).
1Each mean represents values from 8 replicates (8 birds/replicate). Birds were challenged on day 14 and lesions were scored on day 21. Lesion scoring on day 21 confirmed that there were no lesions in the unchallenged birds.
2Lesions were scored on a scale of 0 to 4, as per the procedures of Johnson and Reid (1970); zero representing no gross lesions and 4 representing extensive haemorrhage or lesions (depending on the Eimeria species).
3No lesions were found in the intestine or ceca of the unchallenged treatments.
4Lesion scoring was analyzed using Pearson chi-square test to identify significant differences between treatments.
Table 6.
Regression parameters for the relationship between total lesion scoring at duodenum, upper jejunum , lower jejunum, and digestibility of dry matter, energy, amino acids (AA) starch, fat, and ash1,2.
| Dry matter | Mean AA | IDE (kcal/kg DM) | Starch | Fat | |
|---|---|---|---|---|---|
| Linear (P-value) | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Quadratic (P-value) | 0.02 | 0.37 | 0.04 | 0.97 | 0.75 |
| Equation | y = 0.7178906 − 0.0273292* x | y = 0.8304746 | y = 3318.991805 − 162.8083535*x | y = 0.9424404 | y = 0.8557188 |
| − 0.0081775*(x−1.83871)2 | − 0.0380651*x | − 30.4154507*(x−1.838709)2 | − 0.0336953*x | − 0.1591196*x | |
| R-square | 0.72 | 0.83 | 0.80 | 0.56 | 0.92 |
| RMSE3 | 0.05 | 0.04 | 0.95 | 0.06 | 0.10 |
1Orthogonal polynomial contrasts were used to assess the significance of linear or quadratic models to describe the response in the dependent variable to lesion scoring.
2Total lesion score was calculated as the sum of lesion score in the three intestinal segments (duodenum, upper jejunum, and lower jejunum).
3Root mean square error.
DISCUSSION
Although diets were formulated to be suboptimal in methionine and total sulphur AA levels compared to strain nutrient recommendations (Ross, 2007), birds in the unchallenged treatments exceeded the performance objective of this breed (Ross, 2012). Betaine is known to be a methyl group donor (Ratriyanto et al., 2009) and in situations where diets are deficient in methionine, the addition of betaine will compensate for methionine function as a methyl donor and improve performance (Sun et al., 2008). This role of betaine as a methyl group donor may explain the beneficial effect of betaine on FCR in both the challenged and unchallenged treatments. Furthermore, under challenge conditions, like coccidia, methyl requirements increase (Metzler-Zebeli et al., 2009) and the importance of betaine as a methyl donor increases.
As expected, the current results showed that challenging the birds with coccidia adversely influenced broiler performance. Reduced nutrient digestibility and increased immune costs associated with coccidia infection are likely to be responsible for the reduced broiler performance (Williams, 2005). Coccidia challenge reduced the FI and WG by 8.5 and 17.8%, respectively, and increased the FCR by 12.5% compared with the unchallenged group. In a more severe challenge, Parker et al. (2007) reported reductions in average FI and WG of all treatments infected with mixed Eimeria spp. by 21 and 45%, respectively, whereas the FCR was increased by 43% compared with the average of the unchallenged control.
During coccidiosis, sporozoites infect the cells of the intestinal lining causing tissue damage and trauma to the intestinal mucosa and submucosa (Perez-Carbajal et al., 2010). Furthermore, coccidia challenge negatively influences the morphology of the intestine, especially the shortening of villi (Kettunen et al., 2001) and reduces the activities of digestive enzymes (Williams, 2005). The overall effect is reduction in the digestion and absorption of nutrients. For example, the negative effects of coccidia challenge on AA digestibility have been reported by Persia et al. (2006) and Parker et al. (2007). Parker et al. (2007) reported a 8.5% reduction in mean AA digestibility in vaccinated broilers challenged with coccidia compared to the 16% reduction observed in the current study. Although coccidia challenge increased the undigested fraction of all AA, the degree of response varied between individual AA (Figure 1), with Glu and Asp being the most influenced. Persia et al. (2006) also found inconsistent response to coccidia challenge among AA, which also differed according to diet composition and the type of infection. Coccidia infection is known to induce a host intestinal mucogenic response (Collier et al., 2008), which can increase the endogenous losses of mucin-associated AA. Mucin glycoprotein is rich in Pro, Glu, Asp, Thr, and Ser (Lien et al. 1997). A recent study by Adedokun et al. (2012), however, showed that the endogenous AA flow in coccidia-challenged birds was lower compared to unchallenged birds. It was suggested that intestinal inflammation could have negatively affected intestinal morphology leading to a reduction in mucus secretion. These observations highlight the need for further studies to understand the effect of coccidia challenge on AA digestibility and endogenous AA losses.
In the current work, coccidia challenge resulted in 29.5, 24.7, 18.8, and 96.2% reductions in the apparent ileal digestibility of DM, N, starch, and fat, respectively. The profound effect of coccidia challenge on fat digestibility suggests additional mechanisms to the ones mentioned earlier. Adams et al. (1996) reported a reduction in bile salt secretion during coccidia challenge. The mechanism by which coccidia challenge reduces bile salt secretion is possibly due to damage of cells in the intestinal mucosa that produce cholecystokinin (Soede, 2005). Cholecystokinin is responsible for the stimulation of gallbladder contraction and pancreatic enzyme secretion (Wang and Cui, 2007).
In the current study, betaine supplementation improved the digestibility of energy, DM, N, fat, and AA in birds challenged with coccidia, and of starch and ash in both the challenged and unchallenged groups. Kettunen et al. (2001) reported that betaine decreased the crypt to villus height ratio both in healthy and coccidia challenged broilers, which may partly explain the improvements in nutrient digestibility observed with betaine supplementation. In addition, betaine reduced the duodenal and total lesion scores, which may have positively influenced the nutrient digestion and absorption. Interestingly, the highest apparent uplifts of the undigested AA fraction due to betaine supplementation in coccidia challenged groups were for mucin-associated AA, Glu, and Asp (Lien et al. 1997), which is suggestive of the protective role of betaine on intestinal mucosal structure. This protective effect during coccidial infection can be due to the function of betaine both as an osmolyte and as a methyl group donor (Ratriyanto et al., 2009). As an osmolyte, betaine reduces the effect of osmotic stress in the intestinal tract occurring during coccidiosis (Eklund et al., 2005). Augustine et al. (1997) reported that betaine caused a significant reduction in intestinal intracellular invasion by E. tenella or E. acervulina sporozoites as compared with control chicks. As for the methyl donor function of betaine, it is believed that damaged tissues require more methyl groups than healthy tissues (Chiang et al., 1996). Klasing et al. (2002) concluded that increased chemotaxis of monocytes and nitrous oxide release by macrophages may explain the improved intestinal pathology when betaine was fed during the coccidia challenge.
The orthogonal polynomial contrasts showed linear reductions in the digestibility of fat, mean AA, and starch with increasing total lesion scores. Of these nutrients, fat digestibility was found to be the most influenced by the intestinal damage. For each unit increase in total lesion score, digestibility of fat was reduced by 16% compared to mean AA and starch, which were reduced by 3.8 and 3.4, respectively.
In conclusion, our results support previous findings that supplementation with natural betaine reduced the impact of coccidia challenge on intestinal lesion scores and positively affected nutrient digestibility and feed efficiency in broilers. Increasing intestinal lesion scores were associated with reductions in nutrient digestibility.
REFERENCES
- Adams C., Vahl A. A., Veldman A. Interaction between nutrition and E. acervulina infection in broilers chickens: Diet composition that improve fat digestion during E. acervulina infection. Br. J. Nutr. 1996;75:875–880. doi: 10.1079/bjn19960193. [DOI] [PubMed] [Google Scholar]
- Adedokun S. A., Ajuwon K. M., Romero L. F., Adeola O. Ileal endogenous amino acid loss: Response of broiler chickens to fiber and mild coccidial vaccine challenge. Poult. Sci. 2012;91:899–907. doi: 10.3382/ps.2011-01777. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) Official methods of analysis. 15th edition. Arlington, VA, USA: AOAC; 2005. [Google Scholar]
- Augustine P. C., Mcnaughton J. L., Virtanen E., Rosi L. Effect of betaine on the growth performance of chicks inoculated with mixed cultures of avian Eimeria species and on invasion and development of Eimeria tenella and Eimeria acervulina in vitro and in vivo. Poult. Sci. 1997;76:802–809. doi: 10.1093/ps/76.6.802. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Cordon R. K., Tal J., Zeng G. C., Doctor B. P., Pardhasaradhi K., Mccann P. P. S-adenosylmethionine and methylation. The FASEB Journal. 1996;10:471–480. [PubMed] [Google Scholar]
- Collier C. T., Hofacre C. L., Payne A. M., Anderson D. B., Kaiser P., Mackie R. I., Gaskins H. R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J. Strategic selection of exogenous enzymes for corn/soy-based poultry diets. Jpn. Poult. Sci. 2010;47:1–7. [Google Scholar]
- Dalloul R. A., Lillehoj H. S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- De Gussem M. Proc. 16th Eur. Symp. on Poult. Nutr. Beekbergen, The Netherlands: World's Poultry Science Association; 2007. Coccidiosis in poultry: Review on diagnosis, control, prevention and interaction with overall gut health; pp. 253–261. [Google Scholar]
- Eklund M., Bauer E., Wamatu J., Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: Lesion scoring techniques in battery and floor pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kettunen H., Tiihonen K., Peuranen S., Saarinen M. T., Remus J. C. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and Coccidia-infected broiler chicks. Comp. Biochem. Phys. A. 2001;130:759–769. doi: 10.1016/s1095-6433(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Kipper M., Andretta I., Lehnen C. R., Paulo A. L., Gonzalez M. S. Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Anim. Vet. Parasitol. 2013;196:77–84. doi: 10.1016/j.vetpar.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Klasing K. C., Adler K. L., Remus J. C., Calvert C. C. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increased functional properties of phagocytes. J. Nutr. 2002;132:2274–2282. doi: 10.1093/jn/132.8.2274. [DOI] [PubMed] [Google Scholar]
- Korver D. R. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012;173:54–64. [Google Scholar]
- Lien K. A., Sauer W. A., Fenton M. Mucin output in ileal digesta of pigs fed a protein-free diet. J. Anim. Sci. 1997;72:1737–1743. doi: 10.1007/BF01611398. [DOI] [PubMed] [Google Scholar]
- McCleary B. V., Gibson T. S., Mugford D. C. Measurement of total starch in cereal products by amyloglucosidase vs. amylase method: collaborative study. J. Assoc. Off. Anal. Chem. 1997;80:571–579. [Google Scholar]
- Metzler-Zebeli B. U., Eklund M., Mosenthin R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. World's Poult. Sci. J. 2009;65:419–442. [Google Scholar]
- Parker J., Oviedo-Rondon E. O., Clack B. C., Clemente-Hernandez S., Osborne J., Remus J. C., Kettunen H., Makivuokko H., Pierson E. M. Enzymes as feed additives to aid in response against Eimeria species in coccidia-vaccinated broilers fed corn-soybean meal diets with different protein levels. Poult. Sci. 2007;86:643–653. doi: 10.1093/ps/86.4.643. [DOI] [PubMed] [Google Scholar]
- Perez-Carbajal C., Caldwell D., Farnell M., Stringfellow K., Pohl S., Casco G., Pro-Martinez A., Ruiz-Feria C. A. Immune response of broiler chickens fed different levels of arginine and vitamin E to a coccidiosis vaccine and Eimeria challenge. Poult. Sci. 2010;89:1870–1877. doi: 10.3382/ps.2010-00753. [DOI] [PubMed] [Google Scholar]
- Persia M. E., Young E. L., Utterback P. L., Parsons C. M. Effects of dietary ingredients and Eimeria acervulina infection on chick performance, apparent metabolizable energy, and amino acid digestibility. Poult. Sci. 2006;85:48–55. doi: 10.1093/ps/85.1.48. [DOI] [PubMed] [Google Scholar]
- Price K. R. Use of live vaccines for coccidiosis control in replacement layer pullets. J. Appl. Poult. Res. 2012;21:679–692. [Google Scholar]
- Ratriyanto A., Mosenthin R., Bauer E., Eklund M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian-Aust. J. Anim. Sci. 2009;22:1461–1476. [Google Scholar]
- Ravindran V., Morel P. C. H., Rutherfurd S. M., Thomas D. V. Endogenous flow of amino acids in the avian ileum is increased by increasing dietary peptide concentrations. Brit. J. Nut. 2009;101:822–828. doi: 10.1017/S0007114508039974. [DOI] [PubMed] [Google Scholar]
- Ross. Ross 308 Broiler: Nutrition Specifications, June 2007. Newbridge, Midlothian, Scotland, UK: Ross Breeders Limited; 2007. [Google Scholar]
- Ross. Ross 308 Broiler: Performance objectives. Newbridge, Midlothian, Scotland, UK: Ross Breeders Limited; 2012. [Google Scholar]
- SAS Institute. SAS User's Guide. Statistics. Version 9.1 ed. Cary, NC: SAS Inst. Inc.; 2004. [Google Scholar]
- Short F. J., Gorton P., Wiseman J., Boorman K. N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Soede J. Fat digestive physiology and exogenous emulsifiers. World Poult. 2005;21(4):14–16. [Google Scholar]
- Sun H., Yang W. R., Yang Z. B., Wang Y., Jiang S. Z., Zhang G. G. Effects of betaine supplementation to methionine deficient diet on growth performance and carcass characteristics of broilers. Am. J. Anim. Vet. Sci. 2008;3:78–84. [Google Scholar]
- Vermeulen A. N., Schaap D. C., Schetters T. M. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001;100:13–20. doi: 10.1016/s0304-4017(01)00479-4. [DOI] [PubMed] [Google Scholar]
- Wang B. J., Cui Z. J. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:666–678. doi: 10.1152/ajpregu.00131.2006. [DOI] [PubMed] [Google Scholar]
- Williams R. B. A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int. J. Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams R. B. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159- 180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]