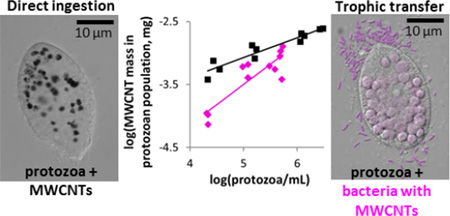
Abstract
Consumer goods contain multiwall carbon nanotubes (MWCNTs) that could be released during product life cycles into the environment, where their effects are uncertain. Here, we assessed MWCNT bioaccumulation in the protozoan Tetrahymena thermophila via trophic transfer from bacterial prey (Pseudomonas aeruginosa) versus direct uptake from growth media. The experiments were conducted using 14C-labeled MWCNT (14C-MWCNT) doses at or below 1 mg/L, which proved subtoxic since there were no adverse effects on the growth of the test organisms. A novel contribution of this study was the demonstration of the ability to quantify MWCNT bioaccumulation at low (sub µg/kg) concentrations accomplished by employing accelerator mass spectrometry (AMS). After the treatments with MWCNTs at nominal concentrations of 0.01 mg/L and 1 mg/L, P. aeruginosa adsorbed considerable amounts of MWCNTs: (0.18 ± 0.04) µg/mg and (21.9 ± 4.2) µg/mg bacterial dry mass, respectively. At the administered MWCNT dose of 0.3 mg/L, T. thermophila accumulated up to (0.86 ± 0.3) µg/mg and (3.4 ± 1.1) µg/mg dry mass by trophic transfer and direct uptake, respectively. Although MWCNTs did not biomagnify in the microbial food chain, MWCNTs bioaccumulated in the protozoan populations regardless of the feeding regime, which could make MWCNTs bioavailable for organisms at higher trophic levels.
Graphical abstract
Introduction
Worldwide production capacity of carbon nanotubes (CNTs) has been reported to exceed several thousand tons per year, and CNT powders have already been incorporated into many commercial applications such as catalysts, water purification systems, coatings, and composites.1 It has been proposed that CNT release during product lifecycles occurs by abrasion from nanocomposites and matrix degradation.2, 3 These processes could introduce the largely biodegradation-resistant CNTs into soils, sediments and sewage sludge4 where they could sorb and modulate the toxicity of other contaminants or vice versa.5 In addition, weathering factors such as UV irradiation and precipitation could alter physico-chemical properties of CNTs and thereby change their bioavailability and toxicity.6
Studies regarding CNT environmental hazards indicate that the bioaccumulation potential of CNTs varies with exposure conditions, test organisms and physico-chemical properties of the CNTs.7 At various exposure concentrations, single-wall carbon nanotubes (SWCNTs) were neither toxic nor bioaccumulative in marine benthic organisms (at up to 100 mg SWCNTs/kg sediment for 14 days),8 marine bivalves (100 mg and 1000 mg SWCNTs/kg dry algae for 28 days),9 earthworms (up to 100 mg SWCNTs/kg soil for 28 days),10 or in aquatic plants and vertebrates in a wetland mesocosm over the 10 month incubation (2.5 mg/L SWCNTs).11 Similarly, MWCNTs did not bioaccumulate in oligochaetes when ingested from MWCNT-spiked soils (30 mg/kg and 300 mg/kg dry soil) or sediments (37 mg/kg and 370 mg/kg dry sediment) into the organism guts, as there was no apparent absorption into tissues after the 28 day exposure and 6 h depuration phases.10, 12, 13 Still, Daphnia magna, exposed to a non-toxic concentration of MWCNTs (up to 0.4 mg/L) for 24 h, retained nanotubes in the gut when placed in clean water for up to 48 h, and excreted most nanotubes only after feeding on algae.14, 15 Recently, MWCNTs were shown to adsorb to algal cells grown for 48 h with MWCNTs, with some nanotubes also entering in the cytoplasm.16 Also, a 2 week exposure of zebrafish to a non-toxic MWCNT concentration of 1 mg/L resulted in uptake and retention of approximately 5 mg MWCNTs/kg dry fish.17 In the latter study, small fractions of MWCNTs accumulated in the fish blood and muscles, indicating the potential for CNT transfer in the food chain. While such studies suggest the potential for trophic transfer and bioaccumulation, most have used relatively high exposure concentrations. As such, understanding the fate of released CNTs is still limited for low (µg/L) concentrations that are estimated to be present in aqueous environments.18
The assessment of trophic transfer and bioaccumulation at the low CNT concentrations predicted to occur in the environment has generally been hindered by the lack of suitable quantification methods of CNTs in complex environmental matrixes.19 To overcome this challenge, we used 14C-labeled MWCNTs (14C-MWCNTs) to study their accumulation and trophic transfer in a microbial food chain of prey, the bacterium Pseudomonas aeruginosa, and predator, the protozoan Tetrahymena thermophila. The use of a sensitive detection method – accelerator mass spectrometry (AMS) - allowed for tracing 14C-MWCNTs in the biological matrices at low (sub µg/kg) levels; this is the lowest detection level obtained to date for CNT quantification in tissues to our knowledge.19, 20 Since MWCNTs were not expected to biodegrade under the experimental laboratory conditions of this study, quantification of 14C could be used to trace MWCNTs in biota. Two environmentally relevant scenarios of CNT transfer to ciliates were compared at the same MWCNT doses: (i) MWCNT uptake via bactivory of MWCNT-encrusted bacteria, and (ii) grazing on medium-dispersed MWCNTs. The potential for MWCNT bioaccumulation and biomagnification in protozoa was assessed.
Materials and Methods
MWCNT Synthesis and Characterization
MWCNTs and 14C-MWCNTs were synthesized using a modified chemical vapor deposition technique, purified by bath sonication with concentrated hydrochloric acid, and surface-modified with a 3:1 v:v ratio of concentrated nitric and sulfuric acid as described previously.10, 13 The specific activity of the 14C-MWCNTs was 0.015 mCi/g (555 kBq/g) as measured by liquid scintillation counting (LSC). For safety reasons, the physico-chemical characterization was performed with unlabeled MWCNTs, synthesized by the same method as the 14C-MWCNTs. More than 90 % of the nanotubes were under 500 nm long, and the average diameter was 36.5 nm ± 12.7 nm as reported previously.21 The Supporting Information (SI, Figure S1) provides additional characterization information.
Preparation and Characterization of MWCNT Stock Suspensions
Stock suspensions of MWCNTs and 14C-MWCNTs were prepared at 200 mg/L in Nanopure water. To prepare the stocks, both MWCNTs and 14C-MWCNTs were weighed into acid-washed and autoclaved 118-mL flasks to which water (70 mL) was added. The flasks were placed in an ice bath and the suspensions sonicated to disperse (40 % amplitude for 1 h, pulsing for 30 s on and 10 s off), using a Cole-Parmer 750-Watt Ultrasonic Homogenizer with a 13-mm diameter probe and replaceable tip, fabricated from titanium alloy Ti-6Al-4V. The output power, measured as described previously,22 was 27 W. Probe sonication was not expected to shorten the MWCNTs, since similar sonication procedures were used previously for similarly-synthesized MWCNTs, and no change in the length distribution was observed.21, 23 The stock suspensions were maintained at room temperature in the dark until addition to the experimental test media. Most (88 % ± 1.4 %; n = 3, uncertainty indicates standard error of the mean) of the MWCNTs were stably dispersed in Nanopure water four days after sonication and remained dispersed over six months, as confirmed by the 14C-MWCNT specific activity measurements. Hydrodynamic diameters and zeta-potential of MWCNTs were measured as described in the SI.
Assessment of MWCNT Effects on P. aeruginosa and T. thermophila
MWCNT toxicity to P. aeruginosa was assessed by measuring membrane integrity using the LIVE/DEAD Bac Light Bacterial Viability Kit L7012, reductase activity using the BacLight™ RedoxSensor™ Green Vitality Kit (both from Molecular Probes, Invitrogen, CA, USA) and growth by measuring the time course optical density (600 nm). Viability of T. thermophila upon direct exposure to MWCNTs in acute conditions (non-growing culture) was assessed by cell counting and membrane integrity as in P. aeruginosa above. Experimental details are in the SI.
Preparation of P. aeruginosa for Trophic Transfer Experiments
A Gram-negative bacterial strain, P. aeruginosa PG201,24–27 was used for 14C-MWCNT sorption studies and for T. thermophila feeding (trophic transfer) experiments. As detailed in the SI, P. aeruginosa was cultured (18 h, 30 °C) with shaking at 26 rad/s (250 rpm) in Erlenmeyer flasks containing half-strength 21C growth medium (50 mL) until late exponential growth phase (optical density at 600 nm [OD600] 0.7, Figure S2A). The 14C-MWCNT stock dispersion (mixed with 2× concentrated bacterial growth medium at a ratio of 1:1, v:v) was added to bacterial culture in the medium with undefined chemistry, due to bacterial growth and excretion of metabolites, yielding a final nominal 14C-MWCNT concentration of either 0.01 mg/L or 1 mg/L (Table S1). Replicates with unlabeled MWCNTs were included for cell counting. Bacteria were incubated at 30 °C, while shaking at 26 rad/s (250 rpm), for 1 h with or without MWCNTs, then harvested by differential centrifugation (9, 715g, 10 min). Bacteria were separated from unassociated MWCNTs by density gradient centrifugation (SI) using sucrose which was biocompatible for T. thermophila trophic transfer experiments. 14C-MWCNT concentrations associated with bacteria were quantified as described below. Bacterial cell numbers were determined by direct counting using epifluorescence microscopy (SI). The mass of an individual dry bacterial cell was determined in a prior study.26
Exposure of T. thermophila to MWCNTs with P. aeruginosa Prey and in Axenic Cultures
T. thermophila strain SB210E26 was cultured in Dryl’s medium (SI) with P. aeruginosa to determine protozoan growth rates and yields, and to quantify the uptake of 14C-MWCNTs when bacterial prey was the only food source. P. aeruginosa, with or without MWCNTs, recovered from sucrose density gradients and resuspended in Dryl’s medium (10 mL), were pipetted into sterile polystyrene Petri plates (10 cm by 15 mm). MWCNT doses supplied to protozoa via MWCNT-encrusted bacteria were 0.004 mg/L and 0.3 mg/L, following nominal exposure concentrations to bacteria of 0.01 mg/L and 1 mg/L, respectively. For exposures in axenic cultures, the MWCNT stock was diluted to a final concentration of either 0.3 mg/L (to equal one of the two MWCNT doses in the trophic transfer experiment) or 1 mg/L in a proteose peptone-based growth (SSP) medium (10 mL in Petri plates; SI). Starved T. thermophila cells were added to achieve an initial cell density of ca. 104 cells/mL. Replicate Petri plates were prepared for each treatment and time point of culture harvest (Table S2). More Petri plates were prepared for sampling at earlier time points when the cell concentrations were low because larger volumes were needed to harvest sufficient biomass for analysis (Table S2). T. thermophila was cultured in the dark in a humidity chamber (30 °C) without agitation. At 2 h, 8 h, 16 h, and 22 h, the cultures were subsampled for microscopy, cell counting, and for total 14C-MWCNT quantification; for the remaining volume of the culture, protozoa were separated from bacteria, fecal pellets, and unassociated MWCNTs by density gradient centrifugation in OptiPrep™ (Axis-Shield, Oslo, Norway) as described in the SI.
Quantification of 14C-MWCNTs
Either LSC or AMS was used to quantify high or low 14C-MWCNT concentrations, respectively, associated with bacteria and protozoa (Table S1).
LSC
Bacterial or protozoan pellets, recovered using density gradient centrifugation (as per the SI), were digested in 2.5 mL of 0.1 % sodium dodecyl sulfate (SDS) in 0.1 mol/L NaOH by vortexing28 and incubating the samples (55 °C, 45 min).29 Two and one half mL of UltimaGold XR (Perkin Elmer, Groningen, The Netherlands) liquid scintillation cocktail were added to the digested samples and the mixtures were kept in the dark for 1 h before LSC (LS 6500, Beckman Coulter Inc., Fullerton, CA) with the counting time set to 10 min. For quantification of 14C-MWCNTs in the total bacterial or protozoan cultures, 1 mL of 0.1 % SDS in 0.1 mol/L NaOH was added to 1.5 mL of the culture, vortexed, then heated and mixed with the cocktail, similarly to how cell pellets were treated. Measured counts per minute (CPM) were converted to disintegrations per minute (DPM) by subtracting the background CPM from the sample CPM and dividing this net CPM by the fractional efficiency (0.95). Quenching of 14C by bacterial and protozoan samples was between 5 and 10 % which was accounted for by spiking the unamended samples (cell pellets or suspensions) with a known mass of 14C-MWCNTs. MWCNT mass in the MWCNT-exposed bacterial and protozoan samples was then calculated as follows:
| (1) |
where DPM(sample) is the activity of the sample in DPM, m(MWCNTs, spiked) is the mass of MWCNTs added to the unamended samples, and DPM(spiked sample) is the activity of the MWCNT-spiked sample in DPM.
AMS
Each liquid sample (supernatant or suspended pellet) containing at least 30 µg carbon was transferred by pipet to a prebaked (900 °C for 3.5 h) quartz tube (≈ 6 mm × 30 mm, 4 mm i.d.) located inside two borosilicate glass culture tubes (10 mm × 75 mm in 12 mm × 100 mm) and dried overnight in a vacuum centrifuge. An excess of CuO (≈ 40 mg) was added and the inner quartz vials were transferred to quartz combustion tubes, evacuated and sealed with a torch. The samples were combusted at 900 °C for 3.5 h to oxidize all organic carbon to CO2 and then reduced to filamentous carbon as previously described.30 Carbon samples were packed into sample holders and carbon isotope ratios were measured on a National Electrostatics Corporation (Middleton, WI) compact 250 kV AMS spectrometer at the Lawrence Livermore National Laboratory. Typical AMS measurement times were 5 min/sample to 10 min/sample, with a counting precision (relative standard deviation, RSD) of 0.5 % to 3 % and a standard deviation among 3 to 10 measurements of 1 % to 3 %. The 14C/13C ratios of the samples were normalized to measurements of four standard samples prepared using the same method of known isotope concentration (IAEA C-6 also known as ANU sucrose) and converted to units of g MWCNTs/g sample.31 The limit of quantitation (LOQ) of 14C-MWCNT in bacteria and protozoa was typically 0.05 µg/kg to 0.07 µg/kg based on the average of 3–9 undosed controls (samples without 14C-MWCNTs) plus 3 times their standard deviation. Undosed controls were analyzed with each batch of samples to establish the LOQ for each set of exposures. The carbon content of each sample type was determined with 3 to 5 replicates using a CE-440 elemental analyzer (Exeter Analytical, Inc. North Chelmsford, MA).
MWCNT concentrations in bacteria and protozoa, were calculated as described in SI. Both volumetric bioconcentration factors (VCF, unitless) and bioconcentration factors (BCF, L/kg) were calculated for all the treatments: for the direct (via the media) bacterial and protozoan exposures to MWCNTs and for protozoan exposures to MWCNTs via bacteria (dietary exposure, SI). Trophic transfer factors (TTF) were also calculated for protozoan exposures to MWCNTs via bacteria (SI). MWCNT mass in protozoa was also estimated by analyzing optical microscopy images (SI) and the results were compared to 14C-MWCNT concentrations quantified by LSC.
Statistical Analysis
After testing the normality using quantile-quantile plot statistical significances of means differences were determined using one-way analysis of variance (ANOVA) and post hoc Tukey’s multiple comparisons test (R, http://www.r-project.org/) or regression analysis (Microsoft Excel, Microsoft Corporation) with a p-value < 0.05 considered statistically significant. The values reported throughout the text are the mean values of at least 3 replicate samples ± standard deviation.
Results and Discussion
MWCNT Characteristics in Media and Effects on Bacterial Growth
The MWCNTs were relatively short (under 500 nm)21 and well dispersed both in Nanopure water and bacterial growth medium (half-strength 21C; Table S3). The acid treatment during the MWCNT purification and surface-modification process added O-containing groups as indicated by the X-ray photoelectron spectroscopy (XPS) performed previously21 and the negative ζ-potential values at neutral pH (Table S3). This contributed to the MWCNTs’ high aqueous dispersibility and stability. Previously, short functionalized MWCNTs have exhibited strong antibacterial effects when deposited on filters,32 although acid-treated MWCNTs in suspensions had no antimicrobial activity up to concentrations of 500 mg/L to 875 mg/L.33 Here, MWCNTs suspended in bacterial growth medium at 0.1 mg/L to 1 mg/L did not affect the specific growth rate and maximum yield of P. aeruginosa (Figure S2B). Similar results showing a lack of a toxic effect on specific algal growth rate at a comparable dose of MWCNTs (1 mg/L) were recently observed.16
Quantification of MWCNTs Associated with P. aeruginosa
At the nominal 14C-MWCNT concentrations of 0.01 mg/L and 1 mg/L, the measured total 14C-MWCNT concentrations in the bacterial suspensions were (0.0058 ± 0.0005) mg/L and (0.64 ± 0.12) mg/L, respectively, indicating that approximately 40 % of added MWCNTs had adsorbed to the flask walls during the incubation and vigorous shaking (250 rpm [26 rad/s]) of the cultures. Thus, in the P. aeruginosa cultures prepared for trophic transfer, the recovery of 14C label after 1-h incubation with 14C-MWCNTs was approximately 60 %.
After separating unbound MWCNTs from bacteria by sucrose density gradient centrifugation, the 14C-MWCNT mass associated with the bacterial cells was measured and normalized to the bacterial cell count in the harvested culture ([1.9×108 ± 2×107] cells/mL and [1.7×108 ± 3×107] cells/mL, in the 0.01 mg/L and 1 mg/L of MWCNTs treatments, respectively). At nominal concentrations of 0.01 mg/L and 1 mg/L, (76 ± 17) % and (70 ± 15) % of the recovered total MWCNT mass in the cultures was adsorbed to the bacterial cells. The calculated MWCNT masses per P. aeruginosa cell were (0.022 ± 0.005) fg and (2.7 ± 0.5) fg, respectively. Assuming a bacterial cell mass of 0.12 pg as determined previously26 (SI, p. S10), the respective MWCNT masses per dry mass of bacteria were (0.18 ± 0.04) µg/mg and (21.9 ± 4.2) µg/mg. In comparison, when the alga Desmodesmus subspicatus was grown with 1 mg/L of 14C-MWCNTs, the mean MWCNT concentration associated with algae increased over time, and reached 4.98 µg/mg dry mass of algae by 72 h.16 This value is approximately 20 % of that measured for bacteria in this study at the dose of 1 mg/L of MWCNT and can likely be explained by the lower surface area per unit dry mass of algae available for MWCNT association. Although some MWCNTs were shown to enter the algal cytoplasm, most were agglomerated around the cell,16 which was also the likely association between bacteria and MWCNTs in this study. The retention of the 14C label, as a tracer for MWCNTs, in the bacterial pellet after density gradient centrifugation indicates that MWCNTs and bacteria were strongly associated, possibly facilitated by interactions with extracellular polymeric substances (EPS).34–36 MWCNT association with cell envelopes of bacteria without internalized MWCNTs has been demonstrated by other researchers using transmission electron microscopy.37, 38 Since MWCNTs did not damage the bacterial membranes (Figure S3), the MWCNTs were assumed not to enter bacterial cells. Thus, MWCNT adsorption to the cell surface rather than accumulation inside bacteria is a plausible scenario for the trophic transfer of MWCNTs.
Influence of Feeding Regime on T. thermophila Growth and MWCNT Effects on the Protozoa
Trophic transfer of MWCNTs by bacteria to protozoa was studied in comparison to direct uptake of MWCNTs from the medium. At the MWCNT concentrations tested (0.004 mg/L to 1 mg/L), T. thermophila population growth was unaffected either during axenic growth in rich medium or in Dryl’s medium with P. aeruginosa, indicated by the fact that the specific growth rates and maximum yields were not significantly different from control cultures (Table S4 and Figure S4). The growth of T. thermophila was exponential between 2 h and 16 h both in rich medium and in Dryl’s medium containing P. aeruginosa (Figure S4). However, T. thermophila grew significantly (two-sample t-test, p ≤ 0.05) faster and yielded higher cell numbers in rich growth medium than when feeding on P. aeruginosa, despite the longer lag phase in rich medium (Table S4 and Figure 1). The latter was likely caused by the adaptation phase after transferring protozoan cultures, which had been previously starved overnight in Dryl’s medium, to the rich medium. In other studies that used different media, SWCNTs at concentrations above 6.8 mg/L induced cell death in T. thermophila incubated in non-nutrient medium,39 and MWCNTs administered at 100 mg/L were growth inhibitory to T. pyriformis in filtered pond water.40 In the current study, besides not affecting T. thermophila population growth in either feeding regime (i.e. in either rich medium, or in starvation medium with bactivory), MWCNT exposure also did not impair membrane integrity and was not lethal in Dryl’s medium at concentrations up to 1 mg/L and 5 mg/L, respectively (Figure S5).
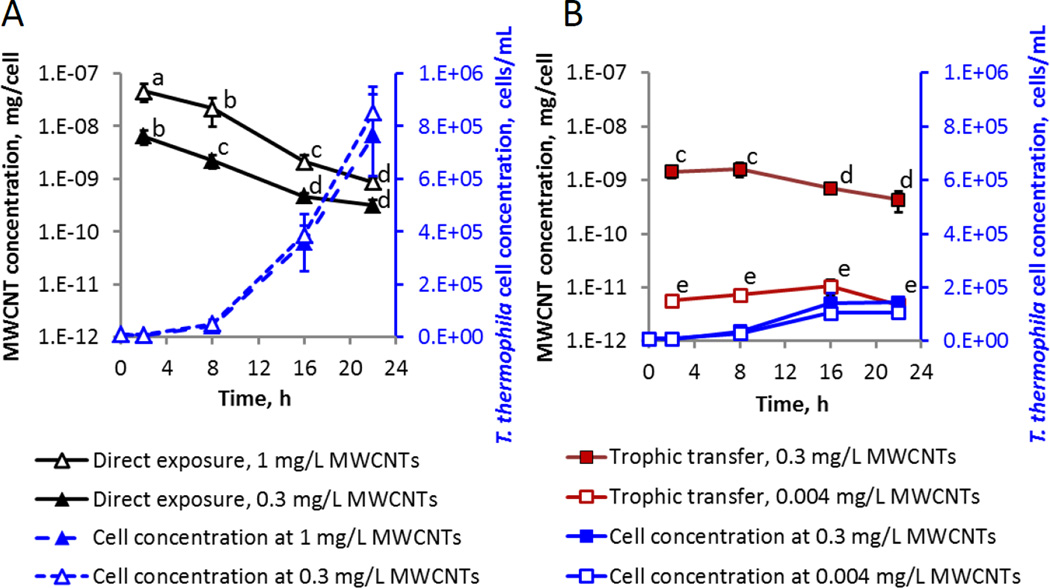
Figure 1.
MWCNT masses per T. thermophila cell and the cell densities of T. thermophila during the direct exposure to (A), and trophic transfer of (B), MWCNTs. Data points are average values of at least 3 replicates; error bars indicate standard deviation. In cases of very small standard deviations, error bars are not visible beyond the symbol. Data points with the same letter are not significantly different from one another; Tukey’s multiple comparisons test, p ≤ 0.05. Note the logarithmic scale of the left vertical axis. MWCNT doses listed in the legend are the nominal doses in the case of the direct exposures, and bacterial cell-associated doses in the trophic transfer experiments (Table S1). Note that the T. thermophila growth curves corresponding to the control (no MWCNTs) treatments in each media (SSP for direct exposure, or Dryl’s medium with P. aeruginosa for trophic transfer) are not shown for simplicity, since the exposure to MWCNT within each feeding regime did not affect the T. thermophila specific growth rate (Figure S4 and Table S4).
MWCNT Uptake by T. thermophila Administered Directly in the Medium
MWCNT mass per cell was measured for T. thermophila exposed to 0.3 mg/L or 1 mg/L of MWCNTs over the course of a 22-h growth period in the rich medium (Figure 1A). The MWCNT mass per cell clearly depended on MWCNT dose during the first 16 h of exposure. For both MWCNT doses, the MWCNT mass per protozoan cell was the highest at 2 h and then decreased as the cell concentration increased over time (Figure 1A). The trend is clearly shown in the scatter plot of logarithm-transformed MWCNT masses and protozoan cell densities (Figure S6A). The decreasing cellular content of MWCNTs, as the biomass increased while the mass of MWCNTs in the system remained the same, was also apparent in Nomarski microscopy images of T. thermophila acquired over the time course of direct feeding of MWCNTs in rich media (Figure 2).
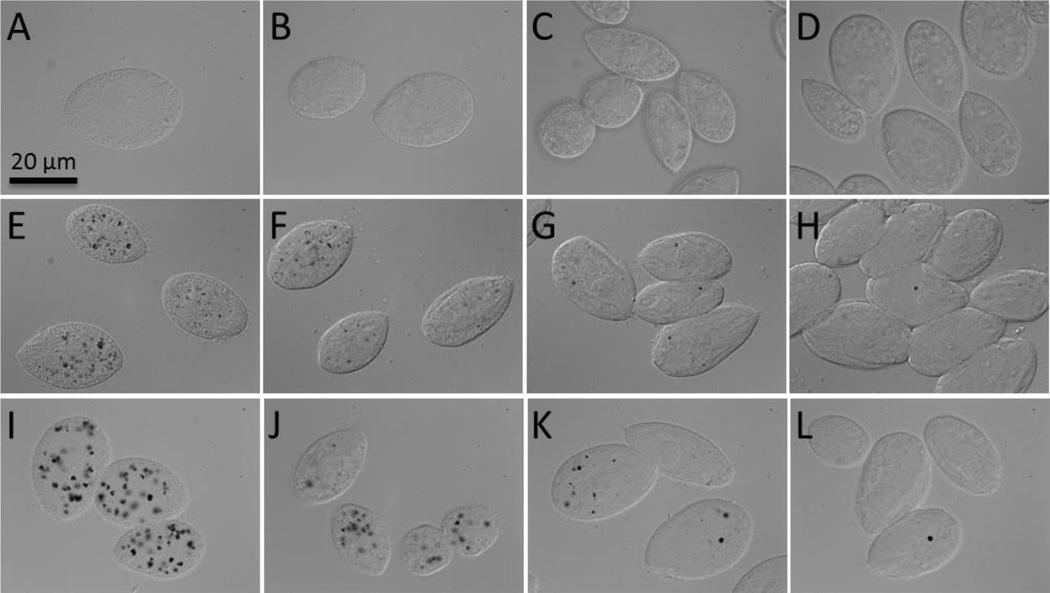
Figure 2.
Nomarski images of T. thermophila grown without MWCNTs (A–D), with 0.3 mg/L (E–H) and 1 mg/L (I–L) MWCNTs in the rich growth medium for 2 h (A, E, I), 8 h (B, F, J), 16 h (C, G, K) and 22 h (D, H, L). MWCNT aggregates internalized by phagocytosis appear as black areas in the food vacuoles of the cells grown with MWCNTs (E –L) while no black spots were detected in the control cells (A–D).
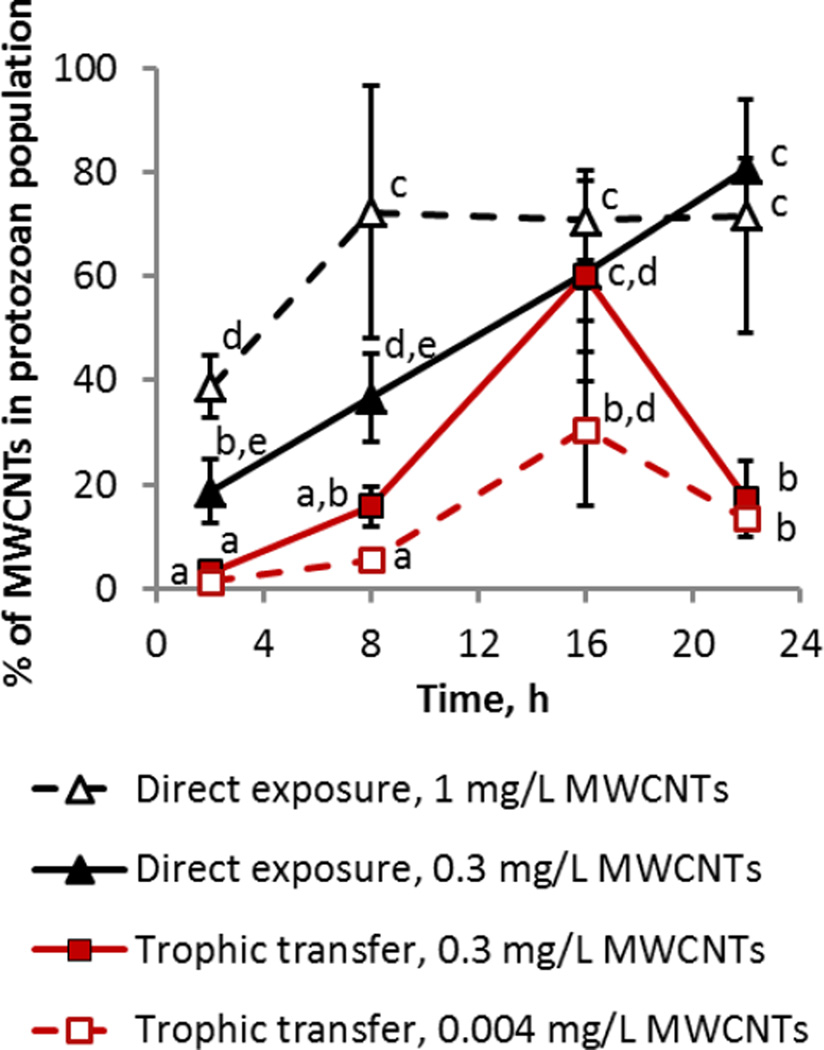
However, at the population level, the MWCNT mass retained in the protozoa correlated positively with the cell number (Figure S6B). The fraction of total administered MWCNTs in protozoan populations increased over the first 8 h independently of administered MWCNT dose (Figure 3). The maximum percentage of MWCNTs in the population was reached twice as quickly for the 1 mg/L (8 h) compared to for the 0.3 mg/L concentration (16 h). The final MWCNT masses within the entire population were (0.003 ± 0.0004) mg and (0.007 ± 0.002) mg for the 0.3 mg/L and 1 mg/L doses, respectively. These statistically similar masses constituted between 70 % to 80 % of the initially added MWCNTs and did not statistically change between 8 h and 16 h (Figure 3), indicating a maximum uptake level of the administered MWCNTs by the growing protozoan populations. That the MWCNT mass in the total population remained below 100 % is likely a result of the dynamics of ingestion, egestion and reuptake of particulate matter by protozoa as discussed in more depth in subsequent sections. This was also evident in a TiO2 nanoparticle (NP) direct uptake study, where, at a comparable cell density to this study, 35 % of the total administered TiO2 at a dose of 100 mg/L was within the total population by 22 h.27 However, in the prior study where the supply of NPs was not limited (at 100 mg/L of TiO2 NPs), protozoa were capable of ingesting a 60-fold higher mass of NPs (0.42 mg TiO2 NPs versus 0.007 mg MWCNTs). Thus, even when taking into account the difference in densities of TiO2 (3.97 g/cm3) and MWCNTs (1.5 g/cm3), we conclude that the dose of MWCNTs was a limiting factor to the uptake, and most of the MWCNTs were ingested by the protozoa by 8 h.
Figure 3.
Percent of administered MWCNT mass retained in the T. thermophila population. Average values of at least 3 replicates are graphed and the error bars indicate the standard deviation. In the case of very small standard deviations, the error bar is not visible beyond the symbol. Data points with the same letter are not significantly different from one another; Tukey’s multiple comparisons test, p ≤ 0.05.
Uptake of MWCNTs by T. thermophila Trophically Transferred via MWCNT-Encrusted P. aeruginosa
In the trophic transfer experiments, P. aeruginosa that had been pre-exposed to 0.01 mg/L or 1 mg/L of MWCNTs and suspended in Dryl’s medium at respective concentrations of (1.8×108 ± 1.8×107) cells/mL and (1.2×108 ± 2×107) cells/mL, resulted in doses to T. thermophila of 0.004 mg/L and 0.3 mg/L of MWCNTs, respectively (Table S1). As in the direct exposures, the MWCNT mass per T. thermophila cell was dose-dependent at each time point measured (Figure 1B). The MWCNT uptake trends over the 22-h growth period differed from those of direct uptake, but also differed at lower and higher MWCNT concentrations within the feeding regime: T. thermophila grazing on bacteria with 0.3 mg/L MWCNTs contained significantly higher levels of MWCNTs per cell at 2 h and 8 h of growth than at 16 h and 22 h, while there was no significant difference in the mass of MWCNTs per cell during growth when protozoa were fed bacteria with 0.004 mg/L of MWCNTs. Similarly to direct uptake, a decrease in MWCNT mass per T. thermophila cell occurred over time. The trend was statistically significant during trophic transfer of 0.3 mg/L of MWCNTs, but not for the lower MWCNT dose (0.004 mg/L, Figure S6A and Figure 1B).
Across the whole population, the retained MWCNT mass increased with higher protozoan cell numbers (Figure S6B). The fraction of total administered MWCNTs in protozoan populations increased over the first 8 h during the trophic transfer experiments for both MWCNT doses, and the maximum was reached at 16 h (Figure 3). Differently from the direct uptake of MWCNTs, the fraction of MWCNTs in the protozoan populations decreased to approximately 15 % by 22 h. Although the total cell number of T. thermophila grown with P. aeruginosa was approximately 1/6 of that in rich medium at 22 h, all cultures had reached stationary growth phase by the end of the experiment (Figure S4). Thus, the difference in MWCNT accumulation in protozoan populations during the two feeding regimes can be explained by the feeding patterns of T. thermophila and the availability of MWCNTs for reuptake after cellular excretion. In the trophic transfer experiments, the protozoan food vacuoles were packed with bacteria which limited the amount of MWCNTs internalized by protozoa, while there was no such physical restriction in the direct uptake exposure conditions. Accumulation of fecal pellets and agglomerated bacteria was evident in the Nomarski images at later trophic transfer time points (16 h and 22 h; Figure 4), suggesting that excreted MWCNTs were incorporated into fecal pellets that were not reingested by protozoa. This explains the decrease in the relative MWCNT mass in the protozoa at 22 h (Figure 3). Accumulation of fecal pellets in the medium was not evident in the images of T. thermophila grown in rich medium (Figure 2), indicating that MWCNTs were excreted as aggregates that were small enough for reuptake, resulting in a higher percentage of administered MWCNTs in the protozoan population (Figure 3). Comparatively, Chan et al.41 showed that initial ingestion of subtoxic amounts of SWCNTs by T. thermophila impaired subsequent digestion of Escherichia coli and increased the number of egested fecal pellets. Here, grazing on MWCNT-amended P. aeruginosa did not appear to alter the numbers of fecal pellets compared to control cultures (Figures 4 and S7).
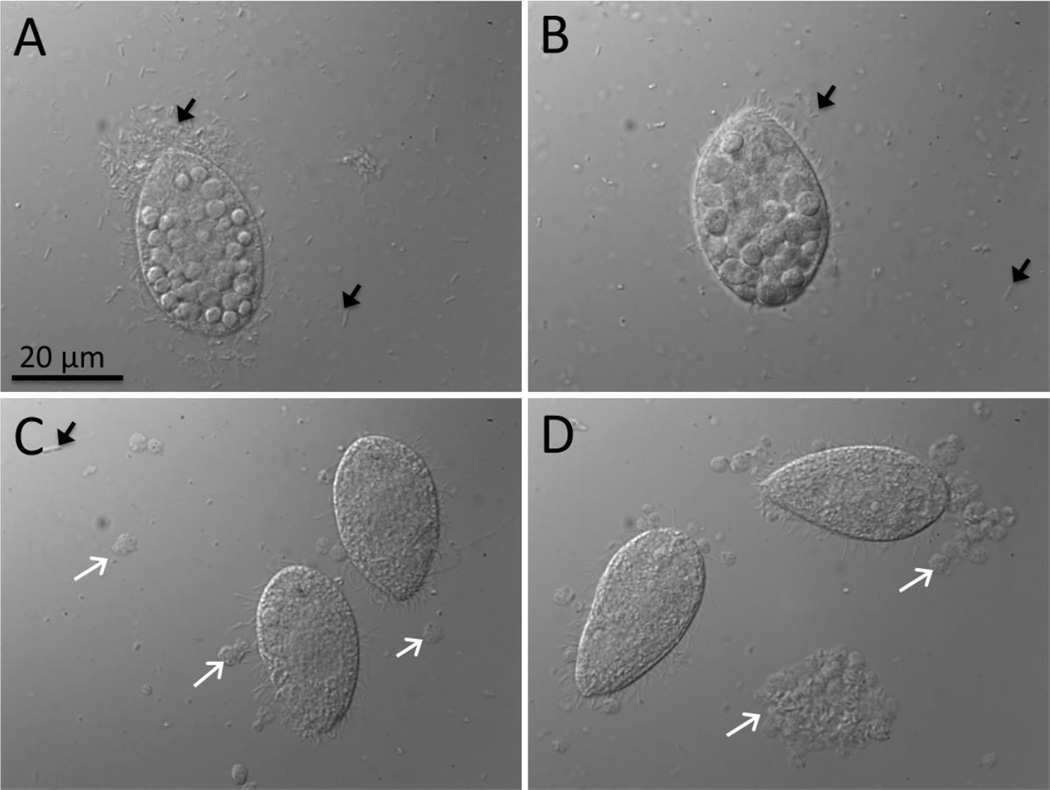
Figure 4.
Nomarski images of T. thermophila grown with MWCNT-encrusted P. aeruginosa as prey (MWCNT dose: 0.3 mg/L) for 2h (A), 8h (B), 16h (C) and 22h (D). Black arrows indicate bacteria which are abundant at 2 and 8 h and white arrows show fecal pellets evident at 16 and 22 h. The round shapes inside T. thermophila, well visible in A and B, are food vacuoles filled with P. aeruginosa.
Quantification of MWCNT Bioaccumulation and Biomagnification
Classical risk assessment of dissolved chemicals defines bioconcentration as increase in the concentration of a chemical substance in or on an organism relative to the concentration of the chemical in the surrounding medium, and bioaccumulation as a process in which the chemical concentration in an organism exceeds that in the medium and the diet.42 However, it has been acknowledged that quantification and interpretation of NP bioaccumulation requires a different approach because of properties of NPs that are distinct from those of hydrophobic organic contaminants (HOC) or metals.43, 44 Translocation of NPs, particularly carbonaceous ones, across epithelial cells (e.g., microvilli) and into organisms’ tissues is generally limited, but NPs may become trapped in the digestive tract and not eliminated even after organismal feeding;14, 45, 46 in these cases, NPs could still be considered as being accumulated.47
In the current study, MWCNTs became adsorbed to the surface of P. aeruginosa. MWCNTs were accumulated in the food vacuoles of T. thermophila when they were directly exposed to MWCNTs in the medium or fed MWCNT-encrusted bacteria. To demonstrate the magnitude of association between MWCNTs and test organisms, and to compare with the published literature, bioconcentration factors (BCF) were calculated in two ways (SI). The first followed the definition conventionally used in risk assessment of chemicals (BCF expressed in L/kg dry mass)42 and the second was the unitless volumetric concentration factor (VCF).26, 27
The BCFs of MWCNTs for P. aeruginosa were (230,000 ± 180,000) L/kg dry mass and (130,000 ± 50,000) L/kg dry mass of bacteria after exposure to 0.01 mg/L and 1 mg/L MWCNTs, respectively. These two BCFs, which are not statistically different, indicate a high propensity of MWCNTs to associate with bacterial cells. The corresponding VCFs were 40,000 ± 30,000 and 35,000 ± 10,000 after exposure to 0.01 mg/L and 1 mg/L MWCNTs, respectively. In comparison, CdSe quantum dots that damaged bacterial membranes and bioaccumulated in cells resulted in much lower VCF of 70.26 However, 100 mg/L TiO2 NPs that, similarly to this study, did not enter cells, fully adsorbed to bacterial membranes.27 In the latter case, the putative BCF is infinity and thus not meaningful, but — despite the difference in NP morphologies — the comparison may indicate that BCFs could have been greater at higher MWCNT exposure concentrations. A direct comparison for MWCNTs was only available for unicellular algae, with a BCF of 5000 L/kg dry mass.16 This value is two orders of magnitude lower than in this study, likely because of the lower available surface area per unit dry mass of algae compared to bacteria.
In prior studies, NP-amended P. aeruginosa were fed to T. thermophila, and NPs accumulated in protozoa through dietary intake, with biomagnification of QDs26 and without biomagnification of TiO2 NPs.27 Herein, MWCNTs in the same microbial food chain were trophically transferred similarly to TiO2 NPs in that MWCNTs accumulated in T. thermophila but did not biomagnify, as indicated by trophic transfer factors (TTF) below 1 (ranging from 0.01–0.04) for both MWCNT doses and all time points (Table S6). MWCNTs, like TiO2 NPs, accumulated in the cells but were confined to the food vacuoles and were continuously excreted into the surrounding medium. The fact that localization of MWCNTs was likely limited to protozoan food vacuoles was supported by significant linear correlations between MWCNT mass versus MWCNT area per cell as measured in the Nomarski images after direct MWCNT uptake (Figure S8), and MWCNT mass versus the total number of food vacuoles in T. thermophila population in trophic transfer experiments (Figure S9). Among other test systems where NPs have been shown to be trophically transferred,48–50 only a few have indicated biomagnification.51, 52
The BCFs calculated herein for T. thermophila grown in MWCNT-amended medium or when grazing on MWCNT-encrusted bacteria, and when sampled at different times, ranged from 35,000 L/kg [log BCF = 4.5] to 800 L/kg [log BCF = 2.9] (Tables S5 and S6, Figure 5). These values are within the same order of magnitude as the logarithm-transformed BCF values of 3.74 to 5.64, calculated for CNTs in daphnids after exposure to between 0.04 mg/L and 0.4 mg/L of 14C-labeled CNTs.53 Considering that “very bioaccumulative” substances, as defined by regulatory agencies in the Unites States, the European Union and Canada, have log BCF values ≥ 3.7,54 the values calculated herein and also those reported in the literature for daphnids53 suggest that NPs have a high propensity for bioaccumulation both in protozoa and daphnids. However, considering that MWCNTs have a low potential for crossing the cell membranes or for absorption into tissues,55, 56 the accumulated MWCNTs are likely retained in the digestive system. Thus, the BCFs are not directly comparable to those calculated for HOCs or metals.
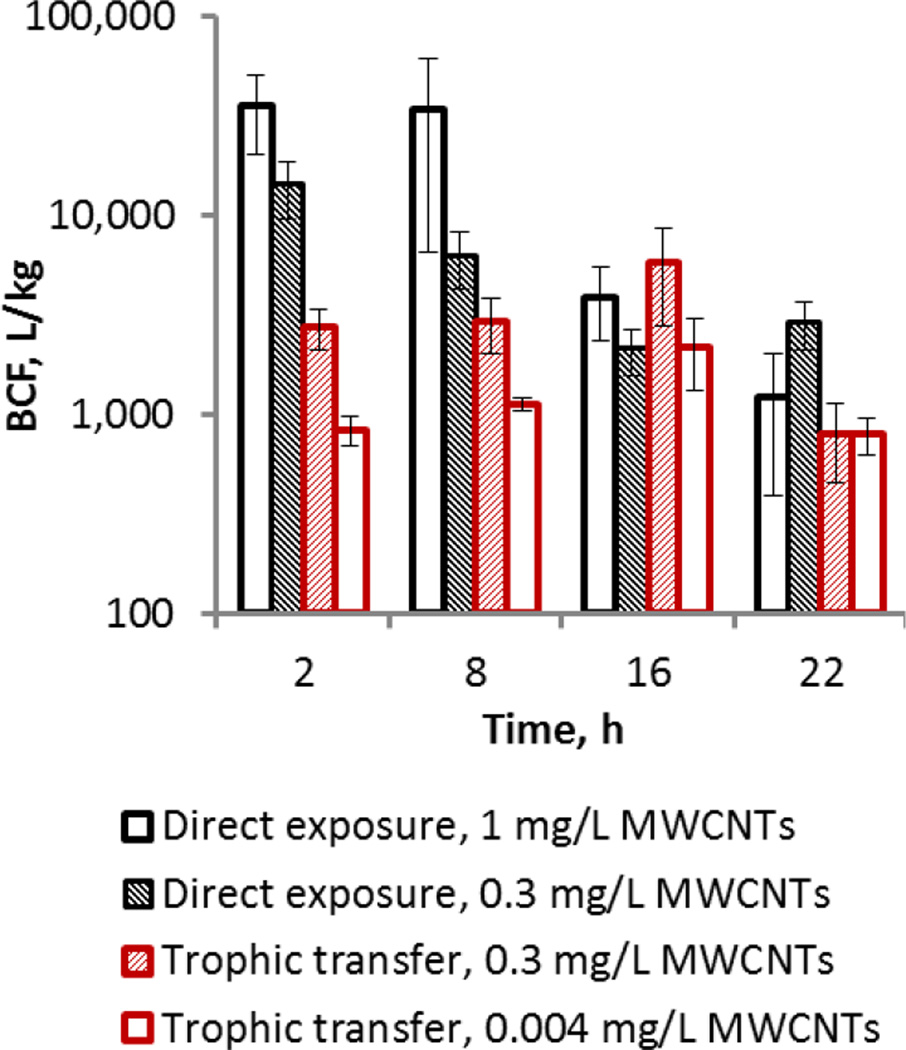
Figure 5.
Bioconcentration factors (BCFs) of MWCNTs at different time points during T. thermophila growth in the presence of MWCNTs, administered either directly in the medium (direct exposure) or with MWCNT-encrusted P. aeruginosa (trophic transfer). The bars indicate BCFs calculated using the mean MWCNT concentration values of three replicates (equations 11 and 12 in SI; Tables S5 and S6) and error bars indicate errors propagated using standard methods.
Comparison of the BCFs calculated for T. thermophila at different time points during direct exposure and trophic transfer of MWCNTs indicated higher bioaccumulation of MWCNTs when taken up directly from the medium than by bactivory at 2 h and 8 h (Figure 5). However there appeared to be no BCF dependence on dose or feeding regime at 16 h and 22 h. Higher accumulation of NPs in the case of direct aqueous exposure compared to trophic transfer has been reported previously for gold NP transfer from algae to mussels,57 and for TiO2 NPs from daphnids to zebrafish.58 However, marine mussels accumulated CeO2 NPs in equal amounts, regardless of whether the NPs were associated with phytoplankton or as free particles in the water column59 and freshwater snails accumulated higher amounts of CuO NPs via dietary intake compared to waterborne exposure.60 T. thermophila accumulated similar masses of TiO2 NPs by direct exposure in the medium and via feeding TiO2 NP-encrusted bacteria.27 For a fast growing unicellular organism, like T. thermophila, and in the limiting MWCNT exposure concentrations used here, the decrease of calculated BCF values observed as a function of time during population growth in direct feeding on MWCNTs (Figure 5) likely reflects the changing ratio between the biomass and MWCNT mass in the system: as the biomass increased over time (from 2 h to 22 h, Figure 1 and S6), the BCF values generally decreased at each administered MWCNT dose (Figure 5). Still, both direct exposure and trophic transfer of MWCNTs resulted in similar BCFs by the end of exposure (22 h), indicating that regardless of MWCNT dose and feeding regime, MWCNTs bioaccumulated in protozoa.
Environmental Implications
T. thermophila was exposed to MWCNTs via direct feeding in rich media or via trophic transfer by bactivory of MWCNT-encrusted P. aeruginosa. Nominal exposure concentrations of MWCNTs in media were on the same order of magnitude as those predicted in aquatic environments by modeling, i.e. down to the µg/L level.18 Working with such low concentrations was enabled by the novel application of AMS to quantify very low levels of 14C from 14C-MWCNTs sorbed to bacteria or bioaccumulated in protozoa. At low exposure concentrations of MWCNTs, T. thermophila indiscriminately ingested and bioaccumulated MWCNTs in a closed system, regardless of whether MWCNTs were made available as free agglomerates or as coatings on bacterial prey. Since for either feeding regime there was bioaccumulation of MWCNTs during population growth, protozoa would be reliable vectors for transferring MWCNTs to the next trophic level. This research also showed that, depending on the objective, future studies can be simplified by focusing on quantitative image analysis to assess T. thermophila bioaccumulation of carbonaceous nanoparticles.
Supplementary Material
Acknowledgments
This work was supported by the UC CEIN with funding from the NSF and EPA under Cooperative Agreements DBI-1266377 and DBI-0830117 and by NIH/NIGMS P41GM103483. The project was additionally supported by funds from the trust of Mr. Henry H. Wheeler, Jr. M.M. acknowledges the Estonian Research Council grant PUTJD16. Work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344; reviewed and released as LLNL-JRNL-681494. Manu Chopra is acknowledged for assistance in experiments and image analysis, Judy D. Orias for media preparation and Sage Davis for performing ESEM in the Micro-Environmental Imaging and Analysis Facility at the University of California Santa Barbara (www.bren.ucsb.edu/facilities/MEIAF/). We acknowledge the use of the NRI-MCDB Microscopy Facility and Materials Research Laboratory at UCSB. Certain commercial equipment, instruments and materials are identified in order to specify experimental procedures as completely as possible. In no case does such identification imply a recommendation or endorsement by the National Institute of Standards and Technology nor does it imply that any of the materials, instruments or equipment identified are necessarily the best available for the purpose.
Footnotes
Supporting Information. Additional materials and methods of MWCNT characterization, test organism growth and media, acute toxicity assays, cell number determination, density gradient centrifugation, calculations of VCFs, BCFs and TTFs, microscopy and image analysis; figures and tables as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- 1.De Volder MFL, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: Present and future commercial applications. Science. 2013;339(6119):535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 2.Petersen EJ, Zhang LW, Mattison NT, O'Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang QG, Henry TB, Holbrook RD, Chen KL. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011;45(23):9837–9856. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- 3.Schlagenhauf L, Buerki-Thurnherr T, Kuo YY, Wichser A, Nuesch F, Wick P, Wang J. Carbon nanotubes released from an epoxy-based nanocomposite: quantification and particle toxicity. Environ. Sci. Technol. 2015;49(17):10616–10623. doi: 10.1021/acs.est.5b02750. [DOI] [PubMed] [Google Scholar]
- 4.Sarma SJ, Bhattacharya I, Brar SK, Tyagi RD, Surampalli RY. Carbon nanotube-bioaccumulation and recent advances in environmental monitoring. Crit. Rev. Env. Sci. Tec. 2015;45(9):905–938. [Google Scholar]
- 5.Holden PA, Gardea-Torresdey JL, Klaessig F, Turco RF, Mortimer M, Hund-Rinke K, Cohen Hubal EA, Avery D, Barcelo D, Behra R, Cohen Y, Deydier-Stephan L, Ferguson PL, Fernandes TF, Herr Harthorn B, Henderson WM, Hoke RA, Hristozov D, Johnston JM, Kane AB, Kapustka L, Keller AA, Lenihan HS, Lovell W, Murphy CJ, Nisbet RM, Petersen EJ, Salinas ER, Scheringer M, Sharma M, Speed DE, Sultan Y, Westerhoff P, White JC, Wiesner MR, Wong EM, Xing B, Steele Horan M, Godwin HA, Nel AE. Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Ecological Hazards of Engineered Nanomaterials. Environ. Sci. Technol. 2016;50(12):6124–6145. doi: 10.1021/acs.est.6b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132–147. doi: 10.1016/j.envint.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Jackson P, Jacobsen NR, Baun A, Birkedal R, Kuhnel D, Jensen KA, Vogel U, Wallin H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013;7 doi: 10.1186/1752-153X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, Ho KT, Chandler GT, Ferguson PL. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ. Toxicol. Chem. 2013;32(6):1270–1277. doi: 10.1002/etc.2174. [DOI] [PubMed] [Google Scholar]
- 9.Parks AN, Burgess RM, Ho KT, Ferguson PL. On the likelihood of single-walled carbon nanotubes causing adverse marine ecological effects. Integr. Environ. Asses. 2014;10(3):472–474. [Google Scholar]
- 10.Petersen EJ, Huang QG, Weber WJ. Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida. Environ. Sci. Technol. 2008;42(8):3090–3095. doi: 10.1021/es071366f. [DOI] [PubMed] [Google Scholar]
- 11.Schierz A, Espinasse B, Wiesner MR, Bisesi JH, Sabo-Attwood T, Ferguson PL. Fate of single walled carbon nanotubes in wetland ecosystems. Environ.-Sci. Nano. 2014;1(6):574–583. [Google Scholar]
- 12.Petersen EJ, Huang QG, Weber WJ. Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus. Environ. Health. Persp. 2008;116(4):496–500. doi: 10.1289/ehp.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen EJ, Huang QG, Weber WJ. Relevance of octanol-water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes. Environ. Toxicol. Chem. 2010;29(5):1106–1112. doi: 10.1002/etc.149. [DOI] [PubMed] [Google Scholar]
- 14.Petersen EJ, Akkanen J, Kukkonen JVK, Weber WJ., Jr Biological uptake and depuration of carbon nano-tubes by Daphnia magna. Environ. Sci. Technol. 2009;43(8):2969–2975. doi: 10.1021/es8029363. [DOI] [PubMed] [Google Scholar]
- 15.Petersen EJ, Pinto RA, Mai DJ, Landrum PF, Weber WJ. Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna. Environ. Sci. Technol. 2011;45(3):1133–1138. doi: 10.1021/es1030239. [DOI] [PubMed] [Google Scholar]
- 16.Rhiem S, Riding MJ, Baumgartner W, Martin FL, Semple KT, Jones KC, Schaffer A, Maes HM. Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells. Environ. Pollut. 2015;196:431–439. doi: 10.1016/j.envpol.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Maes HM, Stibany F, Giefers S, Daniels B, Deutschmann B, Baumgartner W, Schaffer A. Accumulation and distribution of multiwalled carbon nanotubes in zebrafish (Danio rerio) Environ. Sci. Technol. 2014;48(20):12256–12264. doi: 10.1021/es503006v. [DOI] [PubMed] [Google Scholar]
- 18.Holden PA, Klaessig F, Turco RF, Priester JH, Rico CM, Avila-Arias H, Mortimer M, Pacpaco K, Gardea-Torresdey JL. Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: Are they relevant? Environ. Sci. Technol. 2014;48(18):10541–10551. doi: 10.1021/es502440s. [DOI] [PubMed] [Google Scholar]
- 19.Petersen EJ, Flores-Cervantes DX, Bucheli TD, Elliott LCC, Fagan JA, Gogos A, Hanna S, Kagi R, Mansfield E, Bustos ARM, Plata DL, Reipa V, Westerhoff P, Winchester MR. Quantification of carbon nanotubes in environmental matrices: current capabilities, case studies, and future prospects. Environ. Sci. Technol. 2016;50(9):4587–4605. doi: 10.1021/acs.est.5b05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JLRS, Reed RB, Fairbrother DH, Ranville JF. Analysis of single-walled carbon nanotubes using spICP-MS with microsecond dwell time. NanoImpact. 2016;1:65–72. [Google Scholar]
- 21.Zhang L, Petersen EJ, Huang Q. Phase distribution of C-14-labeled multiwalled carbon nanotubes in aqueous systems containing model solids: Peat. Environ. Sci. Technol. 2011;45(4):1356–1362. doi: 10.1021/es1026097. [DOI] [PubMed] [Google Scholar]
- 22.Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment - issues and recommendations. Nanotoxicology. 2011;5(4):711–729. doi: 10.3109/17435390.2010.528846. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LW, Petersen EJ, Zhang W, Chen YS, Cabrera M, Huang QG. Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water. Environmental Pollution. 2012;166:75–81. doi: 10.1016/j.envpol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Priester JH, Stoimenov PK, Mielke RE, Webb SM, Ehrhardt C, Zhang JP, Stucky GD, Holden PA. Effects of soluble cadmium salts versus CdSe quantum dots on the growth of planktonic Pseudomonas aeruginosa. Environ. Sci. Technol. 2009;43(7):2589–2594. doi: 10.1021/es802806n. [DOI] [PubMed] [Google Scholar]
- 25.Horst AM, Neal AC, Mielke RE, Sislian PR, Suh WH, Madler L, Stucky GD, Holden PA. Dispersion of TiO2 nanoparticle agglomerates by Pseudomonas aeruginosa. Appl. Environ. Microb. 2010;76(21):7292–7298. doi: 10.1128/AEM.00324-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werlin R, Priester JH, Mielke RE, Kramer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat. Nanotechnol. 2011;6(1):65–71. doi: 10.1038/nnano.2010.251. [DOI] [PubMed] [Google Scholar]
- 27.Mielke RE, Priester JH, Werlin RA, Gelb J, Horst AM, Orias E, Holden PA. Differential growth of and nanoscale TiO2 accumulation in Tetrahymena thermophila by direct feeding versus trophic transfer from Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2013;79(18):5616–5624. doi: 10.1128/AEM.01680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowakowska J, Griesser HJ, Textor M, Landmann R, Khanna N. Antimicrobial properties of 8-hydroxyserrulat-14-en-19-oic acid for treatment of implant-associated infections. Antimicrob. Agents. Ch. 2013;57(1):333–342. doi: 10.1128/AAC.01735-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sample preparation for LSC. http://www.perkinelmer.com/Resources/TechnicalResources/ApplicationSupportKnowledgebase/radiometric/sample_prep.xhtml. [Google Scholar]
- 30.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of C-14 via accelerator mass spectrometry. Anal. Chem. 2003;75(9):2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 31.Vogel JS, Love AH. Quantitating isotopic molecular labels with accelerator mass spectrometry. Method. Enzymol. 2005;402:402–422. doi: 10.1016/S0076-6879(05)02013-6. [DOI] [PubMed] [Google Scholar]
- 32.Kang S, Mauter MS, Elimelech M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008;42(19):7528–7534. doi: 10.1021/es8010173. [DOI] [PubMed] [Google Scholar]
- 33.Arias LR, Yang LJ. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir. 2009;25(5):3003–3012. doi: 10.1021/la802769m. [DOI] [PubMed] [Google Scholar]
- 34.Adeleye AS, Keller AA. Long-term colloidal stability and metal leaching of single wall carbon nanotubes: Effect of temperature and extracellular polymeric substances. Water Res. 2014;49:236–250. doi: 10.1016/j.watres.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 35.Verneuil L, Silvestre J, Mouchet F, Flahaut E, Boutonnet JC, Bourdiol F, Bortolamiol T, Baque D, Gauthier L, Pinelli E. Multi-walled carbon nanotubes, natural organic matter, and the benthic diatom Nitzschia palea : "A sticky story". Nanotoxicology. 2015;9(2):219–229. doi: 10.3109/17435390.2014.918202. [DOI] [PubMed] [Google Scholar]
- 36.Luongo LA, Zhang XQ. Toxicity of carbon nanotubes to the activated sludge process. J. Hazard. Mater. 2010;178(1–3):356–362. doi: 10.1016/j.jhazmat.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 37.Simon-Deckers A, Loo S, Mayne-L'Hermite M, Herlin-Boime N, Menguy N, Reynaud C, Gouget B, Carriere M. Size-, Composition- and Shape-Dependent Toxicological Impact of Metal Oxide Nanoparticles and Carbon Nanotubes toward Bacteria. Environmental Science & Technology. 2009;43(21):8423–8429. doi: 10.1021/es9016975. [DOI] [PubMed] [Google Scholar]
- 38.Umbuzeiro GA, Coluci VR, Honorio JG, Giro R, Morales DA, Lage ASG, Mazzei JL, Felzenszwalb I, Souza AG, Stefani D, Alves OL. Understanding the interaction of multi-walled carbon nanotubes with mutagenic: organic pollutants using computational modeling and biological experiments. Trac-Trend Anal Chem. 2011;30(3):437–446. [Google Scholar]
- 39.Ghafari P, St-Denis CH, Power ME, Jin X, Tsou V, Mandal HS, Bols NC, Tang XW. Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nat. Nanotechnol. 2008;3(6):347–351. doi: 10.1038/nnano.2008.109. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Ran TC, Li YG, Guo JX, Li WX. Dependence of the cytotoxicity of multi-walled carbon nanotubes on the culture medium. Nanotechnology. 2006;17(18):4668–4674. doi: 10.1088/0957-4484/17/18/024. [DOI] [PubMed] [Google Scholar]
- 41.Chan TSY, Nasser F, St-Denis CH, Mandal HS, Ghafari P, Hadjout-Rabi N, Bols NC, Tang X. Carbon nanotube compared with carbon black: effects on bacterial survival against grazing by ciliates and antimicrobial treatments. Nanotoxicology. 2013;7(3):251–258. doi: 10.3109/17435390.2011.652205. [DOI] [PubMed] [Google Scholar]
- 42.Organisation for Economic Co-operation and Development. OECD Guidelines for the Testing of Chemicals. Bioaccumulation in Fish: Aqueous and Dietary Exposure. Paris: 2012. [Google Scholar]
- 43.Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, Hanna SK, Hartmann NB, Hund-Rinke K, Mader B, Manier N, Pandard P, Salinas ER, Sayre P. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: Key issues and consensus recommendations. Environ. Sci. Technol. 2015;49(16):9532–9547. doi: 10.1021/acs.est.5b00997. [DOI] [PubMed] [Google Scholar]
- 44.Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines. Paris: Organisation for Economic Co-operation and Development; 2014. [03/05/2014]. pp. 1–84. [Google Scholar]
- 45.Chen QQ, Yin DQ, Li J, Hu XL. The effects of humic acid on the uptake and depuration of fullerene aqueous suspensions in two aquatic organisms. Environ. Toxicol. Chem. 2014;33(5):1090–1097. doi: 10.1002/etc.2539. [DOI] [PubMed] [Google Scholar]
- 46.Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JVK. Analysis of fullerene-C-60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ. Toxicol. Chem. 2010;29(5):1072–1078. doi: 10.1002/etc.124. [DOI] [PubMed] [Google Scholar]
- 47.Bour A, Mouchet F, Silvestre J, Gauthier L, Pinelli E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J. Hazard. Mater. 2015;283:764–777. doi: 10.1016/j.jhazmat.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 48.Holbrook RD, Murphy KE, Morrow JB, Cole KD. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat Nanotechnol. 2008;3(6):352–355. doi: 10.1038/nnano.2008.110. [DOI] [PubMed] [Google Scholar]
- 49.Unrine JM, Shoults-Wilson WA, Zhurbich O, Bertsch PM, Tsyusko OV. Trophic transfer of Au nanoparticles from soil along a simulated terrestrial food chain. Environ. Sci. Technol. 2012;46(17):9753–9760. doi: 10.1021/es3025325. [DOI] [PubMed] [Google Scholar]
- 50.De la Torre Roche R, Servin A, Hawthorne J, Xing BS, Newman LA, Ma XM, Chen GC, White JC. Terrestrial trophic transfer of bulk and nanoparticle La2O3 does not depend on particle size. Environ. Sci. Technol. 2015;49(19):11866–11874. doi: 10.1021/acs.est.5b02583. [DOI] [PubMed] [Google Scholar]
- 51.Judy JD, Unrine JM, Bertsch PM. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 2011;45(2):776–781. doi: 10.1021/es103031a. [DOI] [PubMed] [Google Scholar]
- 52.Chen JY, Li HR, Han XQ, Wei XZ. Transmission and accumulation of nano-TiO2 in a 2-step food chain (Scenedesmus obliquus to Daphnia magna) B. Environ. Contam. Tox. 2015;95(2):145–149. doi: 10.1007/s00128-015-1580-y. [DOI] [PubMed] [Google Scholar]
- 53.Hou WC, Westerhoff P, Posner JD. Biological accumulation of engineered nanomaterials: a review of current knowledge. Environ. Sci.-Proc. Imp. 2013;15(1):103–122. doi: 10.1039/c2em30686g. [DOI] [PubMed] [Google Scholar]
- 54.Arnot JA, Gobas FAPC. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006;14(4):257–297. [Google Scholar]
- 55.Edgington AJ, Petersen EJ, Herzing AA, Podila R, Rao A, Klaine SJ. Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna. Nanotoxicology. 2014;8:2–10. doi: 10.3109/17435390.2013.847504. [DOI] [PubMed] [Google Scholar]
- 56.Edgington AJ, Roberts AP, Taylor LM, Alloy MM, Reppert J, Rao AM, Mao JD, Klaine SJ. The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes. Environ. Toxicol. Chem. 2010;29(11):2511–2518. doi: 10.1002/etc.309. [DOI] [PubMed] [Google Scholar]
- 57.Larguinho M, Correia D, Diniz MS, Baptista PV. Evidence of one-way flow bioaccumulation of gold nanoparticles across two trophic levels. J. Nanopart. Res. 2014;16:2549(8) [Google Scholar]
- 58.Zhu XS, Wang JX, Zhang XZ, Chang Y, Chen YS. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere. 2010;79(9):928–933. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Conway JR, Hanna SK, Lenihan HS, Keller AA. Effects and implications of trophic transfer and accumulation of CeO2 nanoparticles in a marine mussel. Environ. Sci. Technol. 2014;48(3):1517–1524. doi: 10.1021/es404549u. [DOI] [PubMed] [Google Scholar]
- 60.Croteau MN, Misra SK, Luoma SN, Valsami-Jones E. Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures. Environ. Sci. Technol. 2014;48(18):10929–10937. doi: 10.1021/es5018703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.