Abstract
Natural antibody (NAb) levels and survival rates were evaluated in 4 breeds of laying hens in Ethiopia: indigenous, improved indigenous, exotic layer, and crossbred. Titers of NAb isotypes IgG and IgM binding keyhole limpet hemocyanin (KLH) in serum were measured at 20, 26, 35, and 45 wk age. Repeated-measure ANOVA showed that IgG and IgM levels vary with time within each breed (P < 0.05). Indigenous chickens had significantly (P < 0.05) higher NAb levels at all ages. The Cox proportional hazard analysis showed increased hazard with increased levels of NAbs in the exotic layers (P < 0.05). However, the reduced hazards with increased levels of NAbs were not significant in the improved indigenous and crossbred chickens. Indigenous chickens showed increased hazard with increasing levels of NAb (P > 0.05). We concluded that not only the NAb levels but also the effect of Nabs on survival vary between indigenous and improved breeds. The results indicate that NAb levels are associated with survival in elite (improved) breeds, but are associated with increased hazard in indigenous chickens.
Keywords: confinement, indigenous chickens, natural antibody, survival
INTRODUCTION
Indigenous chickens are abundant in village poultry production systems. They have managed to survive and produce under harsh environments where they scavenge for feed. Indigenous chickens are less suitable for confined management systems primarily due to their poor genetic potential for growth and egg production. Raising indigenous chickens in confinement in Ethiopia resulted in high morbidities and mortalities (Duguma et al., 2005; Demeke, 2003). Improving the health management may decrease the mortality and further improve the survival of indigenous chickens in confinement as shown by improved survival of indigenous chickens after applying vaccination for Marek's disease (Duguma et al., 2006).
Innate immunity plays an important role in survival of organisms, although additionally acquired immunity is often required in vertebrates (Beutler, 2004). Natural antibodies (NAb) are found in healthy unimmunized individuals and are an important part of the first line of defense in animals by providing early resistance against infection (Ochsenbein and Zinkernagel, 2000). Low levels of innate immunity may be related with enhanced disease susceptibility and high levels with disease resistance. NAbs are thought to participate in the maintenance of immune homeostasis by exposure to environmental stimulations (Coutinho et al., 1995). In modern housing systems the (opposing) relationships between NAb at early age and survival has been reported for commercial (elite) layer breeds (Star et al., 2007; Sun et al., 2011). However, NAb levels and their association with survival in noncommercial chicken breeds have not been investigated. The aim of the present study is to compare the NAb levels and survival rates in indigenous, improved indigenous, exotic, and crossbred laying hens under confinement in Ethiopia. In addition, the phenotypic relation between NAb and survival was studied.
MATERIALS AND METHODS
Chickens, Housing, and Feed
A total of 480 laying hens from 4 breeds were included in this study: indigenous, improved indigenous, exotic, and crossbred. The indigenous chickens were hatched from eggs collected from Horro region in Ethiopia. The improved indigenous chickens were produced by parents selected from the 7 generations of a selection experiment with Horro chickens on egg production and growth [for details of the experiment see Dana et al. (2011)]. The exotic chickens were commercial brown egg layers which were obtained as fertile eggs from ISA, a Hendrix genetics company (The Netherlands). The crossbred chicks were produced at Debre Zeit Agricultural Research Center poultry research farm by crossing cocks from exotic Rhode Island Red (RIR) strain, which were imported as 1-day-old chickens from ISA, a Hendrix genetics company (The Netherlands) with hens from seventh-generation improved indigenous breed. Both exotic RIR males and hatching egg of exotic layers were imported from the same company a few months apart and their genetic relationship was not known. Eggs of the 4 breeds were hatched at Debre Zeit Agricultural Research Center the same day at the same hatchery. Chickens were provided with a chick feed (20% CP and 2,950 kcal/kg) until 8 wk age, and grower ration (18% CP and 2,850 kcal/kg) from 8 to 18 wks. From 18 wks onwards the birds were provided with ad libitum layer (16% CP and 2,750 kcal/kg) feed. The chickens were kept in open house filled with teff straw concrete floor and deep litter up to 45 wk age. Standard density of 8 and 6 birds/m2 were used during the rearing and laying period, respectively. Twenty-two h to 23 h light was provided during the first 3 d and 10 h light afterwards until Week 8. Natural lighting was used after Week 8 as the day length was more or less the same in Ethiopia during the study period. Infrared lamp of 250 W was used to provide heat. The temperature during the first 3 d was 28 to 30°C and was reduced to 23°C in 4 wks. Afterwards 23 to 25°C temperature was provided throughout the study period. Temperature was monitored using thermometer and ventilation was adjusted by opening the curtains. After 18 wk age approximately 150 pullets from each breed were picked and transferred to the layer house and reared in pens partitioned with mesh wire. The 4 breeds were randomly assigned into twelve pens with a size of 7 m2 in a replication of 3. At 20 wk age 120 pullets of each breed were used for blood sampling. Blood sampling was conducted at 20, 26, 35, and 45 wks age on these birds. All chickens were housed in the same house and managed by one person to minimize environmental variation. All chickens were vaccinated against Newcastle disease (HB1 strain at Day 1 and Lasota at Day 21), Marek's disease (Day 1), Gumboro (Day 7), and fowl pox (Week 14). The description of the experimental chickens is shown in Table 1.
Table 1.
Number of chickens used for blood collection at the start of the experiment (Week 20); average survival of chickens in days (±SD); proportion of survival until end of Week 45 (%) and mean survival time (days) of surviving birds.
| Breed | No. at Week 20 (n) | Mean survival (d ± SD) | Proportion of survival until the end of Week 45 (%) |
|---|---|---|---|
| Indigenous Horro | 120 | 290 (9) | 38 |
| Improved Horro | 120 | 307 (5) | 87 |
| Exotic layer | 120 | 311 (4) | 95 |
| Crossbred | 120 | 309 (4) | 90 |
Experimental Design
The chickens were followed from 20 to 45 wks age. Blood samples from 120 layers of each breed were taken from the wing vein at 20, 26, 35, and 45 wk sage. Collected blood was clotted and serum was separated by gravity. Collected sera were stored at −20°C for ELISA. NAb binding Keyhole limpet hemocyanin (KLH) were determined at 20, 26, 35 and 45 wks age. KLH is a large cooper-containing protein found in the hemolymph of the sea mollusk Megathura crenulata. Antibodies in chickens to this protein are regarded as ‘natural’ as birds are unlikely to encounter KLH.
NAb Isotypes: IgG and IgM
Titers of NAb isotypes IgM and IgG binding KLH were determined in individual serum samples by an indirect ELISA as follows (Sun et al., 2011). Flat-bottomed 96-well medium binding ELISA plates were coated with 100 μL coating buffer (pH 9.6) containing KLH (2 μg/mL, MP Biomedicals Inc., Aurora, OH), and incubated at 4°C overnight. Duplicate standard positive serum samples were stepwise diluted in Columns 11 and 12 per plate, respectively. After subsequent washing the plates were filled with 100 μL PBS containing Tween 20 (0.05%) and horse serum (0.5%) per well. Serum samples were stepwise 3-fold diluted starting at 1:30, and the plates were incubated during 1 h at room temperature (25°C). After washing, plates were incubated with 1:20,000 diluted rabbit-antichicken IgM labeled with peroxidase, or 1: 40,000 diluted rabbit-anti-chicken IgG-Fc, respectively (Bethyl Laboratories, TX), and incubated 1.5 h at room temperature (25°C). After washing, binding of isotype specific antibodies in the serum sample to KLH was visualized by adding 100 μL substrate (70 μg/mL tetramethylbenzidine and 0.05% H2O2). After 10 min, the reaction was stopped with 50 μL 2.5 N H2SO4 solution. Extinctions were measured with a Multiskan (Labsystems, Helsinki, Finland) at wavelength of 450 nm. Titers were calculated based on log 2 values of the dilutions that gave extinction closest to 50% of EMAX, where EMAX represents the mean of the highest extinction of the standard positive serum present on each plate.
Survival: Hazard Analysis
Survival analysis was conducted to establish the relation between natural antibody levels binding KLH and survival. The time to die or survival time (time until death) were used during the follow-up period of Weeks 20 to 45. Chickens that were alive at the end of the each period were treated as censored because information about their survival time was incomplete as mortality was not observed in the chickens until the end of the period. Postmortem study was done on gross abnormalities in major organs including spleen, liver, gizzard, intestines, and caeca (abnormal size, shape, and color). The findings did not indicate that mortalities were due to apparent disease and further investigation was not conducted. Survival time and probability of being dead or alive were used to plot survival trend using the Kaplan–Meier function. The levels of NAb (IgG and IgM) in individual chickens and the survival days were used to conduct survival analysis using a Cox proportional hazard model. The IgG and IgM level of Week 20 was used as explanatory variables. Survival is commonly characterized by a hazard function that represents the instantaneous death rate for an individual surviving to a particular time point (Allison, 1997). The hazard function for individual i at time t, hi(t), can be described using a proportional hazards model with k explanatory variables (xi1, xik):
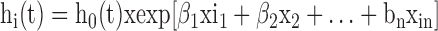 |
where h0(t) denotes an unspecified baseline hazard function and β1,. .., βk are the regression coefficients associated with the n explanatory variables. The baseline hazard function is an arbitrary function common to all observations. The hazard ratio, h(t)/h0(t), provides an estimate of the risk per unit change in the explanatory variables (NAb levels) relative to the baseline hazard function Collet (1994) and Allison (1997) and associated standard errors are approximated as described by Collet (1994). The Cox proportional hazard is one of the most commonly used approaches to model hazard functions (Collet, 1994; Allison, 1997). In the Cox proportional hazards model, the baseline hazard function is unspecified and no assumptions regarding the particular form of this function are required.
Statistical Analysis
A one-way ANOVA was performed to compare the NAb isotype levels binding KLH between breeds at each time point using SAS 9.1.2 (SAS Institute, 2004). Mean differences among breeds was tested with a multiple comparison test (Bonferroni Test). Further repeated measure analysis of variance with 2 factors [breed, and weeks (time) as repeated factor] was conducted to show the between and within subject interactions with time (Table 3).
Table 3.
Statistical result of a repeated measure1 ANOVA with breed and time as factors.
| Effect | SS2 | df | MS | F | P |
|---|---|---|---|---|---|
| Between group | |||||
| Breed | 1,762.4 | 3 | 587.5 | 119.3 | <0.0001 |
| Error | 2,344.8 | 476 | 4.9 | ||
| Within group | |||||
| Time | 2,488.7 | 3 | 829.6 | 295.4 | <0.0001 |
| Time×breed | 114.1 | 9 | 12.7 | 4.5 | <0.0001 |
| Error (time) | 4,010.7 | 1,428 | 2.8 | ||
1NAb levels were measured on the same chickens of each breed at Weeks 20, 25, 35, and 45. Between-groups comparison showed significant effect of breed on the NAb levels. Within-group comparison showed significant effect of time on NAb levels, and the interaction of time with breeds showed that breed had different effect on NAb levels depending on time at (P < 0.0001).
2SS = sum of squares; MS = mean square; F = mean F-value.
The Kaplan–Meier survivor function and cox proportional hazard regression analysis were conducted using STATA 11.1 (StataCorp, 2009). The data were first declared into a survival-time data using survival days as time variable and the probability to die or alive as failure. P < 0.05 was regarded as significant.
RESULTS
Natural Antibody Isotypes in the Various Breeds
Results of analysis of variance of IgG and IgM levels binding KLH in serum in different breeds are presented in Table 2. The average NAb levels of IgG and IgM binding KLH in all breeds was lowest at Week 20, peaked in Week 35 with the exception of IgM level in indigenous chickens. The NAb levels were higher in indigenous chicken than in the other breeds at the different times (Table 2). At most times the difference was significant (P < 0.05) except for difference with the crossbreds in IgG at Week 45 and IgM at Week 35. NAb levels were measured on the same chickens of each breed at Weeks 20, 25, 35, and 45. The repeated-measure ANOVA showed significant (P < 0.001) interactions between breed and time (Table 3).
Table 2.
One-way ANOVA of the least-squares mean (±SE) levels of IgG and IgM antibodies binding KLH in sera from the 4 breeds studied at Weeks 20, 26, 35, and 45.1
| Item | Week | |||
|---|---|---|---|---|
| 20 | 26 | 35 | 45 | |
| IgG titer | ||||
| Indigenous Horro | 5.3 (0.16)a | 7.8 (0.19)a | 8.0 (0.26)a | 7.2 (0.24)a |
| Exotic layer | 3.1 (0.10)b | 5.8 (0.13)b | 6.6 (0.16)b | 6.4 (0.15)a |
| Crossbred | 3.1 (0.10)b | 5.4 (0.1)b | 5.9 (0.17)b,c | 5.7 (0.18)b |
| Improved Horro | 2.8 (0.10)b | 4.7 (0.12)c | 5.4 (0.18)c | 4.9 (0.19)c |
| IgM titer | ||||
| Indigenous Horro | 6.6 (0.11)a | 8.9 (0.19)a | 8.4 (0.28)a | 8.5 (0.27)a |
| Exotic layer | 5.0 (0.10)b | 7.6 (0.10)b | 7.9 (0.19)a,b | 7.7 (0.18)b |
| Crossbred | 4.9 (0.10)b | 6.8 (0.11)c | 7.4 (0.18)b,c | 6.8 (0.19)c |
| Improved Horro | 4.8 (0.10)d | 6.7 (0.11)c | 7.0 (0.20)c | 6.2 (0.21)c |
1IgG and IgM levels of the breeds were compared among each other at each time point separately.
a–dMeans with different superscripts indicate significant difference between breeds (P < 0.05).
Survival Analysis
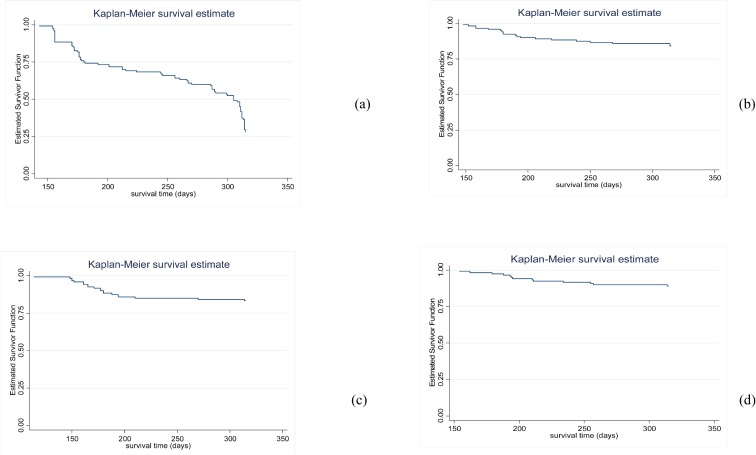
The mean survival patterns resulting from survival analysis (Cox proportional hazards model) in the 4 breeds over the follow-up period (Weeks 20 to 45) are presented in Figure 1. Each time point of the estimated survival function represents the probability that a layer of 20 wks will survive to that given point in time. Marked decrease in survival probabilities were observed for indigenous chickens (Figure 1a). The estimated hazard ratios for NAb levels measured at 20 wk age are presented in Table 4. Generally, the hazard (risk of death) was slightly higher than 1 for both NAb levels in indigenous chickens but not significantly different from 1 (P > 0.05). The hazard decreased with increasing level of IgG and IgM binding KLH in other the breeds (improved indigenous, exotic layer, and crossbred). Only in exotic layer the hazard ratio was significantly lower than 1 (P < 0.05).
Figure 1.
Kaplan–Meier estimated survivor functions for (a) unimproved Horro, (b) improved Horro, (c) crossbred and (d) exotic layer. Each time point of the estimated survival function represents the probability that a layer of 20 wks will survive to that given point in time.
Table 4.
Hazard ratios1 and (± SE) showing the risk of mortality during the follow-up period of 25 wks estimated using increasing titers of IgG and IgM binding KLH at Week 20.
| Effect | Weeks 20 to 45 (140 to 315 d) | P value |
|---|---|---|
| IgG-unimproved Horro | 1.05 (0.06) | 0.505 |
| IgG-improved Horro | 0.70 (0.15) | 0.106 |
| IgG-exotic layer | 0.59 (0.15)2 | 0.040 |
| IgG-crossbred | 0.74 (0.10) | 0.142 |
| IgM-unimproved Horro | 1.05 (0.09) | 0.495 |
| IgM-improved Horro | 0.76 (0.15) | 0.170 |
| IgM-exotic layer | 0.52 (0.12)2 | 0.006 |
| IgM-crossbred | 0.79 (0.16) | 0.264 |
1The hazard ratio explained as the chance of death of chickens when they show high NAb divided by the chance of their death when their NAb was low (measured on Week 20). A ratio of 1 means the ratio of the chances is the same, and the effect of high and low level NAb on hazard is the same. If hazard ratio is more than 1, the numerator is bigger and the hazard is higher. If the numerator is smaller, the hazard ratio will be less than 1 and the hazard is lower.
2Hazard ratio significantly different from 1 (P < 0.05).
DISCUSSION
Natural Antibodies of the IgM and IgG Isotypes
Natural antibody isotypes binding KLH were found in chickens that were not immunized previously with KLH (Parmentier et al., 2004; Star et al., 2007; Sun et al., 2011). Higher NAb levels were associated with high immune responsiveness (Parmentier et al., 2004). In the present study indigenous chickens showed higher NAb levels of both isotypes (Table 2). The lowest NAb level was found in improved indigenous birds, i.e., birds from an indigenous line that was selected for enhanced egg production and growth for 7 generations. This improved indigenous chicken line was started from Horro chickens collected on local farms and is genetically closest to the indigenous chicken. The breeding program resulted in a substantial increase in egg production and growth rate (results not shown). However, there needs to be a study to verify the genetic correlation between NAb and production traits in indigenous chickens and the consequences of selection on productivity and adaptation to confinement.
Indigenous chickens managed to survive stress for generations without proper nutrition and vaccination (Udo, 1997). The higher NAb levels might explain the ability of indigenous chickens to survive disease pressure in the village poultry production systems.
In all breeds, NAb levels were lowest at young age, peaked at 35 wks, and decreased thereafter with age (Table 2). Earlier studies reported mixed effects of ageing. Increasing NAb titers were found with aging of birds by Parmentier et al. (2004) and Star et al. (2007) and decreasing level of NAb with age (Sun et al., 2011). Many studies on the effect of aging on immunity were done in rodent models. These studies reported deficiencies of cellular and humoral immunity with age (Gahring and Weigle, 1990; Frasca et al., 2005; Haynes and Eaton, 2005). However, these studies did not consider NAbs.
Survival Analysis
To study mortality associated with NAb level, both logistic regression and Cox proportional hazard analysis can be used (Southey et al., 2001). However, treating mortality as binary trait portrays deaths as having occurred during a defined period of time, and ignores the continuity of the mortality process and the time of death (Allison, 1997). Similar estimates of the explanatory variables could be obtained from both the survival analysis and logistic analysis, but the survival analysis has lower standard errors than the logistic analysis (Southey et al., 2001). Kaplan–Meier estimated survivor functions revealed reduced probability of survival over time in all breeds. A markedly lower probability of survival was found for indigenous chickens.
The hazard analysis in the present study showed that a unit increase in the levels of both IgG and IgM titers to KLH were associated with increased hazard (risk of mortality) in indigenous chickens albeit not statistically significant. The probability that they were exposed to a certain disease is small due to the fact that all of them were kept under the roof and managed by a single person. In addition, a number of studies (Koelkebeck and Cain, 1984; Roush et al., 1984; Adams and Craig, 1985) showed that mortality increased with an increase in housing density. The primary glucocorticoid secreted by the avian adrenal gland was reported to increase in stressful conditions (Curtis et al., 1980; Siegel, 1980). Stress may result in immunosuppression but the actual mechanisms remain elusive (Schat and Skinner, 2008). The higher mortality of indigenous chickens even in the absence of apparent disease was observed and the relation between higher NAb and increased hazard in confinement may need further study. Antibody titers to human serum albumin were not affected when chickens were treated with immunosuppressive agents like glucocorticoids (El-Lethey et al., 2003). Since the level of stress hormones was not measured, we cannot confirm the link between increased levels of stress hormones and higher mortality.
Wijga et al. (2009) estimated heritabilities in brown and white layer breeds, where in lower heritabilities were found for IgG than IgM, and lower heritabilities were found in white than in brown layers (Sun et al., 2011). In improved indigenous, exotic layer and crossbred chickens, increasing levels of NAbs were associated with decreased hazard, but the effects on hazards were only statistically significant in exotic layer. Higher NAb levels in the exotic layer were associated with better survival. Our findings suggests that NAbs have a positive effect on survival in chickens that have adapted to confinement whereas that effect is not found or even reversed in indigenous birds that are not adapted to confinement. In this respect, we speculate that the effect of confinement, and as a consequence stress, i.e. no homeostasis, may also result in different levels of NAb directed to other antigens than KLH. Previously it was shown that levels of NAb are affected not only by aging, but also by antigenic challenges in different fashion (Berghof et al., 2010)
Acknowledgments
We sincerely thank Koepon foundation for funding this study and providing a scholarship for the first author.
REFERENCES
- Adams A. W., Craig J. V. Effects of crowding and cage shape on productivity and profitability of caged layers: A survey. Poult. Sci. 1985;64:238–242. [Google Scholar]
- Allison P. D. Survival Analysis Using the SAS System. A Practical Guide. Cary, NC: SAS Institute Inc.; 1997. [Google Scholar]
- Berghof T. V. L., De Vries Reilingh G., Nieuwland M. G. B., Parmentier H. K. Effect of aging and repeated intratracheal challenge on levels of cryptic and overt natural antibodies in poultry. Poult. Sci. 2010;89:227–235. doi: 10.3382/ps.2009-00449. [DOI] [PubMed] [Google Scholar]
- Beutler B. Innate immunity: An overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Collet D. Modelling Survival Data in Medical Research. London, U.K: Chapman and Hall; 1994. [Google Scholar]
- Coutinho A., Kazatchkine M. D., Avrameas S. Natural autoantibodies. Curr. Opin. Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Curtis M. J., Flack I. H., Harvey S. The effect of Escherichia coli endotoxins on the concentrations of corticosterone and growth hormone in the plasma of the domestic fowl. Res. Vet. Sci. 1980;28:123–127. [PubMed] [Google Scholar]
- Dana N., van der Waaij E. H., van Arendonk J. A. M. Genetic and phenotypic estimates for body weights and egg production in Horro chicken of Ethiopia. Trop. Anim. Health Prod. 2011;43:21–28. doi: 10.1007/s11250-010-9649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeke S. Growth performance of indigenous and White Leghorn chickens under scavenging and intensive system of management in Ethiopia. Livest. Res. Dev. 2003;15:11. [Google Scholar]
- Duguma R., Dana N., Yami A. A Marek's disease vaccination opened the door to rear indigenous chickens of Ethiopia under confined management. Intern. J. Appl. Res. Vet. Med. 2006;4:121–127. [Google Scholar]
- Duguma R., Yami A., Dana N., Hassen F., Esatu W. Marek's disease in local chicken strains of Ethiopia reared under confined management regime in central Ethiopia. Rev. Med. Vet. 2005;156:541–546. [Google Scholar]
- El-Lethey H., Huber-Eicher B., Jungi T. W. Exploration of stress-induced immunosuppression in chickens reveals both stress-resistant and stress-susceptible antigen responses. Vet. Immunol. Immunopathol. 2003;95:91–101. doi: 10.1016/s0165-2427(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Frasca D., Riley R. L., Blomberg B. B. Humoral immune response for specific antibody response. Poult. Sci. 2005;88:1805–1810. [Google Scholar]
- Gahring L. C., Weigle W. O. The effect of aging on the induction of humoral and cellular immunity and tolerance in two long-lived mouse strains. Cell. Immunol. 1990;128:142–151. doi: 10.1016/0008-8749(90)90013-h. [DOI] [PubMed] [Google Scholar]
- Haynes L., Eaton S. M. The effect of age on the cognate function of CD4+ T cells. Immunol. Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelkebeck K. W., Cain J. R. Performance, behaviour, plasma corticosterone, and economic returns of laying hens in several management alternatives. Poult. Sci. 1984;63:2123–2131. doi: 10.3382/ps.0632123. [DOI] [PubMed] [Google Scholar]
- Ochsenbein A. F., Zinkernagel R. M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Parmentier H. K., Lammers A., Hoekman J. J., Reilingh G. D., Zaanen I. T. A., Savelkoul H. F. J. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev. Comp. Immunol. 2004;28:39–49. doi: 10.1016/s0145-305x(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Roush W. B., Mashaly M. M., Graves H. B. Effect of increased bird population in a fixed cage area on production and economic responses of single comb White Leghorn laying hens. Poult. Sci. 1984;63:45–48. doi: 10.3382/ps.0630045. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS User's Guide. Cary, NC: SAS Inst. Inc.; 2004. [Google Scholar]
- Schat K. A., Skinner M. A. Avian immunosuppressive diseases and immune evasion. In: Davison F., Kaspers B., Schat K. A., editors. Avian Immunology. London, U.K: Academic Publishers; 2008. pp. 299–322. [Google Scholar]
- Siegel H. S. Physiological stress in birds. Bioscience. 1980;30:529–534. [Google Scholar]
- Southey B. R., Rodriguez-Zas S. L., Leymaster K. A. Survival analysis of lamb mortality in a terminal sire composite population. J. Anim. Sci. 2001;79:2298–2306. doi: 10.2527/2001.7992298x. [DOI] [PubMed] [Google Scholar]
- Star L., Frankena K., Kemp B., Nieuwland M. G. B., Parmentier H. K. Natural humoral immune competence and survival in layers. Poult. Sci. 2007;86:1090–1099. doi: 10.1093/ps/86.6.1090. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp; 2009. [Google Scholar]
- Sun Y., Parmentier H. K., Frankena K., van der Poel J. J. Natural antibody isotypes titers as predictors of survival in purebred laying hens. Poult. Sci. 2011;90:2263–2274. doi: 10.3382/ps.2011-01613. [DOI] [PubMed] [Google Scholar]
- Udo H. M. J. Relevance of farmyard animals to rural development. Outlook Agric. 1997;26:25–28. [Google Scholar]
- Wijga S., Parmentier H. K., Nieuwland M. G. B., Bovenhuis H. Genetic parameters for levels of natural antibodies in chicken lines divergently selected for specific antibody response. Poult. Sci. 2009;88:1805–1810. doi: 10.3382/ps.2009-00064. [DOI] [PubMed] [Google Scholar]