Abstract
Deregulation of tumor suppressor genes is associated with tumorigenesis and the development of cancer. In prostate cancer, ID4 is epigenetically silenced and acts as a tumor suppressor. In normal prostate epithelial cells, ID4 collaborates with androgen receptor (AR) and p53 to exert its tumor suppressor activity. Previous studies have shown that ID4 promotes tumor suppressive function of AR whereas loss of ID4 results in tumor promoter activity of AR. Previous study from our lab showed that ectopic ID4 expression in DU145 attenuates proliferation and promotes AR expression suggesting that ID4 dependent AR activity is tumor suppressive. In this study, we examined the effect of ectopic expression of ID4 on highly malignant prostate cancer cell, PC3. Here we show that stable overexpression of ID4 in PC3 cells leads to increased apoptosis and decreased cell proliferation and migration. In addition, in vivo studies showed a decrease in tumor size and volume of ID4 overexpressing PC3 cells, in nude mice. At the molecular level, these changes were associated with increased androgen receptor (AR), p21, and AR dependent FKBP51 expression. At the mechanistic level, ID4 may regulate the expression or function of AR through specific but yet unknown AR co-regulators that may determine the final outcome of AR function.
Keywords: ID4, AR, ADT, p21, FKBP51, bHLH, Cancer, Apoptosis, Migration
Introduction
ID4, a dominant negative helix-loop-helix transcriptional regulator is highly expressed in normal epithelial cells of the prostate [1, 2]. In PCa, ID4 expression is progressively lost with increasing stage of the disease [1] due to promoter hyper-methylation [3]. We have previously reported that knockdown of Inhibitor of differentiation-4 (ID4) in prostate cancer LNCaP cells, promoted tumorigenicity with a gene expression signature that resembles that of constitutively activated AR in castrated mice [4]. Conversely, ectopic ID4 expression induced re-expression of AR that led to decreased proliferation and increased apoptosis in otherwise androgen receptor negative prostate cancer cell line DU145 [5].
Androgen receptor (AR) is a critical survival pathway for prostate cancer cells. AR may act both as a tumor suppressor and a proliferator in the prostate [6]. Over-expression of AR in PC3 cells results in decreased invasion in vivo mouse models whereas mice lacking the prostate epithelial AR (PEARKO) have increased apoptosis in epithelial luminal cells. The PERKO mice developed larger and more invasive metastatic tumors in lymph nodes and died earlier than wild-type littermates [7].
AR activity and function is regulated by many co-factors and chaperones [8]. In this context, ID4 appears to be one of the key regulators of AR function. Results suggest that in the presence of ID4, AR functions as a tumor suppressor whereas loss of ID4 promotes AR to act as a tumor promoter. Identifying new or complex interactions between AR co-/regulators could provide some insight into possible mechanisms by which AR undergoes transition between a tumor suppressor vs. tumor promoter. Here, we report that ID4 acts as a regulator of AR by not only inducing AR expression but promoting its tumor suppressor activity, leading to induction of apoptosis and inhibition of cell migration and growth, in more metastatic PC3 cells.
Materials and Methods
Cell Lines
PC3 prostate cancer cell line was purchased from ATCC and cultured as per ATCC recommendations.
ID4 overexpression in prostate cancer cell line
Overexpression of pCMV +ID4 vector and pCMV vector alone in PC3 and subsequent selection of transfectants was performed in as described previously [5].
Real-time quantitative RT-PCR (qRT-PCR)
Total RNA (2 μg) from cell lines was reverse transcribed in a final volume of 25 μL as per standard protocols [9]. Reverse-transcribed RNA was used for qRT-PCR with gene-specific primers [9] (Supplemental Table 1).
Western Blot Analysis
Western Blot analysis with 30ug of total protein, extracted from cultured prostate cancer cell lines using respective protein specific antibodies (Supplemental Table 2) was performed as described earlier [10].
Proliferation Assay
Cell proliferation was determined using a Cell Titer 96 nonradioactive cell proliferation assay (Promega) according to the manufacturer’s protocol.
Apoptosis Assay
Apoptosis was quantified using Propidium Iodide and Alexa Fluor 488 conjugated Annexin V (Molecular Probes) as described previously [11].
Migration Assay
In vitro cell migration assay was performed using 24-well transwell inserts (8 mm, BD Biosciences, Palo Alto, California). Cells were harvested and centrifuged at 1500 rpm for 5 minutes at room temperature. The pellets were suspended into F12 supplemented with 0.2% BSA. Aliquots of 100μl cell suspension (3×104 cells/inserts) were added to each insert. Chemoattractant solutions were made by diluting EGF (10 ng/ml) into F12 supplemented with 0.2% BSA and the cells were allowed to migrate through a porous membrane coated with rat tail collagen (50 mg/ml) at 37°C for 5 hours. F-12 containing 0.2% BSA served as the control medium. The cleaned inserts were fixed in 4% paraformaldehyde (pH 7.5). Cells on the outside of the transwell insert membranes were stained using DAPI (3μg/ml). The images were captured in five areas using Axiovert 200M, Carl Zeiss microscope. Stained nuclei were counted using image analysis software (ZEN 2012; Carl Zeiss). Results were expressed as migration index defined as: the average number of cells per field for test substance/the average number of cells per field for the medium control.
Immunocytochemistry (ICC)
ICC studies on cells grown in glass chamber slides were performed as described previously [12] using protein specific antibodies (Supplemental Table 2).
Tunel Assay
The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay was used to detect fragmented DNA as marker for apoptosis in FFPE tissue sections using TACS 2 TdT-DAB apoptosis detection kit (Trevigen). The slides were counterstained in hematoxylin and mounted with Immuno-mount (Thermo Scientific).
Luciferase Reporter Assays
Cells were plated in a 96-well plate at a density of 2.5×104 cells/well and experiment was further conducted according to our previous study [10].
Animal Studies
PC3 − CMV and PC3 + ID4 cells (2 × 106) suspended in 100 μL of serum-free F12 medium containing Matrigel (1:1 [v/v]; BD Biosciences) were injected subcutaneously into both flanks of 3-week-old noncastrated (NC) male nu/nu athymic nude mice (Taconic) using a 27-gauge syringe, then followed the procedure previously performed from our lab for harvesting the tumors [13]. The nu/nu mice were maintained at the Mercer University Vivarium. All studies were approved by the Clark Atlanta and Mercer University committee for the use and care of animals.
ChIP Assay
Chromatin immuno-precipitation in cell lines was performed using the ChIP assay kit (Millipore, Billerica, MD) as per manufacturer's instructions. The chromatin (total DNA) extracted from cells was sheared (Covaris S220), subjected to immuno-precipitation with respective antibodies (Supplemental Table 1), reverse cross linked and subjected to quantitative ChIP- PCR in Bio-Rad CFX.
Statistical analysis
qRT-PCR data were analyzed using the ΔΔCt method [1]. The within-group Student t test was used for evaluating the statistical differences between groups.
Results
Previous study from our lab showed that in DU145 cells, ID4 functions as a tumor suppressor [5]. To further elucidate the role of ID4 in prostate cancer, we utilized more metastatic and tumorigenic PC3 cells, which do not express AR and have very low levels of ID4, due to promoter methylation [3].
Generation of ID4 expressing prostate cancer cell line
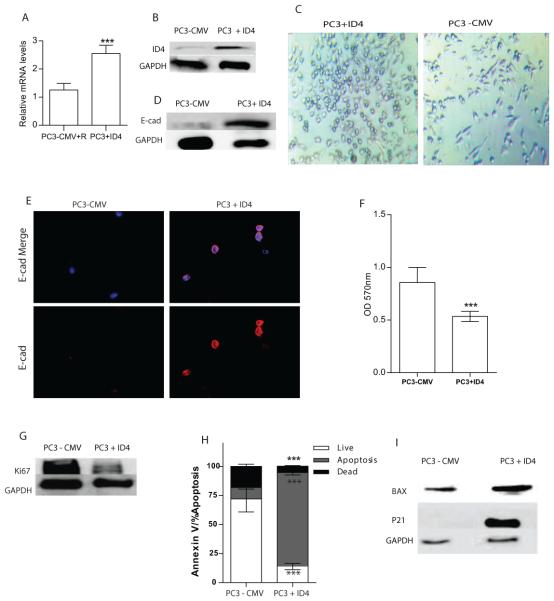
Stably transfected PC3 with pCMV+ID4 vector expressed nearly 2.5 fold higher ID4 expression (PC3+ID4) cells as compared to the control vector (PC3+CMV) transfected cells. The ID4 expression in control vector transfected cells was negligible and was comparable to that in parental PC3 cells. Expression of ID4 was measured by quantitative PCR (Fig. 1A) and western blotting (Fig. 1B).
Figure 1.
Stable Overexpression of ID4 with pCMV-ID4 in PC3 cells. Expression of ID4 was evaluated with real time PCR (A) and Western Blot (B). C: Morphology of the PC3-CMV and PC3+ID4 cells in culture. D and E: Analysis of E-cadherin by Western blot and Immuno-cytochemistry. F: Proliferation rate of PC3-CMV and PC3+ID4 cells expressed as absorption at 570 nm (means ± SEM, n = 3; ***, P < .001). G: Ki-67 expression by Western Blot. H: Percent of cells undergoing apoptosis by flow cytometry (***: P < 0.001). I: Western blot analysis of BAX, and p21. GAPDH was used as loading control.
Effect of ID4 on Morphology, Cell Proliferation and Migration
A change in morphology in PC3+ID4 cells was observed (Fig. 1C, Left panel). PC3+ID4 cells had an "epithelial like" morphology that was associated with increased cell-cell adhesion as compared to a mesenchymal morphology of the PC3-CMV cells (Fig. 1C, Right panel). At the molecular level, the transition towards "epithelial" morphology and increased cell adhesion of PC3+ID4 cells could be due an increase in E-cadherin expression (Fig.1D & E). Overexpression of ID4 also attenuated proliferation of the PC3 cells. The PC3+ID4 cells had a 2 fold decrease in proliferation (Fig. 1F), as compared to control cells. KI-67, a cellular marker for proliferation present during all active phases of cell cycle, was also reduced in PC3+ID4 cells compared to control cells (Fig. 1G). Next we investigated the effect on apoptosis in these cells by Flow cytometry. The rate of apoptosis in PC3+ID4 cells was significantly higher as compared to PC3-CMV control cells (Fig.1H). Western blot analysis showed an increase in BAX expression, a marker associated with apoptosis in PC3+ID4 cells, compared to PC3-CMV cells (Fig. 1I). The Cyclin dependent kinase inhibitor p21, a well-known inhibitor of cyclin-dependent kinases acts as a key factor for the regulation of cell growth is upregulated by AR in prostate cancer cell lines [14]. Consistent with this observation, Western blot analysis showed stable ID4 expression in PC3 cells resulted in upregulation of p21 levels (Fig. 1I), compared to PC3 control cells. Collectively, these results demonstrate that ID4 expression decreases proliferation and increases apoptosis via BAX upregulation.
PC3+ID4 cells showed significantly decreased migration compared to the PC3-CMV control cells (Fig. 2A&B) in a transwell migration assay.
Figure 2.
ID4 regulates Migration. A: ID4 significantly inhibited migration in PC3+ID4 cells compared to PC3–CMV and PC3+EGF cells (positive control). Each bar represents Mean ± SEM (n = 3). (*** p < 0.001 compared to PC3-CMV). B: Representative images of PC3+EGF, PC3 CMV and PC3+ID4 cells after migration of cells through Transwell (×10).
ID4 promotes AR expression
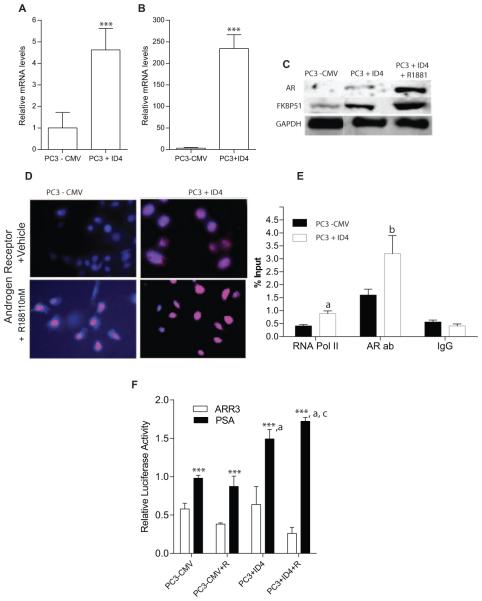
Real time PCR demonstrated that PC3+ID4 cells have 4 fold greater AR expression compared to PC3-CMV cells (Fig. 3A). Western blot analysis further demonstrated greater AR protein expression in PC3+ID4 cells compared to PC3-CMV cells (Fig. 3C). Immuno-cytochemistry further confirmed increased expression of androgen receptor in PC3+ID4 cells (Fig. 3D). The AR expression in PC3+ID4 cells was localized primarily to the nucleus (Fig. 3D2) with an increase in expression and nuclear localization after treatment with 10nM R1881 (Fig. 3D4). In contrast, AR was not expressed in PC3-CMV cells treated with vehicle alone but was expressed only after R1881 treatment (3D3).
Figure 3.
ID4 regulates expression of AR. A and B: Quantitative real time RT-PCR of AR and FKBP51 respectively (mean± SEM, n=3 in triplicate, normalized to GAPDH followed by fold change over PC3-CMV cells ***: P < 0.001). C: Western blot analysis of AR and FKBP51 in PC3– CMV and PC3+ID4 cells (±R1881). GAPDH was used as loading control. D: AR immuno-cytochemistry (red). The cells were counterstained with DAPI (blue) to reveal the nucleus (×200). E: Enrichment of Pol II and AR, on FKBP51 promoter in PC3-CMV, PC3+ID4 cells. The data is expressed (mean±SEM, n=3 in triplicate) as % of input (“a”, and “b” corresponding to Pol II, and AR respectively, p<0.001 compared with PC3 - CMV cells. F: The AR transcriptional activity as determined by PSA AR response element driven luciferase reported plasmid. The data is normalized to Renilla luciferase. The mutant ARR3 luciferase reporter plasmid was used as a negative control (mean ± SEM ***: P < 0.001 as compared to respective ARR3 luciferase, a: p<0.001 as compared to PSA luciferase activity in PC3-CMV, c: p<0.01 as compared to PC3+ID4, n=3).
FKBP51, a well-established AR regulated gene was used to assess the transcriptional activity of AR. ID4 induced FKBP51 expression at both mRNA and protein levels in PC3+ID4 cells (Fig. 3B&C). Androgen-dependent transcription of the FKBP51 is conferred through a non-canonical androgen response element (ARE) element within an intron [15]. Chromatin immuno-precipitation analysis using androgen receptor antibody revealed that binding to FKBP51 promoter is significantly increased (P < 0.001) in PC3+ID4 cells compared to PC3 control cells (Fig. 3E). These results suggest that ID4 promotes binding of AR to FKBP51promoter.
In order to further assess the transcriptional activity of AR, the activity of luciferase driven by the PSA promoter was performed. The relative PSA luciferase activity increased significantly in PC3+ID4 cells as compared to PC3-CMV cells (Fig. 3F), which is consistent with the increased expression of AR in these cell lines. The mutant ARR3 luciferase plasmid (mt-ARR3 RE) used as a negative control, did not result in significant luciferase activity. (Firefly PSA is normalized to Renilla empty vector).
ID4 results in decreased tumor growth in vivo
The influence of ID4 on tumor formation by PC3 was examined by measuring the size and weight of the tumors. PC3-CMV control cell tumors in nude mice were observed within 1 week of injection (Figure 4A & 4B). In contrast, PC3+ID4 cells formed tumors after a latency period of approximately 3 weeks, which led to a significant decrease in tumor growth. At the end of the experiments (6 weeks), the tumors were excised and volume and weights were measured (Fig. 4C&D). The PC3+ID4 cells formed smaller tumors compared to PC3 control cells. Collectively, these results indicate that overexpression of ID4 decreases tumor growth of PC3 in nude mice.
Figure 4.
table overexpression of ID4 in PC3 cells suppresses tumor growth in vivo. Male nude mice were injected with PC3–CMV and PC3+ID4 cells and evaluated for tumor volumes and tumor weights. A: Representative xenograft images with numbers of mice with similar tumor (n) are shown. B: Volumes of the tumors were measured weekly (expressed as mm3, means±SEM, n = 3/group, ***, p < .001, and *p<0.01 between PC3–CMV and PC3+ID4). The mice were sacrificed at 6 weeks. C, D: Relative volumes and weights (means±SEM, n = 3) of the tumors after excision (***, P < .001, between PC3– CMV and PC3+ID4 tumors). E: TUNEL assay demonstrated increased apoptosis in in PC3+ID4 mice xenograft (Brown staining) as compared to the PC3–CMV cells. The TUNEL positive cells were counted in five fields (at 400×) on three different tissue samples.
ID4 regulated apoptosis in subcutaneous xenografts
Xenografts derived from PC3+ID4 cells showed significantly more apoptotic cells compared to xxenografts from PC3-CMV cells, confirming a role for ID4 in promoting apoptosis [16]. The average number of TUNEL positive cells in PC3+ID4 xenograft tissue is significantly higher compared to PC3 control xenograft tissue (Fig. 4E).
Discussion
The role of ID4 in cancer has not been clarified; however, studies support both a pro-tumor and anti-tumor activity of ID4. ID4 promoter methylation demonstrates its role as a potential tumor suppressor and oncogene in a context dependent manner. We have shown that ID4 expression is decreased due to promoter methylation in prostate cancer [3]. Epigenetic silencing of ID4 promoter tend to support its tumor suppressor role in prostate cancer.
In prostate cancer cell lines, ID4 promotes cell cycle arrest and apoptosis, by up-regulating the expression of p21 and p27 [5]. In the present study also, ID4 decreases migration and cell proliferation of PC3 cells, which is associated with an increase in p21, a regulator of cell cycle progression at G1 and S phase and with a significant decrease in Ki67, a marker of proliferation. [17]. PC3+ID4 cells showed increased apoptosis and decreased cell migration compared to control cells. Most importantly we show an up-regulation of a E-cadherin a well-established tumor suppressor and inhibitor of epithelial to mesenchymal transition [18] by ID4. ID4 may directly regulate E-cadherin expression by neutralizing basic helix loop helix E2A proteins that negatively regulate E-cadherin gene expression through E-Box response elements [19]. Alternatively, increased AR expression in PC3+ID4 cells may itself promote e-cadherin expression [20]. Collectively, these results demonstrated that ID4 expression induces a change in cell morphology/adhesion, decreased proliferation and increased apoptosis, possibly mediate via increased AR expression.
AR plays a tumor suppressor role in normal cells and functions as tumor promoter in prostate cancer cells. [8, 21-23]. Various studies have shown that AR mRNA and protein expression are low but detectable in PC-3 cells [24, 25]. Studies with treatment of PC3, DU145, and LAPC4 cells with RI881 and DHT showed increased AR levels only in PC3 cells, with no effect in DU145 and a negative effect in LAPC4 cells suggesting that PC3 retained the necessary cofactors to engage AR as a tumor suppressor [26]. Addition of functional AR in PC3 cells resulted in decreased invasion in bone lesion assay and in mouse models [6]. Induction of AR expression and activity in PC3 cells indeed results in growth suppression [8, 14, 20, 27], which is probably mediated by co-regulators that enable AR’s normal tumor suppressor function.
Our results demonstrate that ID4 may activate the tumor suppressor activity of AR by inducing expression of target genes P21 and FKBP51, and by decreasing expression of KI67, in PC3 cells. At the mechanistic level, ID4 may regulate the expression or function of specific but yet unknown AR co-regulators that may determine the final outcome of AR function.
In conclusion, ID4 may restore AR expression and activity in PC3 cells, which may contribute to the tumor suppressor function of ID4. Therefore, AR-dependent and –independent pathways may contribute to the tumor suppressor function of ID4. Identification of the factors that mediate these effects in these prostate cancer cells will improve our understanding of mechanisms that contribute to tumor invasion and therapeutic resistance associated with androgen deprivation therapy.
Supplementary Material
Highlights.
ID4 expression induces AR expression in PC3 cells, which generally lack AR.
ID4 expression increased apoptosis and decreased cell proliferation and invasion.
Overexpression of ID4 reduces tumor growth of subcutaneous xenografts in vivo.
ID4 induces p21 and FKBP51 expression- co-factors of AR tumor suppressor activity.
Acknowledgements
The work was supported by NIH/NCI CA128914 (JC) and in part by NIH/NCRR/RCMI G12RR03062.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sharma P, Chinaranagari S, Patel D, Carey J, Chaudhary J. Epigenetic inactivation of inhibitor of differentiation 4 (Id4) correlates with prostate cancer. Cancer Med. 2012;1:176–186. doi: 10.1002/cam4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharma P, Knowell AE, Chinaranagari S, Komaragiri S, Nagappan P, Patel D, Havrda MC, Chaudhary J. Id4 deficiency attenuates prostate development and promotes PIN-like lesions by regulating androgen receptor activity and expression of NKX3.1 and PTEN. Molecular cancer. 2013;12:67. doi: 10.1186/1476-4598-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chinaranagari S, Sharma P, Chaudhary J. EZH2 dependent H3K27me3 is involved in epigenetic silencing of ID4 in prostate cancer. Oncotarget. 2014;5:7172–7182. doi: 10.18632/oncotarget.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Patel D, Knowell AE, Korang-Yeboah M, Sharma P, Joshi J, Glymph S, Chinaranagari S, Nagappan P, Palaniappan R, Bowen NJ, Chaudhary J. Inhibitor of differentiation 4 (ID4) inactivation promotes de novo steroidogenesis and castration-resistant prostate cancer. Molecular endocrinology. 2014;28:1239–1253. doi: 10.1210/me.2014-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carey JP, Asirvatham AJ, Galm O, Ghogomu TA, Chaudhary J. Inhibitor of differentiation 4 (Id4) is a potential tumor suppressor in prostate cancer. BMC cancer. 2009;9:173. doi: 10.1186/1471-2407-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- [8].Litvinov IV, Antony L, Dalrymple SL, Becker R, Cheng L, Isaacs JT. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. The Prostate. 2006 doi: 10.1002/pros.20483. [DOI] [PubMed] [Google Scholar]
- [9].Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, MacArthur S, Stark R, Warren AY, Mills IG, Neal DE. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- [10].Knowell AE, Patel D, Morton DJ, Sharma P, Glymph S, Chaudhary J. Id4 dependent acetylation restores mutant-p53 transcriptional activity. Molecular cancer. 2013;12:161. doi: 10.1186/1476-4598-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patel D, Chaudhary J. Increased expression of bHLH transcription factor E2A (TCF3) in prostate cancer promotes proliferation and confers resistance to doxorubicin induced apoptosis. Biochem Biophys Res Commun. 2012;422:146–151. doi: 10.1016/j.bbrc.2012.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharma P, Patel D, Chaudhary J. Id1 and Id3 expression is associated with increasing grade of prostate cancer: Id3 preferentially regulates CDKN1B. Cancer Med. 2012;1:187–197. doi: 10.1002/cam4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel D, Morton DJ, Carey J, Havrda MC, Chaudhary J. Inhibitor of differentiation 4 (ID4): From development to cancer. Biochimica et biophysica acta. 2015;1855:92–103. doi: 10.1016/j.bbcan.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- [15].Makkonen H, Kauhanen M, Paakinaho V, Jaaskelainen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic acids research. 2009;37:4135–4148. doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knowell AE, Patel D, Morton DJ, Sharma P, Glymph S, Chaudhary J. Id4 dependent acetylation restores mutant-p53 transcriptional activity. Molecular cancer. 2013;12:161. doi: 10.1186/1476-4598-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fisher G, Yang ZH, Kudahetti S, Moller H, Scardino P, Cuzick J, Berney DM. G. Transatlantic Prostate, Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. British journal of cancer. 2013;108:271–277. doi: 10.1038/bjc.2012.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- [19].Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276:27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- [20].Huo C, Kao YH, Chuu CP. Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer Lett. 2015;369:103–111. doi: 10.1016/j.canlet.2015.08.001. [DOI] [PubMed] [Google Scholar]
- [21].Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006 doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- [22].Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- [23].Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles' heel for prostate cancer therapy a gain of function in androgen receptor signaling? The Journal of clinical endocrinology and metabolism. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- [24].Edelstein RA, Carr MC, Caesar R, Young M, Atala A, Freeman MR. Detection of human androgen receptor mRNA expression abnormalities by competitive PCR. DNA and cell biology. 1994;13:265–273. doi: 10.1089/dna.1994.13.265. [DOI] [PubMed] [Google Scholar]
- [25].Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, Meisner LF, Chang C, Herman JG, Isaacs WB, Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer research. 1998;58:5310–5314. [PubMed] [Google Scholar]
- [26].Fujimoto N, Mizokami A, Harada S, Matsumoto T. Different expression of androgen receptor coactivators in human prostate. Urology. 2001;58:289–294. doi: 10.1016/s0090-4295(01)01117-7. [DOI] [PubMed] [Google Scholar]
- [27].Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer research. 1993;53:1304–1311. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.