Abstract
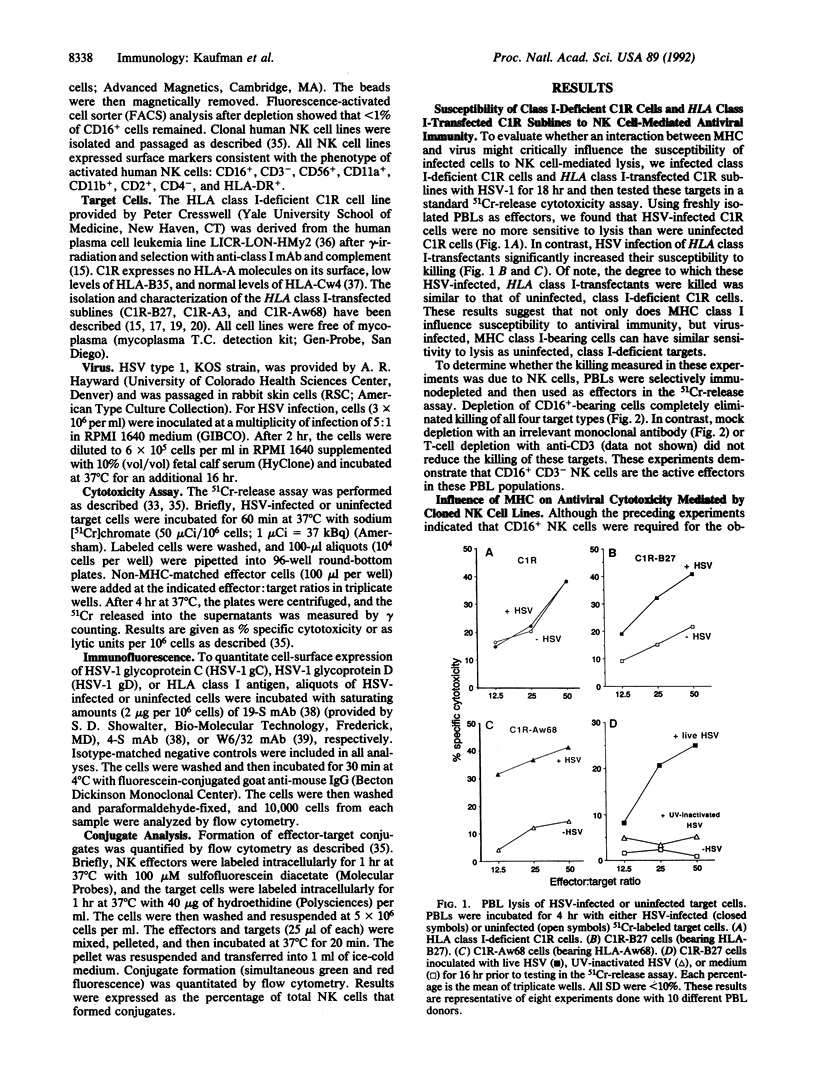
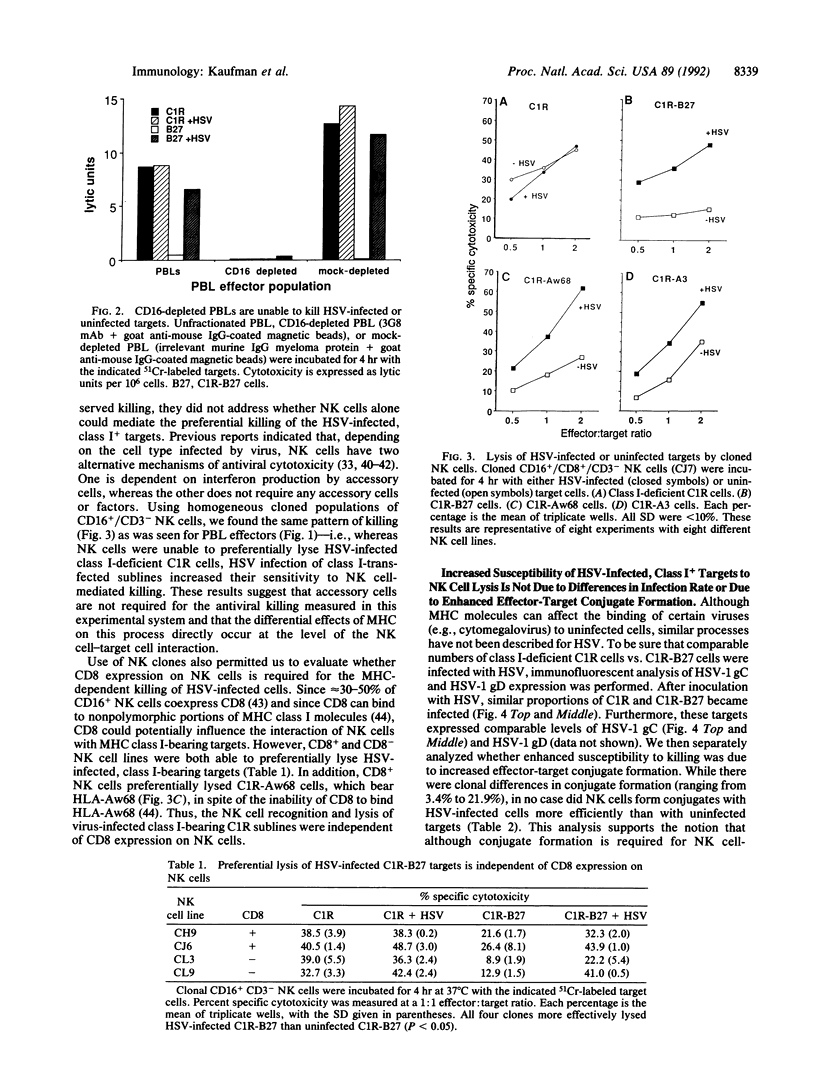
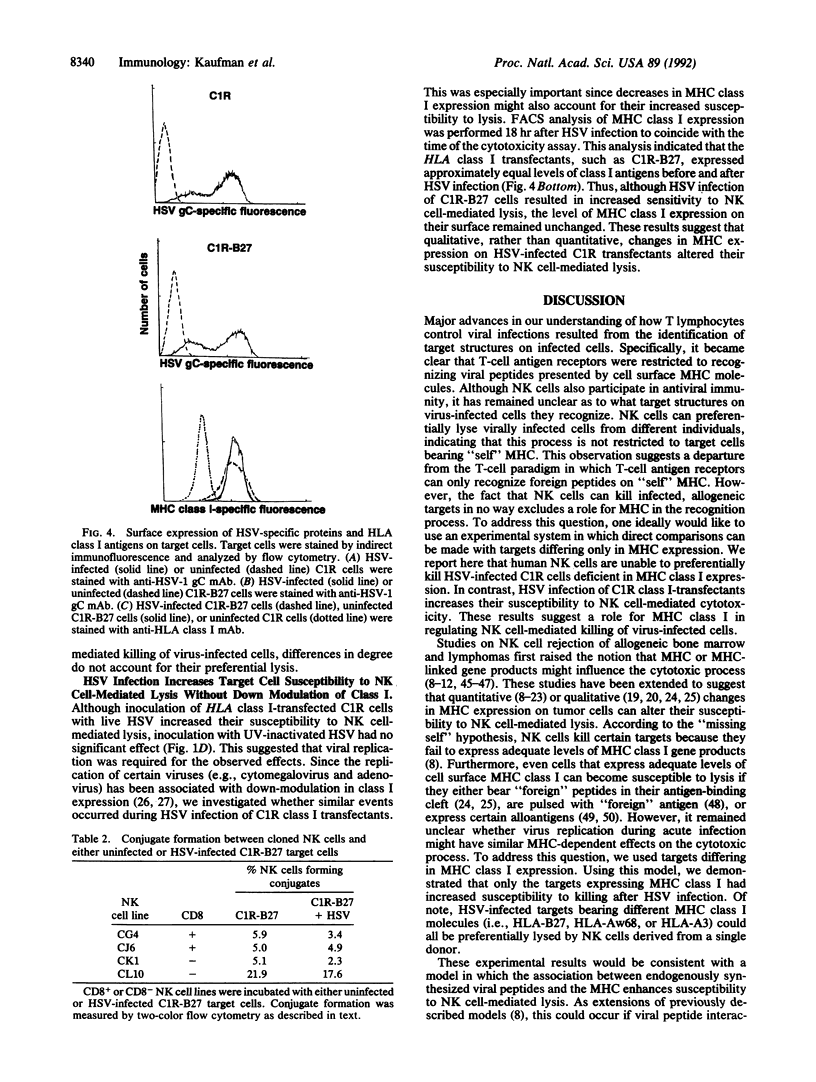
Target structures important for natural killer (NK) cell recognition of virally infected cells are not well defined. Since major histocompatibility complex (MHC) class I molecules bind viral peptides during acute infection, we evaluated whether an interaction between MHC and virus might influence the susceptibility of infected cells to NK cell-mediated lysis. To control for MHC class I expression on target cells, we used either HLA class I-deficient C1R cells or C1R sublines expressing transfected HLA class I gene products. Human NK cells were unable to preferentially lyse class I-deficient C1R cells after infection with herpes simplex virus (HSV). In contrast, HLA class I transfectants were significantly more susceptible to NK cell-mediated cytotoxicity after HSV infection. This occurred for HSV-infected C1R cells expressing any of the three HLA class I gene products tested (i.e., HLA-B27, HLA-A3, or HLA-Aw68), indicating that NK cell recognition in this system does not require "self" MHC and is not unique for a single haplotype. Productive HSV infection is required for the increased killing, since inoculation with UV-inactivated virus did not lead to increased lysis. In addition, since HSV infection of the transfectants did not significantly alter the level of class I expression, the change in susceptibility appears to be due to qualitative changes in the target structures on HSV-infected, HLA class I+ targets. These results demonstrate a role for MHC class I in regulating NK cell-mediated killing of virus-infected cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay S., Perussia B., Trinchieri G., Miller D. S., Starr S. E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986 Jul 1;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bix M., Liao N. S., Zijlstra M., Loring J., Jaenisch R., Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991 Jan 24;349(6307):329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- Chadwick B. S., Miller R. G. Hybrid resistance in vitro. Possible role of both class I MHC and self peptides in determining the level of target cell sensitivity. J Immunol. 1992 Apr 1;148(7):2307–2313. [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. I. Graft rejection by irradiated responder mice. J Exp Med. 1971 Jul 1;134(1):83–102. doi: 10.1084/jem.134.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Keller R., Lee G., Finkel D. Lysis of cytomegalovirus-infected human fibroblasts and transformed human cells by peripheral blood lymphoid cells from normal human donors. Proc Soc Exp Biol Med. 1977 Feb;154(2):259–263. doi: 10.3181/00379727-154-39650. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Smith C. M., Neville A. M., O'Hare M. J. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol. 1982 Aug;12(8):641–648. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Feldman M., Curl S., Schnell J., Denny T. Positively selected Leu-11a (CD16+) cells require the presence of accessory cells or factors for the lysis of herpes simplex virus-infected fibroblasts but not herpes simplex virus-infected Raji. J Immunol. 1989 Aug 15;143(4):1318–1326. [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Howell D. M., Pettera L., Tehrani S., Lopez C. Immediate-early gene expression is sufficient for induction of natural killer cell-mediated lysis of herpes simplex virus type 1-infected fibroblasts. J Virol. 1991 Jun;65(6):3151–3160. doi: 10.1128/jvi.65.6.3151-3160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Fitzgerald P. A., Mendelsohn M., Lopez C. Human natural killer cells limit replication of herpes simplex virus type 1 in vitro. J Immunol. 1985 Apr;134(4):2666–2672. [PubMed] [Google Scholar]
- Gooding L. R., Wold W. S. Molecular mechanisms by which adenoviruses counteract antiviral immune defenses. Crit Rev Immunol. 1990;10(1):53–71. [PubMed] [Google Scholar]
- Harel-Bellan A., Quillet A., Marchiol C., DeMars R., Tursz T., Fradelizi D. Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5688–5692. doi: 10.1073/pnas.83.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Höglund P., Ohlén C., Carbone E., Franksson L., Ljunggren H. G., Latour A., Koller B., Kärre K. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Kärre K. MHC gene control of the natural killer system at the level of the target and the host. Semin Cancer Biol. 1991 Oct;2(5):295–309. [PubMed] [Google Scholar]
- Leibson P. J., Hunter-Laszlo M., Hayward A. R. Inhibition of herpes simplex virus type 1 replication in fibroblast cultures by human blood mononuclear cells. J Virol. 1986 Mar;57(3):976–982. doi: 10.1128/jvi.57.3.976-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985 Dec 1;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Maudsley D. J., Pound J. D. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991 Dec;12(12):429–431. doi: 10.1016/0167-5699(91)90013-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Pende D., Tripodi G., Tambussi G., Viale O., Orengo A., Barbaresi M., Merli A., Ciccone E. Identification of four subsets of human CD3-CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990 Dec 1;172(6):1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya C. V., Schoon R. A., Leibson P. J. Alternative mechanisms of natural killer cell activation during herpes simplex virus infection. J Immunol. 1990 Jun 1;144(11):4370–4375. [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4(3):120–137. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984 Mar;132(3):1410–1415. [PubMed] [Google Scholar]
- Quillet A., Presse F., Marchiol-Fournigault C., Harel-Bellan A., Benbunan M., Ploegh H., Fradelizi D. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression. Direct demonstration using beta 2-microglobulin-transfected Daudi cells. J Immunol. 1988 Jul 1;141(1):17–20. [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Shah P. D., Gilbertson S. M., Rowley D. A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985 Aug 1;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989 Mar;19(3):447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Cresswell P., Dawson J. R. The alpha 1/alpha 2 domains of class I HLA molecules confer resistance to natural killing. J Immunol. 1989 Dec 1;143(11):3853–3857. [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkus W. J., Dawson J. R. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol. 1991;10(5):393–416. [PubMed] [Google Scholar]
- Storkus W. J., Howell D. N., Salter R. D., Dawson J. R., Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987 Mar 15;138(6):1657–1659. [PubMed] [Google Scholar]
- Storkus W. J., Salter R. D., Alexander J., Ward F. E., Ruiz R. E., Cresswell P., Dawson J. R. Class I-induced resistance to natural killing: identification of nonpermissive residues in HLA-A2. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5989–5992. doi: 10.1073/pnas.88.14.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Suzuki T., Engleman E. G. Evidence for the involvement of CD56 molecules in alloantigen-specific recognition by human natural killer cells. J Exp Med. 1991 Jun 1;173(6):1451–1461. doi: 10.1084/jem.173.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978 Nov;121(5):1631–1635. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Windebank K. P., Abraham R. T., Powis G., Olsen R. A., Barna T. J., Leibson P. J. Signal transduction during human natural killer cell activation: inositol phosphate generation and regulation by cyclic AMP. J Immunol. 1988 Dec 1;141(11):3951–3957. [PubMed] [Google Scholar]
- Yu Y. Y., Kumar V., Bennett M. Murine natural killer cells and marrow graft rejection. Annu Rev Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- Zemmour J., Little A. M., Schendel D. J., Parham P. The HLA-A,B "negative" mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992 Mar 15;148(6):1941–1948. [PubMed] [Google Scholar]



