Abstract
Chronic myelogenous leukemia (LML) was recognized as a distinct entity in the mid-1800s. Since Nowell and Hunagerford initiated their research on CML in1960 our understanding in CML has been increasing. Imatinib became the preferred treatment from 2000 onwards as a result of its unprecedented success. The lack of structured Indian data on CML led to the formation of a CML data cansortuim which invited CML data albiet retro spartive form around the country including major cancer service providers both government and private. We provide a summary of published Indian data on CML here.
Keywords: Chronic myeloid leukemia, Indian, published data
Introduction
Chronic myelogenous leukemia (CML) was recognized as a distinct entity in the mid-1800s. The research on CML was initiated by Nowell and Hungerford in 1960. They used newly developed techniques to detect a small chromosome in metaphase preparations of marrow cells from CML patients.[1] CML accounts for 15% of cases of leukemia in the United States. There is a slight male preponderance (male-to-female ratio 1.6:1). Its annual incidence is about 1.5 cases per 100,000 individuals. This incidence has not changed over in the past few decades, and it increases with age. The median age at diagnosis is 55–60 years; it remains uncommon in children and adolescents; only 2.7% of CML cases are younger than 20 years. Incidence remains almost constant worldwide. In India, CML is the most common adult leukemia.[2,3]
In the 1990s, the recommended approach to manage a newly diagnosed patient with CML-chronic phase (CP) was allogeneic stem cell transplantation, especially if the patient was relatively young and had suitable donor; for other patients’ interferon-alpha with or without cytarabine was recommended. From 2000 onward imatinib (IM) at 400 mg daily became the preferred initial treatment[4] and this practice received substantial support from the interim results of the immediate risk-stratification improves survival study published in 2003;[5] the 7-year update for patients who received IM as first-line treatment showed an actuarial overall survival (OS) of 86% and also showed that responding patients whose disease had not progressed in any way in their first 3 years of the study were extremely unlikely to relapse at a later stage and also unlikely to suffer from any late onset side effects. However, the 7-year update also showed that only 57% of the original patient cohort was still in continuing complete cytogenetic response (CCgR) taking IM on study according to the original protocol.[6]
The unprecedented success both in terms of efficacy, convenience, and safety made IM a standard of care, offering initial treatment by transplantation only for the rare patient with a genetically identical twin because of the extremely low risk of transplant-related mortality with syngeneic donors. Until recently, some investigators recommended an initial allograft for younger patients with a high probability of surviving the transplant based on the European scoring system,[7] but even this approach has now fallen out of favor.
The lack of structured Indian data on CML led to the formation of a CML data consortium which invited CML data albeit retrospective from around the country including major cancer service providers both government and private. The concerted effort led to the meeting which was conceived by Indian Cooperative Oncology Network in 2010. In the meeting, 8115 patients’ data were presented, and 18 centers submitted their manuscripts comprising 6677 patients. This data represent large series of patients from all over the country treated on day-to-day clinical practice with several limitations and thence, presents the actual real world outcomes of CML patients in India.
Patient Characteristics
Median age: Median age at presentation in India is a decade younger compared with the age presented in European (median age 55 years) as well as in American (median age 66 years) literature.[8,9] The median age of the population varied from minimum 32 years (Nizam Institute of Medical Sciences [NIMS], Hyderabad, South India) to maximum 42 years (Ashirwad Center, Mumbai, Southwest India).[10,11] This decade younger population was the most consistent fact presented in almost all studies in India. The reason for this early presentation remains elusive
Incidence: As stated in various cancer registries, CML is one of the most common adult leukemias in Indian population accounting for 30% to 60% of all adult leukemias[12] The data presented at CML meeting showed that the incidence of CML cases varied from 70% of all leukemia cases at Indira Gandhi Institute of Medical Sciences (IGIMS), Regional Cancer Centre (RCC), Patna, Bihar, India to 16.6% Gujarat Cancer and Research Institute (GCRI), Gujarat, India.[13,14] This difference in the incidence of CML cases at two different centers is likely due to the fact that these are not population-based registries, and it is accountable to different cancer populations they cater to and referral bias
Sex ratio: There is a male preponderance. The male to female sex ratio varied from 1:08 (Sterling, Gujarat, India) to 3:1 (Tata Memorial Hospital [TMH], Mumbai, Maharashtra, India)[15,16]
Symptoms at presentation: The most common symptom was splenomegaly ranging from 100% (WIA, Chennai, Tamil Nadu, India) to 81% (IGIMS, Patna, Bihar, India) followed by hepatomegaly, fatigue, weakness, dragging pain, pallor, or sometimes asymptomatic seen in 30% cases (HealthCare Global, Bangalore Institute of Oncology, Bengaluru).[13,14,17] No organomegaly was seen in 5.4% patients (Institute of Haematology and Transfusion Medicine, Kolkata, West Bengal, India).[18] In comparison to Western data where approximately 40% of patients are asymptomatic and diagnosed on the basis of abnormal counts, majority of Indian patients are symptomatic and mostly present with dull aching pain in the left hypochondriac region secondary to splenomegaly[7]
Blood counts at presentation: The median hemoglobin ranged from 9 g/dl (IGIMS, Patna, Bihar, India) to 11 g/dl (All India Institute of Medical Sciences [AIIMS], New Delhi, India);[13,19] the median white blood cell count ranged from 0.46 × 109/cumm (Rajiv Gandhi Cancer Institute, New Delhi, India) to 1.86 × 109/cumm (WIA, Chennai, Tamil Nadu, India).[20,21] CML is a myeloproliferative disease, but it can present with as low counts as 0.18 × 109/cumm (Postgraduate Institute [PGI], Chandigarh, India)[22]
CML phase at presentation: The percentage of patients presenting in CP varied from 85% (PGI, Chandigarh, India) to 97% (IGIMS, Patna, Bihar, India) with a median of 89.5%, whereas in European data, the presentation of CML in CP has been reported to be as high as 96.8%[8,13,22]
Sokal risk category: The Sokal risk category data being retrospective are not complete. However, few centers who presented their data with risk stratification, it appears the majority of patients are in Intermediate risk category ranging from 27% to 47%, whereas low-risk category range from 25% to 55% and high-risk category range from 12% to 28% of patients.[16]
Treatment and Monitoring
The predominantly used molecule is IM mesylate, a large number comes through the good offices of Glivec International Patient Assistance Program and a similar large number on the Indian generic brands. All studies reported IM as a safe and well-tolerated drug. Notably, in the era preceding IM, the duration of therapy, response to therapy, and remission rates were short and inadequate, as well as a far more rapid rate of transformation was evidenced in the report of Mishra et al. from AIIMS. Furthermore, high drug toxicity impact on patients affected the compliance and follow-up. The starting dose of IM was uniformly 400 mg in all the studies with all the authors preferring to increase the dose of IM if inadequate response was witnessed or milestones not achieved, probably the alternatives being too expensive.
It is difficult to interpret the treatment response from various centers. As the time taken for response varies and also the treated population is quite heterogeneous. At most of the centers, there is no mention of early CP (ECP) or late CP (LCP) at presentation except TMH and few other centers from Mumbai. In many of the centers, patients were treated with hydroxyurea, interferon at first and then shifted to IM. Details can be found in the individual chapters from various centers. The primary resistance to IM in newly diagnosed patients varied from 0.1% (Omega, Hyderabad) to 3% (Asian Institute of Oncology [AIO], Raheja, Mumbai, Maharashtra, India).[23,24] AIIMS has reported two patients out of 525 patients having primary resistance.[19] The response from various centers is shown in Table 1.
Table 1.
Clinical outcomes of chronic myeloid leukemia treated with imatinib reported from Indian population
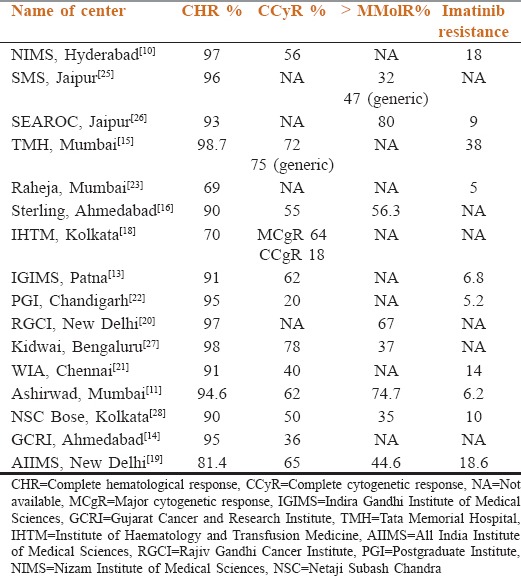
All the reports show complete hematological response (CHR) of 85–98.7% between 1 and 3 months of therapy with most managing to keep the hematological remissions for at least 2–3 years. Data on complete cytogenetic response were forthcoming in all the series, reaching approximately 77%. However, the data on molecular assessment were patchy and incomplete. This is an area which warrants improvement so as to be able to provide a standard of care to all patients. It is noteworthy that patients in lower socioeconomic (SE) class presented with higher Sokal scores and with more disease burden as described from SMS Medical College Hospital, Jaipur, indicating probably a late diagnosis.[25] Interestingly, the data from TMH, Mumbai, Maharashtra, India, showed that irrespective of the Sokal score, the CCyR was similar, i.e., for low risk (76.3%), intermediate risk (73.8%), and high risk (77.3%), suggesting that IM may overcome this aspect of disease.[15]
Studies from TMH and SMS, Jaipur, also compared the responses of innovator Glivec to the Indian IM and found similar hematological responses, cytogenetic and better molecular responses with generic.[15,25] Unfortunately, a significant number of patients in the Glivec arm were not tested for molecular responses for economic reasons making the difference artifactual.
In SMS, Jaipur, 137 (64%) patients were on Glivec, whereas 76 (36%) were on generic IM. The CHR was 88% in Glivec arm, whereas it was 96% in generic arm. Another study from TMH, Mumbai, Maharashtra, India, showed similar findings with 72% CCyR in Glivec arm and 75% CCyR in generic arm.
Safety of IM in pregnancy came from SEAROC, Jaipur, three patients conceived and all babies born did not have any congenital anomaly.[26] AIIMS study showed that ten female became pregnant, whereas on IM, but only three stopped the drug as per instructions. However, there were uneventful outcomes except one baby had meningocele.[19]
Mutation analysis data from SEAROC, Jaipur, performed in five patients in accelerated phase, showed no known mutation in the ABL kinase domain.[26] At Kidwai Bengaluru, mutation analysis was done in 101 patients showing poor response, in 73%, there was no known mutation. The most common mutation seen was T315I (four patients) and M351T (four patients).[27] At NIMS, Hyderabad, 29/90 (32.2%) patients have detectable kinase domain mutation. The most common mutation was T315I in nine (31%) patients. Thirteen (12.2%) of all patients (38% of all mutations) had a P-loop mutation. N374Y was a novel mutation has not been reported before. Dose escalation to 800 mg resulted in CCgR in 25/90 (27.7), partial cytogenetic response in 10 (11.3) with 2-year event-free survival (EFS) in 34%, and 2-year OS rate of 93%.[29]
The importance of compliance was emphasized by Parikh, where the CCyR rate in patients taken with more than or <4 weeks gaps irrespective of brands was 57 and 80%, respectively.[15] Ganesan et al. also looked at the aspect of more than 1 week of nonadherence. The 5-year EFS in adherent and nonadherent patients was 76.7% and 59.8%, respectively (P < 0.011, log rank test). Nonadherent patients were less likely to achieve complete cytogenetic responses (26% vs. 44%; P = 0.004; χ2 test) at any point.[30]
TMH documented resistance or relapse in 372 (38%).[15] Bansal and Advani data, from AIO, showed that primary and secondary resistance was significantly high in the patients registered as old cases, but they were not affected by Sokal scoring.[23]
One such study from Ashirwad, Mumbai, Maharashtra, India, showed that on Cox regression analysis, age under 40 years, low Sokal score, CHR, and CCyR were significant predictive factors for EFS, whereas on multivariate analysis, low Sokal score and ECP were the significant predictive factors for CCyR.[11] Similarly, from AIO, Raheja, Mumbai, Maharashtra, India also reiterated that ECP, better tolerability to the drug, and no primary resistance are significant indicators of better survival of patients with CML.[23] All indicating that early diagnosis and timely treatment can provide better outcomes.
SE status of patients also had an impact on the response to IM. A study by SMS, Jaipur, showed that patients with upper SE status had 100% CHR, whereas LE status had 90.3% CHR, also LE patients with increased disease burden with 25% having high Sokal scores compared with only 6% in upper SE patients.
Toxicity
The most common nonhematological toxicity seen was changes in skin pigmentation to the tune of almost 20%, followed by weight gain, edema, diarrhea, myalgias, arthralgias, and transaminitis. Some have reported ototoxicity, decrease in vision also (RCC, Patna, Bihar, India), and second malignancies (AIO, Raheja, Mumbai, Maharashtra, India).[23] Among hematological toxicity, most common were anemia seen in 30% of patients, thrombocytopenia between 17% and 50%, and neutropenia in 5% to 44%. Grade III/IV toxicity requiring intervention was seen <1% (GCRI, Gujarat, India) and it was reported up to 16% by Ashirwad, Mumbai, Maharashtra, India.[14,23]
Survival
The survival varies from 81% to 100% in various studies as shown in Table 2.
Table 2.
Survival outcomes of chronic myeloid leukemia patients treated in the imatinib era from various centers of India
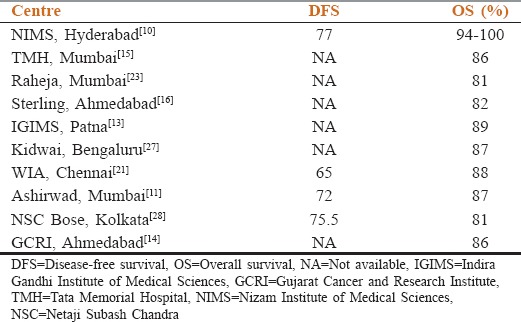
It appears from these studies that Indian CML OS, pattern of response in CP and CCyR with compliance (or noncompliance) is similar to the Western population. This comes despite several limitations in treatment, monitoring of disease, availability of second-generation tyrosine kinase inhibitors (TKIs), late presentations, and significant population coming from low SE strata.
Conclusion and Future Directions
Several issues need to be addressed for our patients of CML. Timely diagnosis can improve outcomes, and we are already witnessing an increasing trend in asymptomatic presentation incidentally detected on routine blood counts. Efforts made to increase awareness and timely referral will certainly pay. The appropriate molecule upfront as is evident in all studies would appear to be IM mesylate for cost reasons, however identifying a subset early that may not be responsive to IM is essential to prevent progressive disease. Such practice would need exquisite monitoring of disease – an area where challenge exists. Strategies of monitoring, especially molecular are deficient either due to cost or availability of reliable testing, something that will have to be worked out by a similar Indian consortium of specialized and cheaper laboratories working in different zones which could be referred the samples as per quality control norms for getting standardized results. Centralization may curtail cost in addition to developing international standards. Inability to routinely perform the molecular tests is highlighted in most of the studies including one from AIO, wherein maximum patients had been followed with blood counts only, cytogenetic study on follow-up was done in few patients only, who could afford it. This will help optimization of treatment by either use of second generation TKI or other strategies such as transplant, a practice which is gradually gaining acceptance with better affordability of newer drugs.
Importantly, to have a national level data bank several variables will have to be matched such as appropriate test for response assessment, frequency of test while on treatment, standardization of tests, implementing uniform response criteria, criteria for describing resistance so as to increase or change treatment, and the appropriate use and timing of allogeneic transplantation, especially in the younger population not responsive to therapy. Relevantly, our ability to recognize the resistant mutations early as per defined criteria would lead to preemptively change therapy, especially in the younger population. The role of allogeneic bone marrow transplant looking into the more common T315I mutation in our population also should be put in its proper perspective.
It appears from the published Indian data on CML that our OS, pattern of response in CP and CCgR is not very different from the Western population. Where we lack is probably monitoring by molecular tests and uniform implementation of response criteria.
Interestingly, various studies also reinforce that the innovator and generic are similar in their efficacy, a fact which is reassuring to all those patients and their physicians who may not be able to afford the innovator brand.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- 2.Bhutani M, Vora A, Kumar L, Kochupillai V. Lympho-hemopoietic malignancies in India. Med Oncol. 2002;19:141–50. doi: 10.1385/MO:19:3:141. [DOI] [PubMed] [Google Scholar]
- 3.Aziz Z, Iqbal J, Akram M, Saeed S. Treatment of chronic myeloid leukemia in the imatinib era: Perspective from a developing country. Cancer. 2007;109:1138–45. doi: 10.1002/cncr.22498. [DOI] [PubMed] [Google Scholar]
- 4.Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood. 2007;110:2828–37. doi: 10.1182/blood-2007-04-038943. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, Radich JP, et al. International randomized study of interferon versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CMLCP) treated with imatinib (IM) Blood. 2008;112:76. [Google Scholar]
- 7.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 8.Tardieu S, Brun-Strang C, Berthaud P, Michallet M, Guilhot F, Rousselot P, et al. Management of chronic myeloid leukemia in France: A multicentered cross-sectional study on 538 patients. Pharmacoepidemiol Drug Saf. 2005;14:545–53. doi: 10.1002/pds.1046. [DOI] [PubMed] [Google Scholar]
- 9.Cortes JE, Richard TS, Hagop K. In: Chronic myelogenous leukemia. Cancer Management: A Multidisciplinary Approach. 10th ed. Padzur R, Coia LR, Hoskins WJ, Wagman LD, editors. Lawrence: CMPMedica; 2007. p. 789. [Google Scholar]
- 10.Rajappa S, Varadpande L, Paul TR, Jacob RT, Digumarti R. Report of chronic myeloid leukemia in chronic phase from Dr. Senthil Rajappa, 2002-2009. Indian J Med Paediatr Oncol. 2013;34:208–10. doi: 10.4103/0971-5851.123745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal MB, Agarwal UM, Rathi SS, Masurkar S, Zaveri B. Report of chronic myeloid leukemia in chronic phase from Ashirwad Hematology Centre, Mumbai, 2002-2009. Indian J Med Paediatr Oncol. 2013;34:199–203. doi: 10.4103/0971-5851.123741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutani M, Kochupillai V. In: Hematological malignancies in India. Progress in Hematologic Oncology. Kumar L, editor. New York: Pub. The Advanced Research Foundation New York; 2003. p. 10. [Google Scholar]
- 13.Prasad RR, Singh P. Report of chronic myeloid leukemia from Indira Gandhi Institute of Medical Sciences, Regional Cancer Center, 2002-2009. Indian J Med Paediatr Oncol. 2013;34:172–4. doi: 10.4103/0971-5851.123719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SA. The treatment of chronic myeloid leukemia, data from Gujarat Cancer and Research Institute, Ahmedabad. Indian J Med Paediatr Oncol. 2013;34:189–92. doi: 10.4103/0971-5851.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh P. Report of chronic myeloid leukemia in chronic phase from Tata Memorial Hospital, Mumbai, 2002-2008. Indian J Med Paediatr Oncol. 2013;34:164–7. doi: 10.4103/0971-5851.123716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deotare UR, Chudgar U, Bhagat E. Report of patients with chronic myeloid leukemia, from hematology clinic, Ahmedabad, Gujarat 2000-2010 at 1 st myelostone meeting: Indian evidence of chronic myelogenous leukemia. Indian J Med Paediatr Oncol. 2013;34:193–5. doi: 10.4103/0971-5851.123734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas KG, Patil S, Shashidhara Epidemiological and clinical profile of patients with chronic myeloid leukemia at Health-Care Global, Bangalore Institute of Oncology. Indian J Med Paediatr Oncol. 2013;34:211–2. doi: 10.4103/0971-5851.123746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray SS, Chakraborty P, Chaudhuri U, Ganesh Report of chronic myeloid leukemia in chronic phase from Eastern India, Institute of Hematology and Transfusion Medicine, Kolkata, 2001-2009. Indian J Med Paediatr Oncol. 2013;34:175–6. doi: 10.4103/0971-5851.123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra P, Seth T, Mahapatra M, Saxena R. Report of chronic myeloid leukemia in chronic phase from All India Institute of Medical Sciences, 1990-2010. Indian J Med Paediatr Oncol. 2013;34:159–63. doi: 10.4103/0971-5851.123712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doval DC, Batra U, Goyal S, Sharma A, Azam S, Shirali R. Chronic myeloid leukemia treatment with Imatinib: An experience from a private tertiary care hospital. Indian J Med Paediatr Oncol. 2013;34:182–5. doi: 10.4103/0971-5851.123725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan P, Rejiv R, Manjunath N, Sanju C, Sagar TG. Report of chronic myeloid leukemia in chronic phase from Cancer Institute (Women India Association), Chennai, 2002-2009. Indian J Med Paediatr Oncol. 2013;34:206–7. doi: 10.4103/0971-5851.123744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra P, Varma N, Varma S. A short report on chronic myeloid leukemia from Post Graduate Institute of Medical Education and Research, Chandigarh. Indian J Med Paediatr Oncol. 2013;34:186–8. doi: 10.4103/0971-5851.123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal S, Advani SH. Report of chronic myelogenous leukemia in chronic phase from, Asian Institute of Oncology, Mumbai, 2002-2010. Indian J Med Paediatr Oncol. 2013;34:168–71. doi: 10.4103/0971-5851.123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dattatreya PS, Nirni SS. Report of chronic myeloid leukemia in chronic phase from Omega Hospital and Indo-American Centre, Hyderabad, 2004-2010. Indian J Med Paediatr Oncol. 2013;34:204–5. doi: 10.4103/0971-5851.123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra H, Sharma R, Singh Y, Chaturvedi H. Report of chronic myeloid leukemia SMS Medical College Hospital, Jaipur. Indian J Med Paediatr Oncol. 2013;34:177–9. doi: 10.4103/0971-5851.123723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maru A. Report of chronic myeloid leukemia from SEAROC experience, Jaipur over a period of 9 years. Indian J Med Paediatr Oncol. 2013;34:180–1. doi: 10.4103/0971-5851.123724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babu G. Report of patients with chronic myeloid leukemia Kidwai Memorial Institute of Oncology, Bangalore over 15 years. Indian J Med Paediatr Oncol. 2013;34:196–8. doi: 10.4103/0971-5851.123736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay A, Dasgupta S, Mukhopadhyay S, Bose CK, Sarkar S, Gharami F, et al. Imatinib mesylate therapy in patients of chronic myeloid leukemia with Philadelphia chromosome positive: An experience from Eastern India. Indian J Hematol Blood Transfus. 2012;28:82–8. doi: 10.1007/s12288-011-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajappa S, Mallavarapu KM, Gundeti S, Paul TR, Jacob RT, Digumarti R. Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate. Indian J Med Paediatr Oncol. 2013;34:221–3. doi: 10.4103/0971-5851.123750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86:471–4. doi: 10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]


