Abstract
Context:
Insulin users have been reported to have a higher incidence of diabetic retinopathy (DR).
Aim:
The aim was to elucidate the factors associated with DR among insulin users, especially association between duration, prior to initiating insulin for Type 2 diabetes mellitus (DM) and developing DR.
Materials and Methods:
Retrospective cross-sectional observational study included 1414 subjects having Type 2 DM. Insulin users were defined as subjects using insulin for glycemic control, and insulin nonusers as those either not using any antidiabetic treatment or using diet control or oral medications. The duration before initiating insulin after diagnosis was calculated by subtracting the duration of insulin usage from the duration of DM. DR was clinically graded using Klein's classification. SPSS (version 9.0) was used for statistical analysis.
Results:
Insulin users had more incidence of DR (52.9% vs. 16.3%, P < 0.0001) and sight threatening DR (19.1% vs. 2.4%, P < 0.0001) in comparison to insulin nonusers. Among insulin users, longer duration of DM (odds ratio [OR] 1.12, 95% confidence interval [CI] 1.00–1.25, P = 0.044) and abdominal obesity (OR 1.15, 95% CI 1.02–1.29, P = 0.021) was associated with DR. The presence of DR was significantly associated with longer duration (≥5 years) prior to initiating insulin therapy, overall (38.0% vs. 62.0%, P = 0.013), and in subjects with suboptimal glycemic control (32.5% vs. 67.5%, P = 0.022).
Conclusions:
The presence of DR is significantly associated with longer duration of diabetes (>5 years) and sub-optimal glycemic control (glycosylated hemoglobin <7.0%). Among insulin users, abdominal obesity was found to be a significant predictor of DR; DR is associated with longer duration prior to initiating insulin therapy in Type 2 DM subjects with suboptimal glycemic control.
Keywords: Diabetic retinopathy, insulin nonusers, insulin users, sight threatening diabetic retinopathy
Insulin therapy is often initiated in subjects with Type 2 diabetes mellitus (DM) who fail to achieve an optimum glycemic control with oral hypoglycemic agents. Besides the influence on carbohydrate, lipid, and protein metabolism, insulin also influences hemostasis, vascular tone, and angiogenesis, and there is evidence that the development of vascular complications can occur independent of the metabolic effects of insulin.[1,2] Multiple clinical trials, including the Diabetes Control and Complications Trial in Type 1 diabetes and many smaller studies have shown that immediately following the initiation of intensive insulin therapy, diabetic retinopathy (DR) can transiently worsen (termed “early worsening” or normoglycemic re-entry phenomenon).[3,4,5,6] Moreover, the usage of insulin has been shown to be associated with the presence of DR and proliferative DR (PDR) in some studies.[7,8,9]
However, despite reported proangiogenic effects of insulin and transient worsening of DR, intensive glycemic control with insulin has been associated with lower occurrence of DR.[10] The purpose of this study was to elucidate the factors associated with DR in insulin users, especially the association between duration prior to initiating insulin therapy and DR in a population-based sample of subjects with Type 2 diabetes. We also observed the prevalence of DR and sight threatening DR (STDR) among insulin users and insulin nonusers.
Materials and Methods
This is a population-based cross-sectional study. The study population was recruited from Chennai, the fourth largest city in India. In our study, the computed sample size is 5830. This estimation is based on the following assumptions: The prevalence of DR in the general population is assumed to be 1.3%, with a relative precision of 25%, a drop-out rate of 20%, and a design effect of 2. The sample size for the prevalence survey was calculated by using the formula 4PQ/d2 where P is the expected prevalence; Q = 1 − P and d is the precision. Chennai city was divided into ten corporation zones of 155 divisions. The sampling was based on the multistage systematic random sampling. Sampling was done in two stages: Selection of divisions and selection of study subjects. Selection of divisions is done using computer generated random numbers; of 155 divisions, ten are selected ensuring that one division per one corporate zone is represented in the sample. Eligible study subjects were randomly selected from each division. To meet the target, 600 individuals are enumerated for each division (a total of 6000 in 10 zones). This sample is thus truly representative of urban Chennai, Tamil Nadu, India. The sampling was done to ensure that data are collected from all socioeconomic groups. Family members living on the same premises and sharing a common kitchen were defined as being the members of one household. A door-to-door survey of all the households on the right side of the street was conducted in the selected division until a number of 600 subjects were reached. Institutional Review Board approval was obtained, and a written informed consent was obtained from the subjects as per the Declaration of Helsinki.[11] Inclusion criteria included individuals aged ≥40 years and residing for a minimum of 6 months at the same residence. The study population was selected by multistage systematic random sampling. Of 5830 subjects enumerated, 1414 with diabetes (both known and newly diagnosed) were analyzed for the study. The epidemiology team was provided with intensive training on a one-to-one basis that on doing a household survey, enumeration, and filling out the study data sheet and usage of the blood pressure (BP) apparatus and glucometer. The training lasted for 7 days with 8 h a day. The main objective was to avoid bias or errors in any of the procedures employed. Each trainee was evaluated individually and allowed to participate in the study only after he/she displayed minimum error rates for the tasks involved in the study. In order to ensure accurate and reliable data, a comprehensive instruction manual was prepared that included instructions on calibrating glucometer every day. The glucometer was calibrated every day and its reproducibility was assessed by measuring the blood glucose for the same patient six times and also with two machines. A similar procedure was undertaken for the sphygmomanometer. The scale for measuring the weight was calibrated with a known weight once a week. The collected data was scrutinized manually before its entry into the computer. Subjects with diabetes were identified based on the American Diabetes Association criteria.[12] All subjects underwent a detailed examination at the base hospital. Glycosylated hemoglobin (HbA1c) fractions were estimated by using Merck Micro Lab 120 semi-automated analyzer (Bio-Rad DiaSTAT HbA1c Reagent Kit).[12] Total serum cholesterol, high density lipoproteins (HDLs), and serum triglycerides (cholesterol oxidase-peroxidase) were estimated. Low serum HDL cholesterol levels were defined as <1.03 mmol/L (<40 mg/dl) for men and <1.29 mmol/L (<50 mg/dl) for women. High serum triglycerides levels were defined as ≥1.7 mmol/L (≥150 mg/L). Microalbuminuria estimation was done by a semi-quantitative procedure (Bayer Clinitek 50 Urine Chemistry Analyzer). Subjects were considered to have microalbuminuria, if the urinary albumin excretion was between 30 and 300 mg/24 h, and macroalbuminuria at more than 300 mg/24 h. The presence of diabetic neuropathy was considered if vibration perception threshold (VPT) value was >20V. VPT was measured using sensitometer by a single observer by placing a biothesiometer probe perpendicular to the distal plantar surface of the great toe of both legs. The VPT was done by a single trained technician. Anthropometric measurements, including weight, height, waist, and hip were obtained using standardized techniques. Abdominal obesity was defined as waist circumference (WC) ≥90 cm for men and ≥80 cm for women. Hypertension was defined as BP ≥130/85 mmHg. The clinical parameters were collected by trained nurses, whereas the interviews were held by trained personnel.
Out of 1414 subjects with Type 2 diabetes, 248 (17.5%) were newly diagnosed and the remaining had known diabetes. Persons with newly diagnosed diabetes were defined as those who had their fasting blood glucose level ≥110 mg/dl on two separate days. Persons with known diabetes were those who were using either oral antiglycemic drugs or insulin or both. Out of 1414, 68 subjects were taking injection insulin. Insulin users were defined as subjects using insulin for glycemic control and insulin nonusers were defined as those either not using any antidiabetic treatment or using diet control or oral hypoglycemic medications. The duration of insulin usage was calculated as the number of years since the patient is using insulin treatment to achieve glycemic control. The duration prior to initiating insulin therapy was calculated by subtracting the duration of insulin usage from the duration of diabetes in years. Optimal control of HbA1c was described based on the World Health Organization and the American Diabetes Association guidelines (optimal HbA1c, <7%; suboptimal HbA1c, ≥7%).[13]
DR was clinically graded using Klein's classification (modified early treatment DR study [ETDRS] scales).[14] Digital photographs were assessed and graded by two independent observers (experienced retinal specialists) in a masked fashion. The photographs were graded against the standard photographs of the ETDRS grading system for the severity of retinopathy. The grading agreement was high (κ = 0.83). STDR was defined as “referable retinopathy” including severe non-PDR, PDR, or clinically significant macular edema.
Statistical analysis
A computerized database was created for all the records. A Statistical Package for the Social Sciences-SPSS (version 9.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The data were examined for normality of distribution. All normally distributed data were compared using a Student's t-test, while those that did not follow normal distribution were examined using nonparametric tests. A Chi-square test was used for comparing proportions. All the data were expressed as mean ± standard deviation or as percentage. The statistical significance was assumed at P ≤ 0.05. We examined the data for collinearity. The data were not linear. For that reason, we utilized univariate and multivariate logistic regression analyses to elucidate the association between insulin usage and DR. The odds ratio (OR), with 95% confidence intervals (CIs), was calculated for the studied variables. Using a logistic regression procedure, we calculated the area under receiver operating characteristic (ROC) curve (AUC) for DR in insulin users and nonusers. Predictive accuracy for logistic regression model was assessed by comparing the observed and the expected retinopathy by using the Hosmer–Lemeshow (HL) goodness-of-fit test. The Chi-square test was carried out to determine if the observed and expected frequencies are significantly different. A P > 0.05 for the HL test was considered suggestive of a calibrated model.
Results
Table 1 represents the baseline characteristics of insulin nonusers versus insulin users. Subjects using insulin had longer duration of diabetes (11.6 vs. 5.2 years, P < 0.0001), lesser age of onset of diabetes (45.9 vs. 50.7 years, P < 0.0001), lower diastolic BP (DBP) (78.1 mmHg vs. 82.2 mmHg., P = 0.005), lower total serum cholesterol (175.6 mg/dl vs. 187.1 mg/dl, P = 0.023), lower serum triglycerides (127.9 mg/dl vs. 155.0 mg/dl, P = 0.030), higher HbA1c (9.0% vs. 8.2%, P = 0.001), higher microalbuminuria (27.9 mg% vs. 15.4 mg%, P = 0.006), higher macroalbuminuria (7.4% vs. 2.5%, P = 0.015), and more neuropathy (29.4% vs. 18.3%, P = 0.002) in comparison to subjects not using insulin.
Table 1.
Baseline characteristics of subjects not using insulin (insulin nonusers) and subjects using insulin (insulin users)
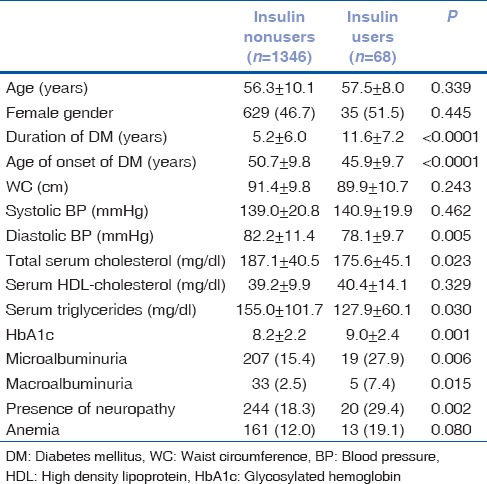
Fig. 1 shows the presence of DR and STDR in insulin nonusers and insulin users. Subjects using insulin were more likely to have DR (52.9% vs. 16.3%, P < 0.0001) and STDR (19.1% vs. 2.4%, P < 0.0001) in comparison to subjects not using insulin.
Figure 1.
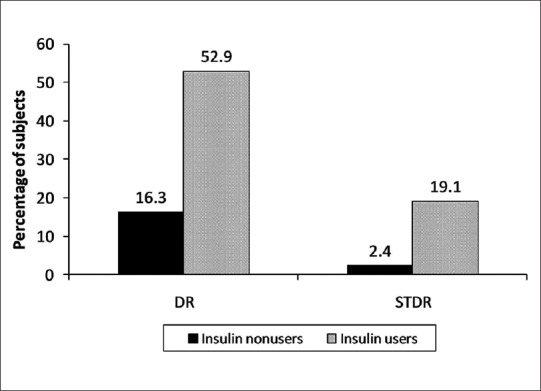
Association of diabetic retinopathy and sight threatening diabetic retinopathy in insulin nonusers and insulin users
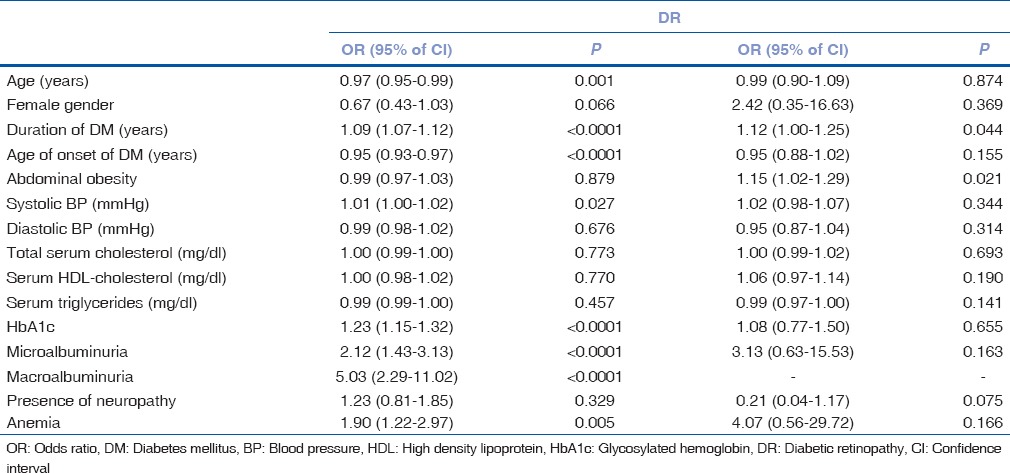
Table 2 shows the multivariate analysis for the factors associated with the development of DR in insulin users and insulin nonusers. In insulin nonusers, older age (OR 0.97, 95% CI 0.95–0.99, P = 0.001), longer duration of diabetes (OR 1.09, 95% CI 1.07–1.12, P < 0.0001), lesser age of onset of diabetes (OR 0.95, 95% CI 0.93–0.97, P < 0.0001), higher systolic BP (OR 1.01, 95% CI 1.00–1.02, P = 0.027), higher HbA1c (OR 1.23, 95% CI 1.15–1.32, P < 0.0001), presence of microalbuminuria (OR 2.12, 95% CI 1.43–3.13, P < 0.0001), presence of macroalbuminuria (OR 5.03, 95% CI 2.29–11.02, P < 0.0001), and presence of anemia (OR 1.90, 95% CI 1.22–2.97, P = 0.005) were associated with the development of DR. In insulin users, longer duration of diabetes (OR 1.12, 95% CI 1.00–1.25, P = 0.044) and abdominal obesity defined by higher WC (OR 1.15, 95% CI 1.02–1.29, P = 0.021) were associated with DR.
Table 2.
Multivariate analysis for the factors associated with development of diabetic retinopathy in insulin nonusers and insulin users
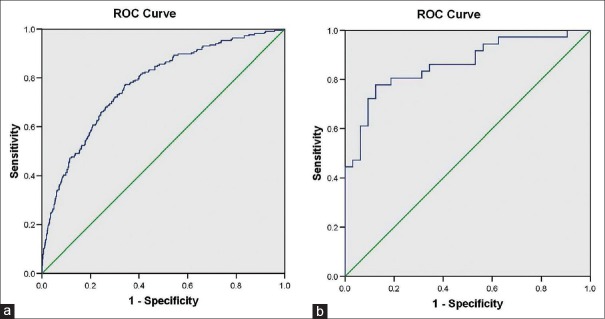
The ROC curves for DR in insulin nonusers and insulin users are shown in Fig. 2. The AUC values and HL – P values for DR for the insulin nonusers were 0.77 (95% CI 0.74–0.81) and 0.67, respectively, and for insulin users were 0.86 (95% CI 0.77–0.95) and 0.54, respectively.
Figure 2.
Receiver operating characteristic curve for presence of diabetic retinopathy in insulin nonusers (a) and insulin users (b)
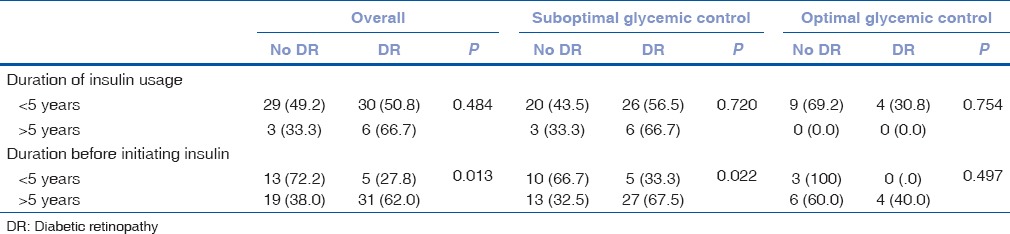
Table 3 shows the association of DR with duration of insulin usage and duration prior to initiating insulin therapy, in relation to glycemic control of the subject. There was no statistically significant association between the presence of DR and duration of insulin usage. The presence of DR was significantly associated with longer duration (≥5 years) prior to initiating insulin therapy overall (38.0 vs. 62.0%, P = 0.013) and in subjects with suboptimal glycemic control (32.5 vs. 67.5%, P = 0.022).
Table 3.
Association of diabetic retinopathy with duration of insulin usage and duration of insulin free period, in relation to glycemic control
Discussion
We found that subjects with Type 2 diabetes with suboptimal glycemic control, when insulin is started late in the course of the disease (longer insulin free duration), the deleterious effects of long-term hyperglycemia due to neglected treatment is probably responsible for the development of DR. It is also evident that poor glycemic control is the immediate cause for DR complication. Over time, most patients with Type 2 diabetes require insulin therapy, either alone or in combination with oral hypoglycemic agents, for satisfactory glycemic control.[15] Since insulin therapy in Type 2 diabetes is often initiated at a stage where glycemic control is suboptimal for the subject, insulin users have been reported to have a higher incidence of DR. Moreover, recently, insulin has been independently implicated in the causation of DR and PDR.[9,16,17]
In the present study, we aimed to elucidate the relationship between insulin usage and DR. For this, we divided the study population into two groups of insulin users and insulin nonusers. On studying the demographic profile of both groups, we observed statistically significant differences. As expected, insulin users had longer duration of diabetes, higher HbA1c, and lesser age of onset of diabetes [Table 1]. The insulin users also had a better lipid profile and lower DBP, when compared with insulin nonusers. Similar presence of more favorable lipid profile in subjects with Type 2 diabetes using insulin in comparison to those using oral hypoglycemic agents has been reported earlier also.[18] The diastolic hypertension is known to be more prevalent among younger subjects and that in our study, the differences in age between insulin nonusers insulin users were not statistically significant.[19] However, the regulation of BP in humans is a complex interplay between several exogenous and endogenous factors such as renin-angiotensin system and the presence of lower DBP among insulin users in the present study is difficult to explain without taking into consideration all these factors. Concerning the microvascular diabetic complications, insulin users had higher albuminuria (micro- and macro-) and neuropathy. Likewise, insulin users also were more likely to have DR and STDR in comparison to insulin nonusers [Fig. 1].
In the present study, we also evaluated the factors associated with DR in insulin users and nonusers separately. Because of the small sample size, however, the same could not be studied in regard to STDR. The factors associated with DR differed among insulin users and insulin nonusers. In insulin users, duration of diabetes and abdominal obesity in terms of WC were associated with DR, whereas HbA1c, age of onset of diabetes, presence of microalbuminuria, macroalbuminuria, and anemia did not have a statistically significant association with DR as was noted in insulin nonusers. In contrast, in insulin nonusers, we did not find an association between abdominal obesity and presence of DR. This suggests that in insulin users, abdominal obesity is a more significant predictor of DR than it is for insulin nonusers. In our previous study,[20] we examined the influence of insulin use as an independent variable in association with the prevalence of DR. We observed that insulin users were at 3.5 times the risk of DR than those who were not using insulin. Physical exercise, which has long been recognized as an effective interventional strategy in the treatment of Type 2 diabetes, may especially prove very useful in insulin users to prevent retinopathy. The usefulness of physical exercise has already been reported in patients with long-standing, insulin-treated Type 2 diabetes with diabetic polyneuropathy.[21]
On considering both the discrimination (AUC) and calibration (HL goodness-of-fit) power of the multivariate analysis model, our study showed that it was appropriate for predicting the association of DR with various factors in both insulin nonusers and insulin users.
Another finding of this study was lack of association between DR and the duration of insulin usage. Rather, association was noted between DR and longer duration before starting insulin, particularly in subjects with suboptimal glycemic control. This finding suggests that insulin is simply a marker of glycemic control/disease severity rather than an independent risk factor for DR. This suggests that in subjects with Type 2 diabetes with suboptimal glycemic control, when insulin is started late in the course of the disease (longer insulin free duration), the deleterious effects of long-term hyperglycemia due to neglected treatment is probably responsible for the development of DR. Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial recommended early insulin initiation in Type 2 diabetic patients with an HbA1c >9% at diagnosis, patients with a fast increase of HbA1c after diagnosis and new onset symptoms, patients with multiple infections and patients with an HbA1c >7% despite maximal metformin treatment and diabetes-related complications.[22] ORIGIN also demonstrated reduced microangiopathy in patients with an HbA1c value of ≥6.4% with basal insulin glargine.[22] Patients with Type 2 diabetes are initiated on additional blood glucose-lowering treatment only when the mean baseline HbA1c reaches a value of 9.0%.[23] Patients started on insulin have even higher mean HbA1c of 9.6% and tend to have more severe baseline complications and co morbidities than those started on oral antidiabetic therapy. In addition, the higher the starting A1c when therapy is initiated or changed, the less likely the patient can achieve adequate glycemic control.[23] Chronic hyperglycemia increases production of reactive oxygen species, and subsequent oxidative stress affects insulin promoter activity (PDX-1 and MafA binding) resulting in diminished insulin gene expression in glucotoxic β-cells.[24] Patients presenting with significant hyperglycemia may benefit from timely initiation of insulin therapy that can effectively and rapidly correct their metabolic imbalance and reverse the deleterious effects of excessive glucose (glucotoxicity) and lipid (lipotoxicity) exposure on β-cell function and insulin action.[25] Glucotoxic effects are reversible with reinstitution of euglycemic conditions and causes greatest recovery of β-cell function with shorter duration of exposure to hyperglycemia.[26]
Hence, in subjects with Type 2 diabetes having suboptimal glycemic control, starting insulin early may be more beneficial in preventing the development of DR in the longer course of the disease. Prospective studies will be required to evaluate the association of duration before starting insulin therapy with future development of DR.
One of the principal shortcomings of the study is its small sample size. Of the 1414 subjects analyzed, only 68 were using insulin. Another major shortcoming is that because of its cross-sectional design, the cause-effect relationship cannot be established between insulin usage and DR. Moreover, there was a lack of information regarding the type of insulin preparation used by the patient. Although we have performed additional analysis pertaining to the effect of duration of insulin usage versus insulin-free period, the numbers are too small, hence making it difficult to generalize from it. The strengths of the study are that it was a well-conducted population-based prevalence study in Type 2 DM, and retinopathy diagnosis was based on the gold standard fundus photography and comprehensive clinical and biochemical evaluation.
Conclusions
That insulin usage in Type 2 diabetes was associated with the presence of DR, STDR, neuropathy, and albuminuria. This finding suggests that insulin is simply a marker of glycemic control/disease severity rather than an independent risk factor for DR. This suggests that in subjects with Type 2 diabetes with suboptimal glycemic control, when insulin is started late in the course of the disease (longer insulin free duration), the deleterious effects of long-term hyperglycemia due to neglected treatment is probably responsible for the development of DR. In insulin users, abdominal obesity was found to be a more significant predictor of DR than it is for insulin nonusers. We also observed an association of DR with the longer duration before initiating insulin therapy, particularly in subjects with suboptimal glycemic control.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
RD Tata Trust, Mumbai, India.
References
- 1.Anfossi G, Russo I, Doronzo G, Trovati M. Relevance of the vascular effects of insulin in the rationale of its therapeutical use. Cardiovasc Hematol Disord Drug Targets. 2007;7:228–49. doi: 10.2174/187152907782793581. [DOI] [PubMed] [Google Scholar]
- 2.Silva PS, Cavallerano JD, Sun JK, Aiello LM, Aiello LP. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat Rev Endocrinol. 2010;6:494–508. doi: 10.1038/nrendo.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;1:200–4. doi: 10.1016/s0140-6736(83)92585-0. [DOI] [PubMed] [Google Scholar]
- 5.Funatsu H, Yamashita H, Ohashi Y, Ishigaki T. Effect of rapid glycemic control on progression of diabetic retinopathy. Jpn J Ophthalmol. 1992;36:356–67. [PubMed] [Google Scholar]
- 6.Agardh CD, Eckert B, Agardh E. Irreversible progression of severe retinopathy in young type I insulin-dependent diabetes mellitus patients after improved metabolic control. J Diabetes Complications. 1992;6:96–100. doi: 10.1016/1056-8727(92)90018-g. [DOI] [PubMed] [Google Scholar]
- 7.Cignarelli M, De Cicco ML, Damato A, Paternostro A, Pagliarini S, Santoro S, et al. High systolic blood pressure increases prevalence and severity of retinopathy in NIDDM patients. Diabetes Care. 1992;15:1002–8. doi: 10.2337/diacare.15.8.1002. [DOI] [PubMed] [Google Scholar]
- 8.Wirta O, Pasternack A, Mustonen J, Laippala P, Lähde Y. Retinopathy is independently related to microalbuminuria in type 2 diabetes mellitus. Clin Nephrol. 1999;51:329–34. [PubMed] [Google Scholar]
- 9.Dills DG, Moss SE, Klein R, Klein BE. Association of elevated IGF-I levels with increased retinopathy in late-onset diabetes. Diabetes. 1991;40:1725–30. doi: 10.2337/diab.40.12.1725. [DOI] [PubMed] [Google Scholar]
- 10.U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: A progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- 11.Touitou Y, Portaluppi F, Smolensky MH, Rensing L. Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol Int. 2004;21:161–70. doi: 10.1081/cbi-120030045. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2003;26(Suppl 1):S106–8. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Summary of revisions for the 2007 clinical practice recommendations. Diabetes Care. 2007;30(Suppl 1):S3. [Google Scholar]
- 14.Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–7. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 15.United Kingdom Prospective Diabetes Study 24: A-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. 1998;128:165–75. doi: 10.7326/0003-4819-128-3-199802010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Amano S, Miyamoto K, Garland R, Keough K, Qin W, et al. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999;40:3281–6. [PubMed] [Google Scholar]
- 17.Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–15. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M, Superko R, Williams P, Lim P, Pan J, Charles MA. The atherogenic lipid profile is associated with type 2 diabetes and some of its treatment modalities. Diabetes Nutr Metab. 2003;16:56–64. [PubMed] [Google Scholar]
- 19.Hozawa A, Ohkubo T, Nagai K, Kikuya M, Matsubara M, Tsuji I, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: The Ohasama study. Arch Intern Med. 2000;160:3301–6. doi: 10.1001/archinte.160.21.3301. [DOI] [PubMed] [Google Scholar]
- 20.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Praet SF, Jonkers RA, Schep G, Stehouwer CD, Kuipers H, Keizer HA, et al. Long-standing, insulin-treated type 2 diabetes patients with complications respond well to short-term resistance and interval exercise training. Eur J Endocrinol. 2008;158:163–72. doi: 10.1530/EJE-07-0169. [DOI] [PubMed] [Google Scholar]
- 22.Hanefeld M, Bramlage P. Insulin use early in the course of type 2 diabetes mellitus: The ORIGIN trial. Curr Diab Rep. 2013;13:342–9. doi: 10.1007/s11892-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Go AS, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007;13:598–606. [PMC free article] [PubMed] [Google Scholar]
- 24.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: Implications for the management of diabetes. Diabetologia. 1985;28:119–21. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 25.Poitout V, Robertson RP. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleason CE, Gonzalez M, Harmon JS, Robertson RP. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000;279:E997–1002. doi: 10.1152/ajpendo.2000.279.5.E997. [DOI] [PubMed] [Google Scholar]