SUMMARY
Strategies that simultaneously enhance the survival and glucose responsiveness of insulin-producing β cells will greatly augment β cell replacement therapies in type 1 diabetes (T1D). We show that genetic and pharmacologic mimetics of the phosphorylated BCL-2 homology 3 (BH3) domain of BAD impart β cell autonomous protective effects in the face of stress stimuli relevant to β cell demise in T1D. Importantly, these benefits translate into improved engraftment of donor islets in transplanted diabetic mice, increased β cell viability in islet grafts, restoration of insulin release, and diabetes reversal. Survival of β cells in this setting is not merely due to the inability of phospho-BAD to suppress pro-survival BCL-2 proteins but requires its activation of the glucose-metabolizing enzyme glucokinase. Thus, BAD phospho-BH3 mimetics may prove useful in restoration of functional β cell mass in diabetes.
Graphical Abstract
INTRODUCTION
Autoimmune destruction of pancreatic β cell mass renders individuals with type 1 diabetes (T1D) insulin-dependent. Strategies that replace or regenerate β cells or preserve remaining β cell mass are potential therapies in T1D (Claiborn and Stoffers, 2008; Halban et al., 2010; Hebrok, 2012; Pagliuca and Melton, 2013). However, the benefits of these approaches can be thwarted by insufficient β cell proliferation, survival, and insulin secretory response to glucose. As such, strategies that simultaneously enhance β cell mass and glucose signaling can be of great therapeutic utility. Beyond stimulating insulin secretion, increased β cell glucose metabolism stimulates β cell mass, at least in part, through mitogenic effects (Levitt et al., 2010; Porat et al., 2011; Terauchi et al., 2007). These observations suggest shared molecular control of both β cell mass and function by glucose.
A high capacity glucose transport system and the high Km glucose-phosphorylating enzyme glucokinase (GK, Hexokinase IV) - the maturity onset diabetes of the young type 2 (MODY2) gene product - enable β cells to sense blood glucose fluctuations and tightly couple insulin secretion to glucose metabolism (Matschinsky, 2009). In addition to stimulating insulin secretion and proliferation, increased glucose metabolism can enhance β cell survival (Porat et al., 2011; Wei et al., 2009), which may additionally contribute to glucose stimulation of β cell mass. However, quantitative and mechanistic evidence for pro-survival effects of glucose in β cells are limited. Moreover, the precise effect of glucose on β cell viability is dependent on the extent and duration of increased glucose flux as well as the levels of free fatty acids that, when abnormally and chronically elevated, can cause β cell apoptosis through gluco- and glucolipo-toxicity (Bensellam et al., 2012).
The above observations indicate a complex link between glucose metabolism and the core apoptotic machinery in β cells. This complexity is further underscored by genetic manipulation of several anti- and pro-survival BCL-2 family regulators of apoptosis (Danial et al., 2008; Luciani et al., 2013; McKenzie et al., 2010; Zhou et al., 2000). In particular, BCL-XL and BCL-2 pro-survival molecules are β cell protective but inhibit glucose signaling and insulin secretion (Luciani et al., 2013; Zhou et al., 2000). This may be explained, in part, by the capacity of BCL-2 and BCL-XL to modulate Ca2+ flux (Danial et al., 2010), which is relevant for metabolic coupling of glucose and insulin secretion in β cells. Importantly, these findings highlight the challenge of targeting BCL-2 proteins for promoting functional β cell mass in a manner that ensures both resistance to apoptosis and proper glucose-modulation of insulin release.
We have previously shown that the BCL-2 family protein BAD stimulates the β cell glucose response and insulin secretion through phosphorylation of a defined residue within an amphipathic α helix known as the BCL-2 Homology (BH)-3 domain: Ser155 in mouse BAD corresponding to Ser118 in the human sequence (Danial et al., 2008). This modification neutralizes BAD’s apoptotic function and simultaneously triggers its ability to activate GK (Danial, 2008; Danial et al., 2008; Gimenez-Cassina et al., 2014; Szlyk et al., 2014). At the molecular and structural level, this is mediated by direct binding of the phospho-BAD BH3 helix near the active site of GK, resulting in the increase of Vmax through a non-allosteric mechanism without changes in the enzyme’s affinity for glucose (Szlyk et al., 2014). Importantly, studies in islets derived from Bad −/− and Bad S155A knockin mice, and human donor islets indicate that the phospho-BAD BH3 helix is required and sufficient for stimulation of insulin secretion in response to glucose (Danial et al., 2008; Szlyk et al., 2014). BAD phosphorylation is sensitive to fed/fasted states and hormones known to regulate β cell survival (Danial et al., 2008; Gimenez-Cassina et al., 2014; Liu et al., 2009), suggesting that BAD’s function may be normally in tune with nutrient and hormonal regulation of functional β cell mass. However, whether beyond neutralizing BAD’s apoptotic activity, BAD phosphorylation has active, cell autonomous effects on β cell survival has not been examined. Furthermore, the extent to which BAD phosphorylation may be protective against stress stimuli relevant to β cell demise in T1D is not known. This is especially relevant given functional redundancies as well as specialization among BCL-2 proteins in the regulation of cell death/survival. In the present studies, we undertook genetic and pharmacologic approaches to mimic BAD phosphorylation within its BH3 helix and determine its acute contribution to β cell survival in vitro, as well as its physiologic effects in replacing and promoting functional β cell mass in an islet transplantation model in vivo.
RESULTS
Survival-promoting effect of the BAD BH3 phospho-mimic variant in β cells
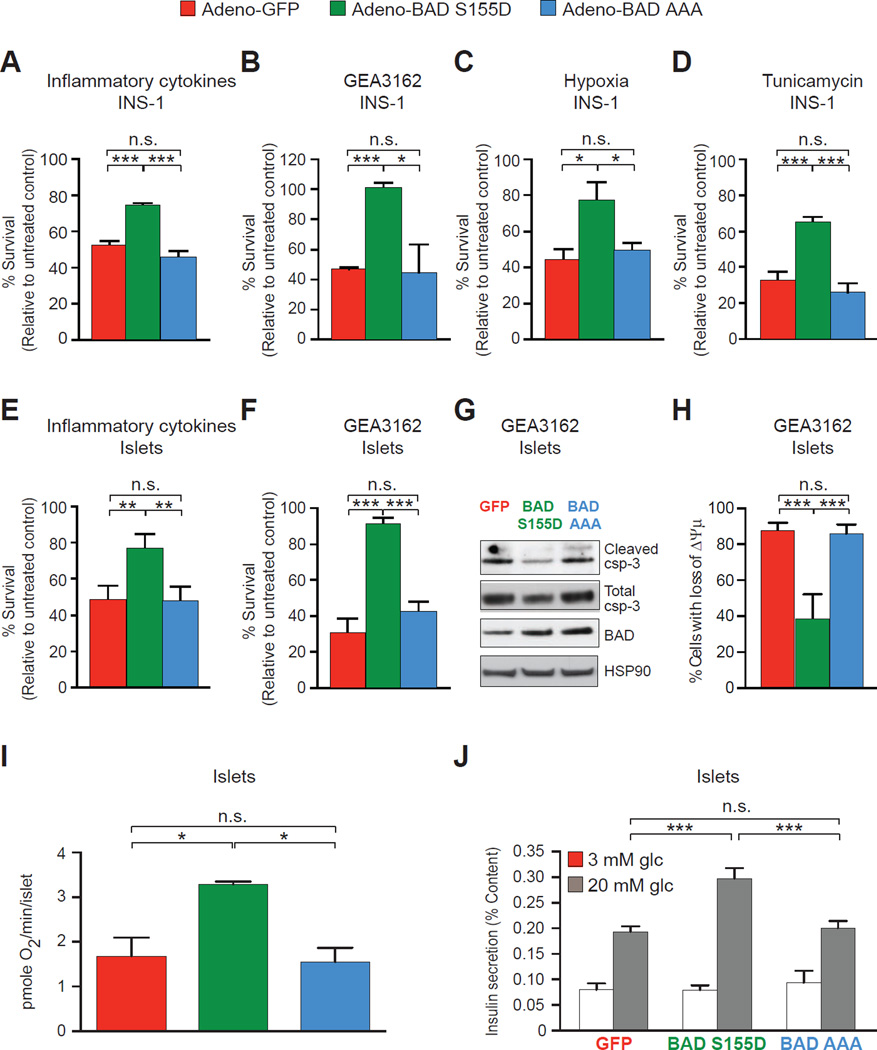
To examine the acute and cell autonomous outcome of BAD phosphorylation in β cell survival, we assessed the effect of the BAD S155D phospho-mimic variant on the viability of INS-1 cells (Figures 1A–D) and primary mouse pancreatic islets (Figures 1E–H) subjected to stress stimuli relevant to β cell death in T1D. These included inflammatory cytokines, oxidative and endoplasmic reticulum (ER) stress (Bedoya et al., 2012; Donath et al., 2008; Eizirik et al., 2013) (Figures S1A–C and Table S1). Compared with GFP-expressing controls, INS-1 cells and primary islets expressing BAD S155D were significantly protected from apoptosis induced by a combination of TNFα, IL-1β and IFNγ (Figures 1A and 1E), the nitric oxide (NO) donor GEA3162 (Figures 1B, F–H, and S1C), hypoxia (Figure 1C), and the ER stress inducer tunicamycin (Figure 1D).
Figure 1. Effect of BAD BH3 phospho-variants on β cell viability and glucose handling.
(A–H) Viability of INS-1 cells (A–D) and primary mouse islets (E–H) expressing GFP, BAD S155D or BAD AAA treated with inflammatory cytokines (combination of 20 ng/ml TNFα, 40 ng/ml IL-1β, and 10 ng/ml IFNγ for 48 hr) (A and E), NO donor GEA3162 (43 µM for 72 hr) (B and F–H), hypoxia (2% O2 for 24 hr) (C), or tunicamycin (5 ng/ml for 48 hr) (D). Cell viability/death was measured by annexin V and 7-AAD staining (A–F), caspase-3 (csp-3) cleavage (G), and loss of mitochondrial membrane potential (ΔΨm) (H).
(I and J) Effect of BAD BH3 phospho-variants on glucose handling as examined by mitochondrial respiration (I) and insulin secretion (J) following stimulation of primary islets with 20 mM glucose. Data are represented as means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., nonsignificant; n=3–8 independent experiments per treatment.
See also Figure S1 and Table S1.
At the molecular level, two scenarios may explain the protective effect of BAD S155D. First, because Ser155 phosphorylation blocks the BAD BH3 domain from binding and neutralizing the pro-survival BCL-2, BCL-XL, BCL-w proteins and subsequent lowering of the threshold for apoptosis (Danial, 2008), the protective effect of BAD S155D may be due to the inhibition of BAD’s pro-apoptotic function. Second, the protective effect of BAD S155D may result from its capacity to bind GK and stimulate glucose metabolism. To distinguish between these possibilities, we examined a second BAD BH3 phospho-mutant, BAD AAA (bearing the triple substitutions L151A, S155A, and D156A). Similar to BAD S155D, the AAA variant is inert in its apoptotic function as it cannot engage and inhibit the pro-survival BCL-2 proteins, but unlike BAD S155D, BAD AAA does not bind or activate GK (Danial et al., 2008; Gimenez-Cassina et al., 2014). If blocking BAD from neutralizing pro-survival BCL-2 proteins is sufficient to protect β cells, then the BAD S155D and AAA variants should be equally protective. If, however, activation of GK is relevant for the protective effect of phospho-BAD, then BAD AAA should lack β cell protective properties. Indeed, unlike BAD S155D, the AAA variant failed to protect β cells (Figures 1A–H), indicating that blocking BAD’s capacity to inhibit pro-survival BCL-2 proteins is not sufficient to protect β cells. Collectively, these results indicate that BAD phosphorylation has an acute and cell autonomous effect on β cell survival that co-segregates with its ability to engage GK.
Acute modulation of the β cell response to glucose by BAD phosphorylation
Processing of glucose-derived metabolites in β cell mitochondria generates metabolic signals such as ATP/ADP, NAD(P)H, and mitochondrial GTP that couple glucose to insulin release (Prentki et al., 2013). To examine the acute modulation of β cell glucose responsiveness by BAD phospho-mutants, we focused on glucose-stimulation of mitochondrial respiration and insulin secretion. Real-time measurements of mitochondrial oxygen consumption rates (OCR) indicated significantly higher respiration in islets expressing BAD S155D compared with GFP- or BAD AAA-expressing islets (Figure 1I). This increase in mitochondrial glucose oxidation was associated with a 35% increase in insulin release (Figure 1J, comparing GFP- and BAD S155D-expressing islets at 20 mM glucose). Notably, BAD S155D expression did not stimulate basal insulin release at 3 mM glucose (Figure 1J), a concentration that does not activate GK (Matschinsky, 2009). Insulin secretion in islets expressing the BAD AAA variant was comparable to that of GFP controls (Figure 1J). Overall, these data indicate an acute effect of BAD phosphorylation on mitochondrial handling of glucose and insulin release in primary islets.
The BAD phospho-BH3 helix is sufficient to protect β cells from apoptosis
The survival-promoting and function-enhancing benefits of BAD phosphorylation in β cells motivated the pursuit of phospho-BAD BH3 mimicry for improving functional β cell mass. We have previously shown that Stabilized Alpha-Helices of BCL-2 domains (SAHBs) generated by hydrocarbon stapling of peptides modeled after the BAD phospho-BH3 helix correct the insulin secretory defect of Bad −/− islets in response to glucose, indicating that this domain is sufficient to emulate BAD’s effect on β cell function (Danial et al., 2008). However, whether BAD SAHBs influence β cell survival is not known.
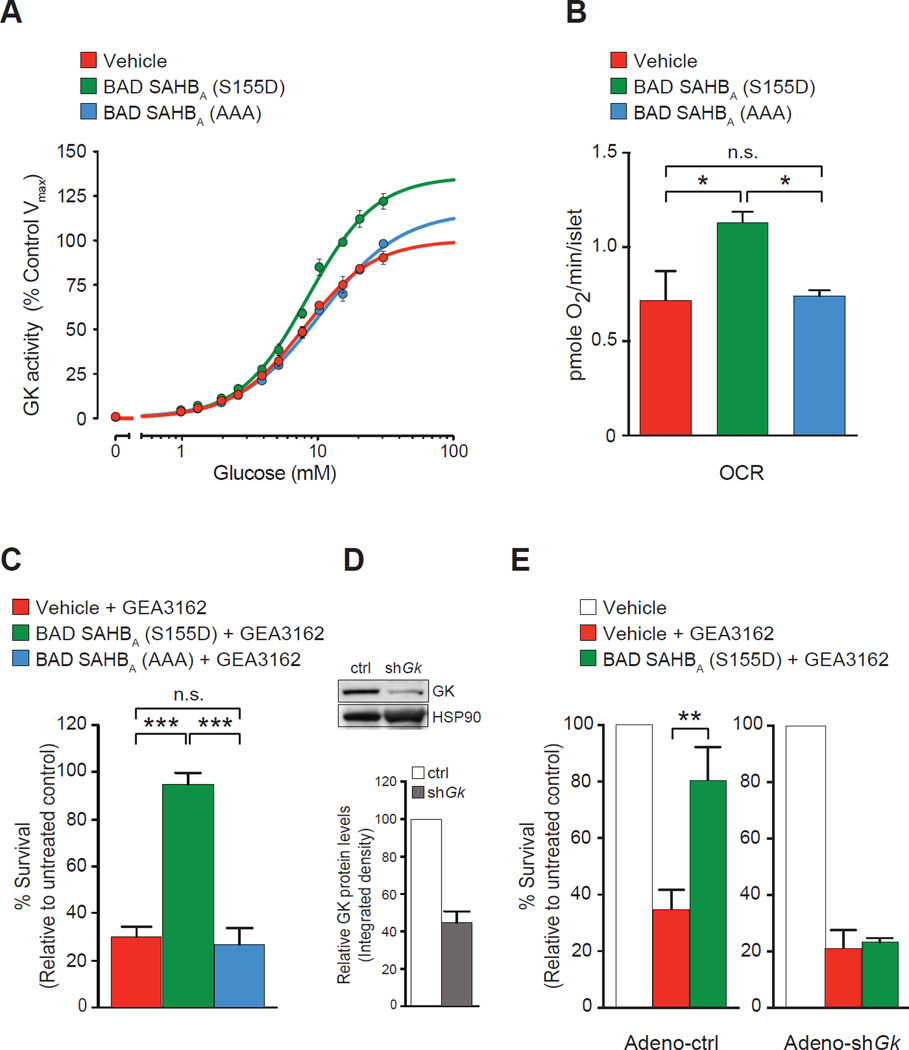
The clear benefits of full-length BAD S155D over BAD AAA in β cell survival and function prompted characterization of their corresponding stapled peptides, BAD SAHBA (S155D) and BAD SAHBA (AAA). Several quality control assays were performed to ensure the differential effect of the BAD BH3 domain on its metabolic target, GK, was preserved following modification by hydrocarbon stapling. In vitro GK activity assays confirmed that BAD SAHBA (S155D) directly activates recombinant GK while BAD SAHBA (AAA) does not as evidenced by changes in Vmax (Figure 2A and Table S2). This distinct GK-activating capacity was consistent with the differential effect of SAHBA (S155D) and SAHBA (AAA) on mitochondrial glucose handling in primary islets (Figure 2B), effectively replicating the phenotype of the full-length BAD S155D and AAA variants (Figure 1I).
Figure 2. GK-dependent protection of islet survival by the phospho-BAD BH3 helix.
(A) Activity of recombinant GK in the presence of vehicle or 5 µM of the indicated BAD SAHBA. Data show the means ± SD of a representative experiment. See Table S2 for the summary of enzyme kinetic parameters derived from 4 independent experiments performed similarly.
(B) Glucose stimulation of mitochondrial respiration in primary islets treated overnight with vehicle or 3 µM of the indicated BAD SAHBA (n=3).
(C) Viability of primary islets pre-treated with 10 µM of the indicated BAD SAHBA that were washed and treated with 43 µM GEA3162 for 72 hr (n=9).
(D–E) Viability of islets subjected to Gk knockdown (D) and treated with GEA3162 as in (C) (n=7). Data in B–E are represented as means ± SEM. *p < 0.05; **p <0.01; ***p < 0.001; n.s., nonsignificant.
See also Figure S2.
To test the protective effects of SAHBA (S155D), we chose the NO-induced islet death paradigm as a representative model of β cell stress. NO production is a prime component of β cell oxidative stress and toxicity caused by inflammatory cytokines (Bedoya et al., 2012). Remarkably, pre-treatment of islets with BAD SAHBA (S155D) but not BAD SAHBA (AAA) was sufficient to provide significant protection against death induced by the NO donor GEA3162 (Figure 2C). Of note, both SAHBA(S155D) and SAHBA (AAA) were taken up by islets with slightly higher uptake of SAHBA (AAA) (Figure S2A), ruling out differences in islet uptake as an explanation for the observed differences in β cell survival. Given the differential GK-activating capacity of BAD SAHBA compounds and the attendant effects on mitochondrial glucose handling (Figures 2A and 2B), we predicted that the survival-promoting function of BAD SAHBA (S155D) would be dependent on glucose metabolism. To test this possibility, islets treated with adenoviruses bearing Gk shRNA were analyzed in parallel (Figure 2D). Molecular depletion of GK curtailed the protective effect of BAD SAHBA (S155D) in this setting (Figures 2E), indicating that GK is required for the survival-promoting effects of the phospho-BAD BH3 mimetic. The protective effect of GK activation in this setting is also consistent with the observation that the GK activator (GKA) compound RO0281675 (Grimsby et al., 2003) rescued NO-induced death in the absence or presence of SAHBA (AAA) (Figure S2B).
Improved engraftment of donor islets treated with the phospho-BAD BH3 mimetic
To examine the physiological significance of phospho-BAD BH3 mimicry in β cell function and survival, we undertook transplantation studies using a marginal islet mass transplantation model. In this model, 150 mouse islets comprise a suboptimal number of donor islets that cannot reverse hyperglycemia when transplanted in mice rendered diabetic after streptozotocin (STZ)-mediated destruction of endogenous β cells (Montana et al., 1993). However, this limited number of donor islets can lead to diabetes reversal when used in conjunction with treatments that improve islet function and viability (Plesner and Verchere, 2011).
We reasoned that the survival-promoting effect of BAD SAHBA (S155D) may protect donor islets in early post-transplant periods (days 2–4), when their viability is severely compromised due to hypoxia prior to revascularization and inflammatory stress associated with tissue trauma during transplantation (Biarnes et al., 2002; Plesner and Verchere, 2011). In addition, because only a small percentage of transplanted islets that survive under these conditions display physiologic insulin secretory characteristics (Plesner and Verchere, 2011), we predicted that improved glucose responsiveness and insulin secretion in donor islets pre-treated with BAD SAHBA (S155D) would impart systemic benefits in transplant recipients.
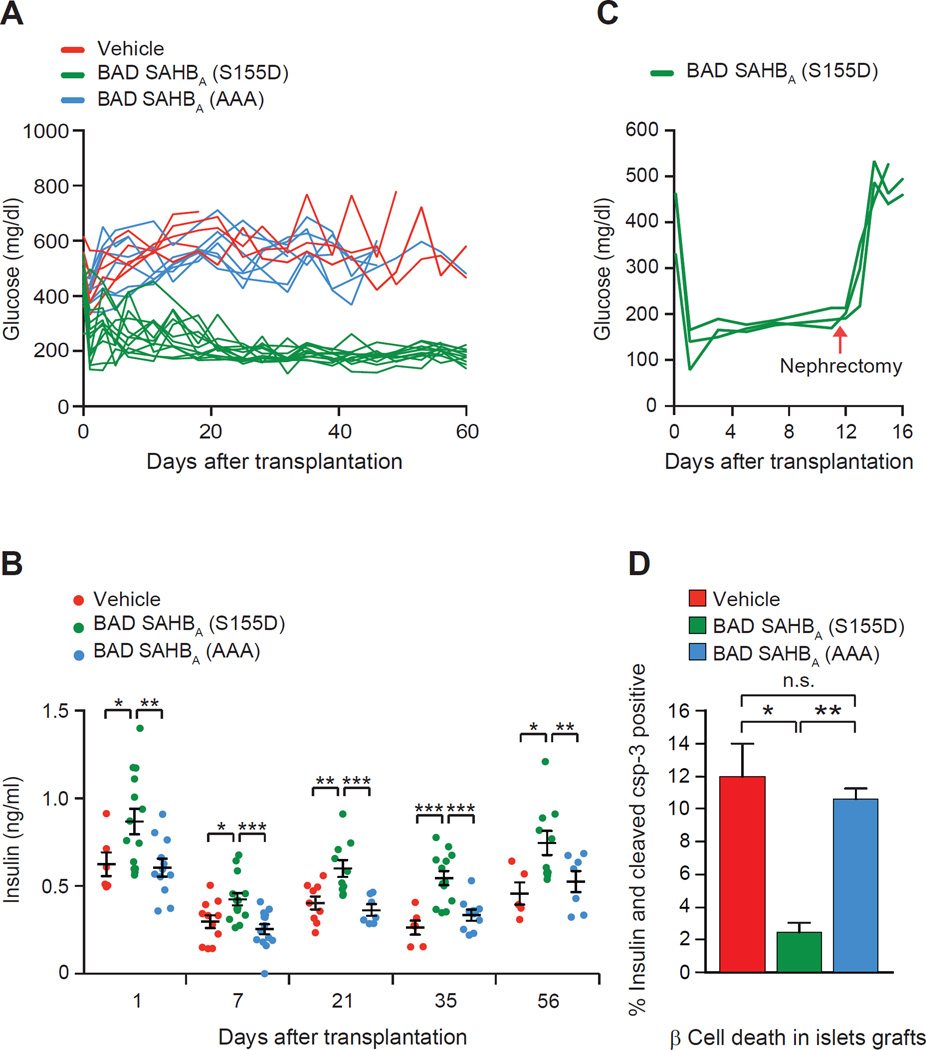
Blood glucose profiles were followed in a large cohort of STZ-treated diabetic mice that were transplanted with 150 wild-type islets pre-treated overnight with BAD SAHBA compounds or vehicle control. Pre-treatment of donor islets with BAD SAHBA (S155D) prior to transplantation markedly improved the glycemic profile in diabetic recipients, whereas mice transplanted with islets pre-treated with BAD SAHBA (AAA) or vehicle remained hyperglycemic with blood glucose values above 400 mg/dl (Figure 3A). Lowering of blood glucose levels in the former cohort was associated with improved plasma insulin levels (Figure 3B). Importantly, removal of islet grafts once normoglycemia was achieved led to hyperglycemia (Figure 3C), indicating that reversal of diabetes in these mice was specifically mediated by BAD SAHBA (S155D)-treated donor islets.
Figure 3. Engraftment of donor islets treated with BAD SAHBA compounds.
(A) Glycemic profiles of diabetic mice transplanted with donor islets pre-treated overnight with vehicle or 10 µM of the indicated BAD SAHBA. Each line represents one transplanted animal (n=6–11 mice per group).
(B) Insulin levels in transplant recipients (n=7–12 mice per group).
(C) Blood glucose levels in diabetic mice transplanted as in (A) and subjected to nephrectomy (red arrow) on day 12 after transplantation. Each line represents one transplanted animal (n=3).
(D) β cell death in islets grafts 2 days after transplantation as quantified by cleaved caspase-3 and insulin co-staining (n =3–5 per group). An average of 762 ± 252 cells were counted per condition. Data in B and D are represented as means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., nonsignificant.
To examine the contribution of islet graft survival to improved glycemia in diabetic mice, we assessed β cell survival in the early period post transplantation, when donor islets are prone to undergo cell death prior to revascularization (Biarnes et al., 2002; Montana et al., 1993; Plesner and Verchere, 2011). Assessment of cells doubly positive for insulin and cleaved caspase-3 in grafts excised on day 2 revealed significantly lower β cell death in grafts from BAD SAHBA (S155D)treated donor islets compared with grafts from vehicle-treated or BAD SAHBA (AAA)-treated controls (Figure 3D). This paralleled the survival-promoting effect of phospho-BAD BH3 mimicry in vitro (Figures 1A–H and 2C).
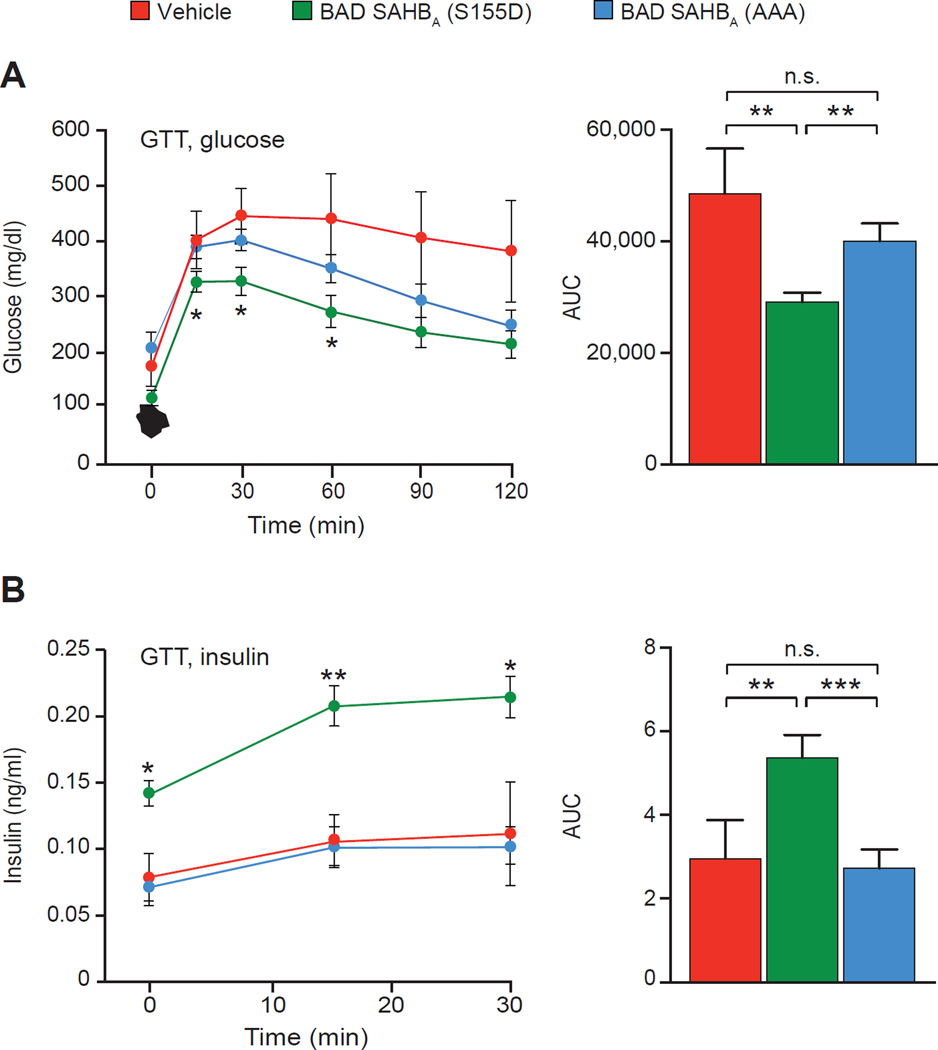
To assess the overall outcomes of BAD SAHB pre-treatment on islet graft performance with respect to glucose homeostasis, mice were subjected to intraperitoneal glucose tolerance test (i.p. GTT) on day 49 after transplantation. Blood glucose levels during GTT were significantly lower in recipients transplanted with BAD SAHBA (S155D)-treated donor islets but not in control cohorts transplanted with vehicle- or SAHBA (AAA)-treated islets (Figure 4A). Improved glucose tolerance was associated with improved insulin release in the first 30 min post glucose injection when insulin normally peaks during i.p. GTT, indicating glucose-responsive insulin secretion by islet grafts in vivo (Figure 4B). In contrast, mice transplanted with vehicle- or SAHBA (AAA)-treated donor islets did not elicit an insulin response to glucose (Figure 4B), which was consistent with their GTT profile (Figure 4A). Overall, these data show that the survival-promoting and function-enhancing benefits of BAD phosphorylation in β cells extend to improved β cell replacement and functional β cell mass in diabetic mice.
Figure 4. Glucose homeostasis in transplant recipients.
(A and B) Blood glucose (A) and insulin (B) levels during i.p. GTT performed 49 days after transplantation (n=11 per BAD SAHBA group). Asterisks in line graphs on the left compare the BAD SAHBA (S155D) and SAHBA (AAA) groups. Area under the curve (AUC) for glucose and insulin is shown on the right.
Data are represented as means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., nonsignificant.
DISCUSSION
Our findings indicate that genetic and pharmacologic approaches to mimic BAD phosphorylation within its BH3 helix impart β cell protective effects in an acute and cell autonomous manner. The short-term benefits of phospho-BAD in β cells include resistance to apoptosis in the face of inflammatory, oxidative and ER stress stimuli, as well as increased glucose handling and insulin secretory capacities. In the long-term, these benefits manifest in superior engraftment of donor islets in transplanted diabetic mice with an attendant increase in functional β cell mass. Collectively, these observations highlight the utility of BAD phospho-BH3 mimetic approaches in augmenting functional β cell mass in diabetes.
At the mechanistic level, parallel comparison of informative phospho-BH3 BAD variants indicates that the effect of BAD phosphorylation on β cell survival is linked to its activation of GK and cannot be solely explained by the lack of its inhibitory effects on pro-survival BCL-2 proteins. Consistent with these observations, the protective effect of BAD SAHBA (S155D) is abrogated following molecular depletion of GK. In addition, GK gain-of-function is β cell protective in islets treated with BAD SAHBA (AAA). The precise branch-points of glucose metabolism downstream of the BAD-GK axis that may promote β cell survival are currently under investigation. Glucose-derived metabolites can feed a number of anabolic reactions that may be relevant in this setting. In addition, changes in ATP and Ca2+ flux following mitochondrial metabolism of glucose-derived pyruvate may provide bioenergetic benefits that can be β cell protective. Furthermore, whether and how the metabolic outputs of glucose that promote β cell survival overlap with those that mediate β cell proliferation such as IRS-2, TORC1/S6K, CaMK, PKC, and sphingosine kinase remain to be determined (Elghazi et al., 2010; Mastrandrea et al., 2010; Sjoholm, 1997; Terauchi et al., 2007). Notably, under the experimental settings used in Figures 3 and 4, β cell proliferation in islet grafts is not altered by pre-treatment with BAD SAHBA compounds (J. C. A.-P., A. G.-O, and N.N.D., unpublished data). Thus, improved β cell mass and function under these specific conditions is predominantly associated with increased survival and insulin secretory capacity of donor islets treated with BAD SAHBA (S155D). However, thorough assessment of the potential connection between phospho-BAD and β cell proliferation requires full examination of other in vivo models of β cell proliferation.
Increased glucose flux through GK activation may have therapeutic utility in promoting β cell mass, however, these benefits have to be carefully weighed against the adverse effects of chronic elevation in glucose metabolism, such as glucotoxicity-induced β cell dysfunction/loss. This warrants characterization of the precise conditions to derive the therapeutic benefits of glucose metabolism for β cell mass restoration. Within this context, the mechanism of GK activation and the duration of increased glucose flux are important considerations. For example, allosteric GKAs increase glucose flux but drastically enhance the enzyme’s affinity for glucose, which may not be beneficial in the long-term (Matschinsky, 2009). A distinct class of GKAs with a non-allosteric mode of action that do not dramatically alter the affinity of GK for glucose, such as phospho-BAD BH3 mimetics (Szlyk et al., 2014), may be beneficial in this setting. In addition, defining the minimal metabolic outputs of glucose that promote β cell mass may provide translational insights into the most effective strategies for harnessing the benefits of glucose signaling to pharmacologically restore functional β cell mass.
EXPERIMENTAL PROCEDURES
Primary islets, cell culture, and insulin secretion assays
Primary mouse islets isolation and culture, as well as insulin release assays were performed as previously described (Danial et al., 2008). INS-1 cells were a kind gift of Dr. Christopher Newgard (Duke University).
Treatment with death stimuli and assessment of cell death/survival
INS-1 cells and primary mouse islets were seeded in 6-well plates at 4×105 INS-1 cells/well and 100 islets/well, respectively, and treated with the following combination of inflammatory cytokines (R&D systems); 20 ng/ml TNFα, 40 ng/ml IL-1β, and 10 ng/ml IFNγ for 48 hr. The NO donor GEA3162 (Enzo Life Sciences) was added at 43 µM in DMSO for 72 hr. Hypoxia was induced by incubating cells at 2% O2 for 24 hr. Tunicamycin (Sigma) was added at 5 ng/ml in DMSO for 48 hr. Control treatment for inflammatory cytokines was PBS, and that for GEA3162 and tunicamycin was 0.1% DMSO. as described below and further detailed in Supplemental Experimental Procedures, cell death/survival was quantified by several parameters, including annexin V and 7-amino-actinomycin D (7-AAD) staining, changes in mitochondrial membrane potential (ΔΨm), and western blotting with an antibody to cleaved (active) caspase-3.
For flow cytometric measurement of cell/death survival, cells were labeled with annexin V (BD Pharmingen) and 7-AAD (BD Biosciences), and subjected to dual color flow cytometry using a FACSCANTO II flow cytometer (Becton Dickinson),and the BD FACSDIVA™ software (BD Biosciences). For each measurement, 104 cells were sorted and gates were applied to generate four quadrants based on the level of annexin V (PE) and 7-AAD (Cy5) staining intensities, and percent survival was calculated based on annexin V and 7-AAD double negativity. Figures S1A and B and Table S1 document percent live cells or those undergoing apoptosis in control INS-1 cells and primary islets treated with the indicated death stimuli. See also Supplemental Experimental Procedures.
For measurement of mitochondrial membrane potential, primary islets were incubated in the presence or absence of GEA3162 as described above, dispersed and live stained with 10 nM tetramethylrhodamine, ethyl ester (TMRE, Molecular Probes) in RPMI for 30 min at 37°C. Treatment with 1 µM of the protonophore carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP, Sigma) was used to dissipate the mitochondrial membrane potential and to define the base line for the analysis of mitochondrial membrane potential (ΔΨm). Cells were rinsed with RPMI (without phenol) containing 1% FBS and analyzed by flow cytometry using a FACSCANTO II flow cytometer (Becton Dickinson)and the BD FACSDIVA™ software (BD Biosciences). Both mean intensities and % cells that showed loss of ΔΨm were analyzed. Representative FACS plots of mitochondrial membrane potential changes in primary islets expressing GFP, BAD S155D or BAD AAA treated with DMSO or GEA3162 are shown in Figure S1C.
Adenovirus production and viral infection
Recombinant adenoviruses carrying GFP, BAD S155D and BAD AAA variants, as well as Gk and control shRNA have been previously described (Gimenez-Cassina et al., 2014). INS-1 cells were infected using a multiplicity of infection of 75 for 48 hr. Adenoviral transduction of primary mouse islets were carried out at 1×105 pfu/islet for 48 hr.
Mitochondrial respirometry
Real-time measurements of mitochondrial oxygen consumption rate (OCR) were performed using the XF24 extracellular flux analyzer instrument (Seahorse Bioscience) as described in Supplemental Experimental Procedures.
SAHB synthesis and treatments
Peptide synthesis, olefin metathesis, FITC derivatization, reverse phase HPLC purification, and amino acid analysis were performed as previously described (Danial et al., 2008; Szlyk et al., 2014). For survival assays and donor islet treatments, islets were treated overnight with 10 µM of the indicated FITC BAD SAHBA in 1% DMSO in RPMI SAHB uptake medium containing 11 mM glucose, 10% serum, and 0.1% Tween 80 pH 6.0 at 37 °C. Control islets were treated with vehicle composed of DMSO and SAHB uptake medium. Islets were then washed and incubated in fresh media for 2 hr before in vitro studies or transplantation. For OCR studies in Figure 1I, islets were treated with 3 µM of the indicated SAHB compounds. For GK activity assays in Figure 2A, BAD SAHBs were added at 5 µM final concentration and 5% DMSO was used as vehicle control. Recombinant GK activity assays were performed as previously described (Szlyk et al., 2014).
Islet transplantation and metabolic studies
150 islets derived from C57BL/6J mice were treated as indicated above and transplanted under the kidney capsule of male STZ-treated diabetic C57BL/6J mice using established procedures (Montana et al., 1993) that are further detailed in Supplemental Experimental Procedures. Blood glucose and insulin measurements, and i.p. GTT were performed as previously described (Danial et al., 2008; Gimenez-Cassina et al., 2014). Islet graft viability was assessed by immunofluorescence as described in Supplemental Experimental Procedures. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Statistical Analysis
Unless otherwise indicated, data are represented as means ± SEM. Statistical significance was calculated using One-way ANOVA. Statistical significance was defined as p < 0.05.
Supplementary Material
Acknowledgments
We thank Elaura Patton and Gabriella Casinelli for technical support, and Jill Fisher for help with islet transplantation surgeries. This work was supported by the U.S. National Institutes of Health grants R01DK078081 (N.N.D.), R01 DK067351 and R01 DK077096 (A. G.-O.), R01GM090299 (L.D.W.), Burroughs Wellcome Fund Career Award in Biomedical Sciences (N.N.D.), Juvenile Diabetes Research Foundation Grant 17-2011-595 (N.N.D.), Barry and Mimi Sternlicht Type 1 Diabetes Research Fund (N.N.D.),Claudia Adams Barr Award in Innovative Basic Cancer Research (N.N.D.), and a Swiss National Science Foundation postdoctoral fellowship (S.L.). The authors also acknowledge generous support from John Lippincott. L.D.W. is a consultant and scientific advisory board member for Aileron Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.L., A.F., and N.N.D. designed experiments. S.L., A.F., K.P., J.M.W., and N.N.D. conducted in vitro studies with INS-1 cells and primary islets, performed islet transplantation, and carried out in vivo studies. B.S. and N.N.D. purified recombinant GK and performed enzyme kinetic analyses. Y. C., J. C. A.-P., and A. G.-O. performed immunofluorescence analyses. G.H.B. and L.D.W. designed and synthesized SAHB compounds. S.L., A.F., and N.N.D. wrote the manuscript, which was reviewed by all authors.
REFERENCES
- Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, Tejedo JR. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets. 2012;4:108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol. 2012;364:1–27. doi: 10.1016/j.mce.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- Claiborn KC, Stoffers DA. Toward a cell-based cure for diabetes: advances in production and transplant of beta cells. Mt Sinai J Med. 2008;75:362–371. doi: 10.1002/msj.20058. [DOI] [PubMed] [Google Scholar]
- Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gimenez-Cassina A, Tondera D. Homeostatic functions of BCL-2 proteins beyond apoptosis. Adv Exp Med Biol. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56:234–241. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- Elghazi L, Balcazar N, Blandino-Rosano M, Cras-Meneur C, Fatrai S, Gould AP, Chi MM, Moley KH, Bernal-Mizrachi E. Decreased IRS signaling impairs beta-cell cycle progression and survival in transgenic mice overexpressing S6K in beta-cells. Diabetes. 2010;59:2390–2399. doi: 10.2337/db09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Cassina A, Garcia-Haro L, Choi CS, Osundiji MA, Lane EA, Huang H, Yildirim MA, Szlyk B, Fisher JK, Polak K, Patton E, Wiwczar J, Godes M, Lee DH, Robertson K, Kim S, Kulkarni A, Distefano A, Samuel V, Cline G, Kim YB, Shulman GI, Danial NN. Regulation of Hepatic Energy Metabolism and Gluconeogenesis by BAD. Cell Metab. 2014;19:272–284. doi: 10.1016/j.cmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- Halban PA, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. The Journal of clinical endocrinology and metabolism. 2010;95:1034–1043. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M. Generating beta cells from stem cells-the story so far. Cold Spring Harb Perspect Med. 2012;2:a007674. doi: 10.1101/cshperspect.a007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, Rosa T, Romano LC, Zou B, O'Donnell CP, Stewart AF, Garcia-Ocana A, Alonso LC. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2010;54:572–582. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H, Shirihai OS, Abel ED, Kulkarni RN. Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PLoS One. 2009;4:e7983. doi: 10.1371/journal.pone.0007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani DS, White SA, Widenmaier SB, Saran VV, Taghizadeh F, Hu X, Allard MF, Johnson JD. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic beta-cells. Diabetes. 2013;62:170–182. doi: 10.2337/db11-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrandrea LD, Sessanna SM, Del Toro A, Laychock SG. ATP-independent glucose stimulation of sphingosine kinase in rat pancreatic islets. J Lipid Res. 2010;51:2171–2180. doi: 10.1194/jlr.M000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- McKenzie MD, Jamieson E, Jansen ES, Scott CL, Huang DC, Bouillet P, Allison J, Kay TW, Strasser A, Thomas HE. Glucose induces pancreatic islet cell apoptosis that requires the BH3-only proteins Bim and Puma and multi-BH domain protein Bax. Diabetes. 2010;59:644–652. doi: 10.2337/db09-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Melton DA. How to make a functional beta-cell. Development. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesner A, Verchere CB. Advances and challenges in islet transplantation: islet procurement rates and lessons learned from suboptimal islet transplantation. J Transplant. 2011;2011:979527. doi: 10.1155/2011/979527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, Girard CA, Karni R, Kaestner KH, Ashcroft FM, Magnuson MA, Saada A, Grimsby J, Glaser B, Dor Y. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Sjoholm A. Glucose stimulates islet beta-cell mitogenesis through GTP-binding proteins and by protein kinase C-dependent mechanisms. Diabetes. 1997;46:1141–1147. doi: 10.2337/diab.46.7.1141. [DOI] [PubMed] [Google Scholar]
- Szlyk B, Braun CR, Ljubicic S, Patton E, Bird GH, Osundiji MA, Matschinsky FM, Walensky LD, Danial NN. A phospho-BAD BH3 helix activates glucokinase by a mechanism distinct from that of allosteric activators. Nat Struct Mol Biol. 2014;21:36–42. doi: 10.1038/nsmb.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Shi M, Barnum S, Cho H, Carlson T, Fraser JD. Effects of glucokinase activators GKA50 and LY2121260 on proliferation and apoptosis in pancreatic INS-1 beta cells. Diabetologia. 2009;52:2142–2150. doi: 10.1007/s00125-009-1446-0. [DOI] [PubMed] [Google Scholar]
- Zhou YP, Pena JC, Roe MW, Mittal A, Levisetti M, Baldwin AC, Pugh W, Ostrega D, Ahmed N, Bindokas VP, Philipson LH, Hanahan D, Thompson CB, Polonsky KS. Overexpression of Bcl-x(L) in beta-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am J Physiol Endocrinol Metab. 2000;278:E340–E351. doi: 10.1152/ajpendo.2000.278.2.E340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.