Abstract
Smoking initiation predominantly occurs during adolescence, often in the presence of peers. Therefore, understanding the neural mechanisms underlying the rewarding effects of nicotine and social stimuli is vital. Using the conditioned place preference (CPP) procedure, we measured immediate early gene (IEG) expression in animals following exposure either to a reward-conditioned environment or to the unconditioned stimuli (US). Adolescent, male rats were assigned to the following CPP US conditions: (1) Saline + Isolated, (2) Nicotine + Isolated, (3) Saline + Social, or (4) Nicotine + Social. For Experiment 1, brain tissue was collected 90 min following the CPP expression test and processed for Fos immunohistochemistry. We found that rats conditioned with nicotine with or without a social partner exhibited CPP; however, we found no group differences in Fos expression in any brain region analyzed, with the exception of the nucleus accumbens core that exhibited a social-induced attenuation in Fos expression. For Experiment 2, brain tissue was collected 90 min following US exposure during the last conditioning session. We found social reward-induced increases in IEG expression in striatal and amydalar subregions. In contrast, nicotine reduced IEG expression in prefrontal and striatal subregions. Reward interactions were also found in the dorsolateral striatum, basolateral amygdala, and ventral tegmental area where nicotine alone attenuated IEG expression and social reward reversed this effect. These results suggest that in general social rewards enhance, whereas nicotine attenuates, activation of mesocorticolimbic regions; however, the rewards given together interact to enhance activation in some regions. The findings contribute to knowledge of how a social environment influences nicotine effects.
Keywords: Conditioned place preference, Immunohistochemistry, Drug, Addiction, Fos, Zif268
1. Introduction
Smoking is a major societal concern, with one out of every five deaths in the United States resulting from health-related consequences of smoking [1]. Initiation of smoking most commonly occurs during adolescence [2–5], where adolescents become dependent at a faster rate and have more difficulty with cessation than adults [3,6–8]. Despite high abuse liability of tobacco products containing nicotine, the reinforcing effects of nicotine itself are relatively weak in pre-clinical animal models of self-administration; however, nicotine has rewarding effects across a range of doses in conditioned place preference (CPP) models [9–12]. In particular, adolescent rodents demonstrate greater sensitivity to the rewarding and reinforcing effects of nicotine [9,11,13–21], and are less sensitive to the aversive properties of nicotine and nicotine withdrawal [10,13,22–26]. Thus, adolescence is a critical period of increased vulnerability to develop nicotine addiction.
Social interaction during adolescence fosters healthy development and appropriate social behavior in adulthood in humans and rodents alike [27–33]. In rodents, social interaction functions as a robust natural reward, as measured by both operant [34–38] and classical conditioning paradigms [12,39–41]. Pro-social interactions exert a substantial influence on drug-related behaviors largely by increasing the rewarding and reinforcing effects of the drugs themselves (see [42,43] for review). For instance, our lab has previously found that social interaction enhances both nicotine and cocaine CPP [12,44]. Additionally, the presence of a conspecific also enhances stimulant self-administration [45–47]. Given that social reinforcement such as ‘group membership’ and ‘peer encouragement’ are cited as the most prevalent reasons for initiation of smoking and tobacco use among adolescents and young adults [48–52], the initial aversive physiological reactions to cigarettes (i.e., coughing, nausea and vomiting) may be overpowered by the strong rewarding and reinforcing effects of social interaction.
The modulatory role social interaction plays in the initial drug experience is crucial for understanding neural processes involved in the development of nicotine addiction. Both nicotine and social reward each independently activate mesocorticolimbic pathways [53–58]; however, little is known about the neural mechanisms involved in processing both stimuli together. A useful approach for addressing this gap in knowledge is to examine immediate early gene expression, which has been widely used as a functional marker of neuronal activation in response to drug- and drug-associated stimuli [59–63].
In the present study, male adolescent rats were conditioned with nicotine and/or social stimuli using previously established experimental parameters from our laboratory [12]. We then used Fos protein expression to examine the neural circuitry involved in the combination of social and nicotine rewards and their reward-associated environments. Our significant effects from Experiment 2 were later examined and confirmed using another immediate early gene, Zif268 (i.e., EGR1). We hypothesized that nicotine and social reward stimuli, as well as exposure to the respective reward-associated environment, would elicit a more robust increase in functional activation within the cortical, striatal, and limbic circuitry when presented in combination than when presented individually.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (Charles River, San Diego, CA) (N = 130) arrived at Arizona State University on postnatal day (PND) 22 (i.e., 22 days old) for both experiments. They were individually housed in a climate-controlled facility with a 12-h light/dark cycle (lights on at 7 PM) with ad libitum access to food and water. Housing and care were conducted in accordance with the 8th ed. Guide for the Care and Use of Laboratory Animals [64]. All experiments were conducted within a conservative estimated timeframe of rodent adolescence: PNDs 28–42 (Spear 2000). Prior to baseline testing, animals were acclimated to handling for 9–11 days. On each of these days, rats were handled for at least 2 min/day.
2.2. Drug preparation
(−)Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in 0.9% sterile saline and the pH was adjusted to 7.2. All injections were given subcutaneously (s.c.) at a volume of 1 mL/kg. Dose is reported as nicotine base.
2.3. Apparatus
Conditioning took place in rectangular Plexiglas chambers as previously described [65]. Each chamber contained a removable solid partition that separated the chamber into two equal-sized compartments, each measuring 35 × 24 × 31 cm high. One compartment had corncob bedding beneath a wire 1 × 1 cm grid floor and alternating black and white vertical stripes on the walls. The other compartment had pine-scented bedding beneath a parallel bar floor (5 mm diameter) and alternating black and white horizontal stripes on the walls. On the pre- and post-conditioning test days, the removable center partition of the apparatus was replaced by a similar partition that contained an opening in the center (28 × 6 cm), allowing the rats free-access to the adjacent compartments simultaneously. A rectangular tower measuring 70 × 24 × 74 cm high of clear Plexiglas was used as an extension of the apparatus to prevent the rats from escaping from the chamber while maintaining the ability to record their behavior via an overhanging video camera. The conditioning room was dimly lit with two overhead lamps, each containing a 25 W light bulb providing equal light distribution for each conditioning chamber. Unpublished data from our laboratory established that adolescent and adult experimentally naïve rats showed no preference for a particular compartment (i.e., unbiased apparatus). A camera (Panasonic WV-CP284, color CCTV, Suzhou, China) used to record testing sessions was mounted 101 cm above the center of the apparatus. A WinTV 350 personal video recorder (Hauppage, NJ, USA) captured live video and encoded it to MPEG streams. A modified version of TopScan Software (Clever Sys., Inc. Reston, VA, USA) used the orientation of an animal's body parts to track its location, which yielded measures of time spent in each compartment.
2.4. Baseline preference
On the first day of the procedure (see Fig. 1A for timeline), rats were placed individually into their assigned CPP apparatus where they had free access to both compartments for 10 min in order to habituate them. This procedure was repeated across the next 2 consecutive days with the starting compartment counterbalanced across days and the time spent in each compartment recorded to assess initial baseline preference. Time in a particular compartment was determined by the software based on the location of the rat's head. Time spent in each compartment was averaged across the two baseline tests to determine each rat's initial side preference. Rats that failed to demonstrate at least five compartment crossovers during either baseline day were excluded from the experiments due to inadequate environmental exploration; however, they were assigned as a physical play partner for experimental rats when initial preferences and body weights did not allow for pairing experimental rats together.
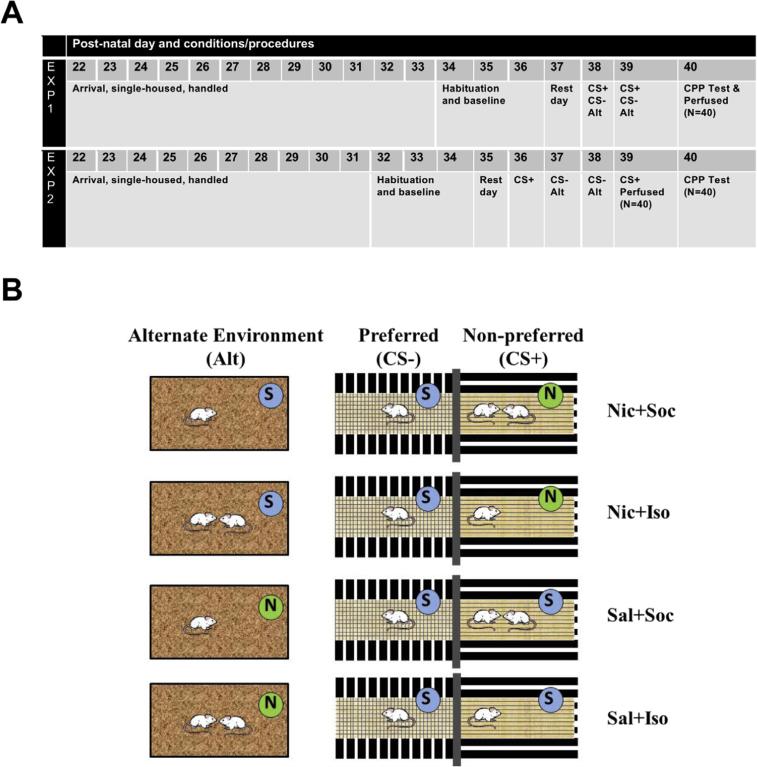
Fig. 1.
Timeline and experimental design. (A) Timeline of the procedures across post-natal days (PNDs) for Experiments 1 and 2 where rats were given 2 conditioning sessions with their assigned unconditioned stimulus (CS+) in their initially non-preferred side of the conditioning place preference (CPP) apparatus, 2 conditioning sessions in the absence of their unconditioned stimulus (CS−) on their initially preferred side, and 2 conditioning sessions in the alternate environment (Alt) with exposure to the unconditioned stimuli that they had not received during CS+ conditioning sessions. (B) Conditioning procedures in the initially preferred, initially non-preferred, and alternate environments. Two conditioning sessions took place in each environment for 10 min each, occurring over 2 or 4 consecutive days. One session took place in the initially preferred side of the apparatus (CS−), during which the rat was alone and received a saline vehicle (S; Sal) injection. Another session took place in the initially non-preferred side (CS+), during which the rat received exposure to the assigned unconditioned stimulus (US). US conditions included either nicotine (N; Nic) or saline (S; Sal) experienced either during isolation (Iso) or in the presence of an age-, sex- and weight-matched social partner (Soc). The final session took place in the alternate environment (Alt) in a separate location from the CPP conditioning chambers. In the alternate environment, rats received whichever US they had not received in their initially non-preferred side in order to control for total reward exposure for Fos and Zif268 protein expression.
2.5. Conditioning and testing
During conditioning sessions in both Experiment 1 and 2, rats were confined either to the initially non-preferred side of the apparatus for 10 min with their assigned unconditioned stimulus (US; i.e., partner rat and/or nicotine) or were confined in the initially preferred side of the apparatus with no US. The initially non-preferred side of the apparatus served as the conditioned stimulus (CS + ) that was exclusively paired with the US and the initially preferred side of the apparatus served as the conditioned stimulus (CS−) that was never paired with the US; these session types alternated. For CS+-US pairings, rats received either saline (Sal) or nicotine (Nic; 0.1 mg/kg/mL, s.c.) and were immediately confined to their initially non-preferred compartment of the chamber either while socially isolated (Iso) or with a social partner (Soc) resulting in 4 groups: (1) Nic + Soc; (2) Nic + Iso; (3) Sal + Soc and (4) Sal + Iso. Socially-conditioned rats were assigned to pairs that were matched for initial compartment preference and body weight within 10 g. All rat partners were unfamiliar with each other prior to conditioning, but remained constant throughout conditioning. All groups received saline and were immediately confined to their initially preferred side alone during their CS− session (no US). Rats also received 10-min sessions during which they were placed into an alternate environment to allow for equal exposure to the rewards across all groups. Thus, rats received exposure to reward(s) that they had not received during conditioning, so that all groups received identical amounts of nicotine and social reward exposure and only the timing and location varied (see Fig. 1B). The alternate environment was an opaque plastic container measuring 34 × 22 × 26 cm high with sani-chip bedding covering the plastic bottom and a perforated blue plastic top to prevent escape while allowing for ventilation. It was located in a separate room away from the dedicated CPP conditioning room.
For Experiment 1 (n = 40), conditioning took place over 2 consecutive days on PNDs 38-39 (Fig. 1A). Each conditioning session type (i.e., CS+, CS− and ALT sessions) occurred on the same day, repeated across 2 consecutive days. The order of the session type was counterbalanced across animals and 6 h intervened between the CS+ and CS− sessions. The ALT sessions occurred at least 2 h after the last CS conditioning session. Rats were given a 10-min place preference test the following day and then were once again returned to their home cages. They were sacrificed 90-min after their CPP expression test as described below. The 90-min time point was chosen for optimal stimulus-induced Fos protein expression [66].
For Experiment 2 (n = 80), conditioning took place over 4 consecutive days on PNDs 36-39 (Fig. 1A). The CS+ and CS− sessions occurred on separate days. The CS+ sessions occurred on the first and fourth day of conditioning to allow the brains to be harvested after the last CS+ session. The CS− and ALT sessions occurred on the second and third day of conditioning. Thus, each rat received only 2 exposures to each of the environments. All rats were placed back into their home cages and either sacrificed 90-min following the end of the last CS+ session to investigate Fos and Zif268 expression in response to US exposure (n = 40) or remained in their home cages until the following day for a 10-min place preference test (n = 40) to verify establishment of CPP in this experiment.
2.6. Tissue preparation
Ninety min following the last CS+ conditioning session or CPP expression test, rats were deeply anesthetized with sodium pento-barbital (100 mg/kg, i.p.). Approximately 200 mL of ice-cold 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 250 mL of ice-cold 4% paraformaldehyde in PBS was perfused through the circulatory system transcardially. Brains were removed and post-fixed in 4% paraformaldehyde for ~24 h and then transferred to 15% and 30% sucrose for ~24 h each. The brains were then sectioned using a microtome (Microm International, Walldorf, Germany) connected to a filtered water freezing stage (Physitemp, Clifton, NJ). Serial coronal 40-μm sections were collected, separated by 160 μm, centered at anatomical locations +1.6, −2.56, and −5.6 mm relative to bregma [67]. The tissue sections were then placed in 0.02 M PBS cryoprotectant solution comprised of 30% sucrose, 10% polyvinyl pyrrolidone, and 30% ethylene glycol and stored at 4 °C.
2.7. Immunohistochemistry
Immunohistochemistry was carried out as previously described [60]. Briefly, free floating tissue sections were first washed in 0.1 M PB (9 × 10 min). The tissue was next incubated for 30 min in 1% H2O2 and followed by incubation for 30 min in 0.1 M PB containing 3% normal goat serum (NGS) (Vector Laboratories, Burlingame, CA, USA). The tissue was then incubated for 72 h at 4 °C with either anti-Fos rabbit polyclonal antibody (1:2000; sc-52, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-EGR1 rabbit polyclonal antibody (1:2000; c-19, Santa Cruz Biotechnology, Santa Cruz, CA), diluted in PBH solution containing 2% NGS, 0.1% bovine serum albumin (Sigma, St. Louis, MO, USA, #A9647) and 0.2% Triton X-100 (Sigma, St. Louis, MO, USA). Following incubation, tissue sections were washed in 0.01 M PB (3 × 10 min) and then incubated for 1 h in biotinylated goat anti-rabbit IgG antibody (Vector Laboratories), diluted 1:500 in PBH solution. The tissue was then washed in 0.01 M PB (3 × 10 min) and then incubated for 90 min in avidin-biotinylated horseradish peroxidase complex (ABC Elite Kit; Vector Laboratories) diluted 1:1000 in PBH. The sections were again washed in 0.1 M PBS (9 × 10 min) and incubated for 20 min in 0.1 M PB containing 0.02% 3,3′-diaminobenzidine (DAB; Sigma), 2% nickel ammonium sulfate, 20% d-glucose, and 0.4% ammonium chloride. Immunoreactivity was visualized with glucose oxidase (1 μL/mL) for 10 min and then the tissue was washed with 0.01 M PB (6 × 10 min). Stained tissue sections were immediately mounted onto gelatin-coated slides, air-dried, and dehydrated before cover slipping.
2.8. Immunoreactivity analysis
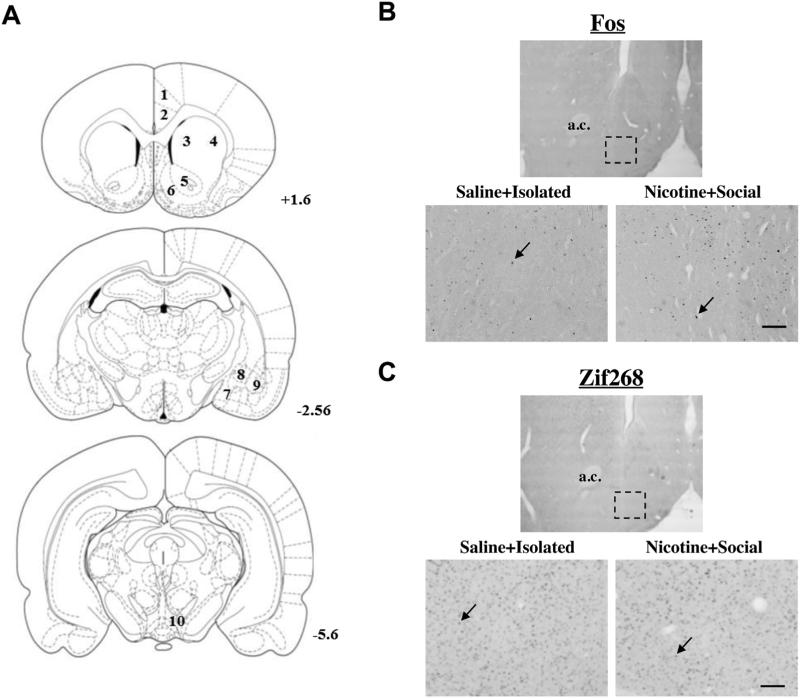
Fig. 2A illustrates the brain regions analyzed [67]. Sections taken at +1.6 mm contained the Cg1 and Cg2 regions of the anterior cingulate cortex, the dorsal lateral (dlCPu) and dorsal medial caudate putamen (dmCPu), nucleus accumbens core (NAcC) and shell (NAcSh); sections taken at −2.56 mm contained the medial amygdala (MeA), central amygdala (CeA), and basolateral amygdala (BLA); and sections taken at −5.6 mm contained the ventral tegmental area (VTA). Fos and Zif268 immunoreactivity was examined using a Nikon Eclipse E600 (Nikon Instruments, Melville, NY) microscope set at 20 × magnification. A range of 4–10 bilateral sample areas were counted per region of interest for each subject (i.e., 1 sample area/2 hemispheres/5 sections maximum), depending on tissue quality and preservation. Fos and Zif268 immunoreactivity were identified by a brown-black oval-shaped nucleus distinguishable from background (see Fig. 2B, C) and quantified using Image J software (U.S. National Institutes of Health, Bethesda, MA) by an observer blind to treatment conditions. Counts were averaged per subject for each region to provide a mean number of immunoreactive nuclei per sample area (0.26 mm2).
Fig. 2.
Brain regions analyzed for IEG immunohistochemistry. (A) Schematic representation of coronal sections of the rat brain taken at +1.6, −2.56, and −5.6 mm from Bregma [67]. Numbers in the sections represent the regions analyzed for Fos as follows: (1) Cg1 region of the anterior cingulate cortex (Cg1); (2) Cg2 region of the anterior cingulate cortex (Cg2); (3) dorsal medial caudate-putamen (dmCPu); (4) dorsal lateral caudate-putamen (dlCPu); (5) nucleus accumbens core (NAcC); (6) nucleus accumbens shell (NAcSh); (7) medial amygdala (MeA); (8) central amygdala (CeA); (9) basolateral amygdala (BLA); (10) ventral tegmental area (VTA). Representative photomicrographs from Experiment 2 showing coronal sections at 4 × (above; dashed rectangle represents sample area, 0.26 mm2) and 20 × (below) magnification in the nucleus accumbens shell (NAcSh) demonstrating Fos (B) and Zif268 (C) protein labeling (black arrows) in representative rats that were sacrificed following the last US exposure from the saline + isolated and nicotine + social conditions. Scale bar is equal to 100 μm. a.c. = anterior commissure.
2.9. Data analysis
CPP was operationally defined as a significant increase in time spent in the initially non-preferred side (i.e., US-paired side) on the post-conditioning test relative to the average of the preconditioning tests (i.e., baseline), with more than half of the total test time (i.e., >300 s) spent in the US-paired side. For each experiment, time spent in the initially non-preferred side of the CPP apparatus was analyzed using a mixed-factor ANOVA with Day (baseline vs. test day) as a within-subjects factor and Drug (saline vs. nicotine), and Social Condition (isolation vs. social partner) as between-subjects factors. Significant interactions were further analyzed using smaller ANOVAs and tests of simple effects. Additionally, comparisons of interest to examine hypotheses regarding preference for nicotine and/or a social partner (i.e., Nic + Soc, Nic + Iso, and Sal + Soc) compared to negative controls (Sal + Iso) were analyzed using student t-tests with Bonferroni correction for multiple comparisons (alpha level/number of comparisons). Fosand Zif268-positive nuclei were analyzed using two-way ANOVAs with Drug (saline vs. nicotine) and Social Condition (isolation vs. social partner) as between-subjects factors. Significant interactions were further analyzed using tests of simple effects.
3. Results
3.1. Conditioned place preference
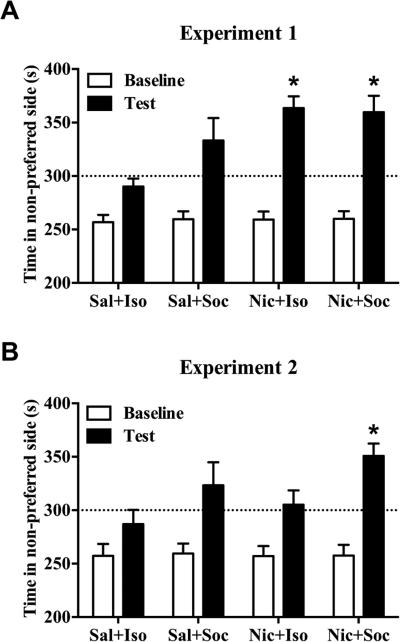
The CPP results for both experiments are shown in Fig. 3. For Experiment 1, the ANOVA of time spent in the initially non-preferred side revealed a main effect of Day (F(1,36) = 90.78, p < 0.001), a main effect of Drug (F(1,36) = 9.10, p < 0.01) and a Day × Drug interaction (F(1,36) = 8.81, p < 0.01). Post-hoc simple effects tests revealed that the Day × Drug interaction was due to an increased amount of time spent in CS+ side on test day in nicotine-conditioned groups relative to their saline-conditioned counterparts (p < 0.01), whereas there were no differences on baseline tests. All groups, with the exception of the Sal + Iso negative control group, spent >50% of the time on the CS+ on test day (Fig. 3A). However, post-hoc comparisons of interest revealed that only the Nic + Iso and Nic + Soc groups spent significantly more time in the CS+ side on test day relative to the Sal + Iso group (p < 0.0167, Bonferroni correction). For Experiment 2, the ANOVA of time spent in the CS+ side revealed a main effect of Day (F(1,36) = 35.32, p < 0.001), a main effect of Social Condition (F(1,36) = 6.38, p < 0.05) and a significant Day × Social Condition interaction (F(1,36) = 90.78, p ≤ 0.05). The only group to exhibit significant CPP was the Nic + Soc group (Fig. 3B), where post-hoc comparisons of interest revealed that only this group spent significantly more time in the CS+ side on test day relative to the Sal + Iso group (p < 0.0167, Bonferroni correction).
Fig. 3.
Effect of nicotine and social stimuli on CPP expression. Nicotine (0.1 mg/kg S.C.) and/or social reward-CPP shown as time (means + SEM) spent in the partner and/or nicotine-paired side pre-conditioning (i.e., Baseline, white bars) vs. postconditioning (i.e., Test, black bars) across groups. The dotted line represents 50% of the total test time (i.e., 300 s). (A) In Experiment 1 (n = 9–11/group), conditioning took place across two consecutive days. Nicotine-conditioned groups exhibited greater CPP compared to saline-conditioned groups (p < 0.05, ANOVA). (B) In Experiment 2 (n = 10/group), conditioning took place across 4 consecutive days with CS+ sessions occurring on the 1st and 4th day and CS− sessions occurring on the 2nd and 3rd day. Only the Nic + Soc group exhibited significant CPP compared to the Sal + Iso group. Asterisk (*) represents an increase compared to Sal + Iso test (ps < 0.0167, Bonferroni).
3.2. Immunohistochemistry
3.2.1. Experiment 1: exposure to conditioned environmental stimuli
The means of Fos-labeled cells for each group in each brain region are shown in Table 1. For rats that were sacrificed following the CPP expression test in Experiment 1, ANOVAs of Fos protein expression revealed a main effect of Social Condition (F(1,36) = 4.15, p < 0.05) in the NAcC following the CPP expression test, where rats that were socially-conditioned exhibited reduced Fos protein expression relative to groups that were isolate-conditioned, regardless of drug exposure (Table 1). A non-significant significant trend toward a main effect of nicotine was observed in the Cg1 (F(1,36) = 3.64, p = 0.06), where nicotine appeared to increase Fos expression relative to saline, regardless of social condition. No effects for Fos expression were observed in any other brain region analyzed (Table 1).
Table 1.
Mean (±SEM) of Fos-positive nuclei for each region for Experiments 1 and 2.
| Brain Regionb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 Groupa | Cg1 | Cg2 | dmCPu | dlCPu | NAcC | NAcSh | MeA | CeA | BLA | VTA |
| Sal + Iso | 95 ± 10.1 | 115 ± 13.9 | 17 ± 4.3 | 3 ± 0.8 | 73 ± 7.6 | 38 ± 3.7 | 62 ± 4.8 | 33 ± 3.7 | 67 ± 5.7 | 7 ± 1.6 |
| Sal + Soc | 92 ± 6.3 | 91 ± 8.8 | 16 ± 2.4 | 4 ± 1.1 | 67 ± 3.9* | 33 ± 2.6 | 56 ± 3.7 | 33 ± 2.8 | 52 ± 2.2 | 8 ± 1.2 |
| Nic + Iso | 106 ± 13.2 | 99 ± 8.9 | 21 ± 4.2 | 5 ± 1.1 | 72 ± 9.1 | 32 ± 4.1 | 59 ± 3.0 | 37 ± 3.6 | 64 ± 5.4 | 7 ± 2.5 |
| Nic + Soc | 119 ± 8.0 | 99 ± 7.5 | 17 ± 2.9 | 4 ± 0.7 | 52 ± 3.7* | 36 ± 2.9 | 64 ± 4.0 | 34 ± 3.2 | 64 ± 4.7 | 8 ± 1.0 |
| Experiment 2 Groupa | Cg1 | Cg2 | dmCPu | dlCPu | NAcC | NAcSh | MeA | CeA | BLA | VTA |
|---|---|---|---|---|---|---|---|---|---|---|
| Sal + Iso | 92 ± 10.5 | 87 ± 16.0 | 19 ± 4.0 | 3 ± 0.6 | 36 ± 4.6 | 25 ± 3.1 | 13 ± 1.8 | 10 ± 2.0 | 28 ± 4.0 | 6 ± 0.6 |
| Sal + Soc | 102 ± 7.4 | 98 ± 13.1 | 26 ± 4.7 | 10 ± 1.7* | 52 ± 6.1* | 37 ± 2.5* | 13 ± 1.8* | 14 ± 2.3* | 20 ± 2.3 | 4 ± 0.5#† |
| Nic + Iso | 55 ± 8.2+ | 54 ± 8.0+ | 12 ± 2.8 | 1 ± 0.3+ | 23 ± 5.2+ | 20 ± 3.1 | 9 ± 1.0 | 11 ± 2.0 | 15 ± 1.9#† | 4 ± 0.8# |
| Nic + Soc | 77 ± 6.3+ | 62 ± 5.3+ | 17 ± 3.9 | 5 ± 1.6*+ | 38 ± 6.2*+ | 34 ± 2.5* | 17 ± 2.5* | 17 ± 2.7* | 24 ± 2.5 | 6 ± 0.8 |
Asterisk (*) indicates a main effect of Social Condition, p < 0.05; plus sign (+) indicates a main effect of Drug, p < 0.05; pound sign (#) indicates a decrease relative to Sal + Iso negative control group (ps ≤ 0.05, post-hoc independent samples t-test following a Drug × Social Condition interaction); dagger (†) represents a decrease relative to Nic + Soc (ps < 0.05, post-hoc independent samples t-test following a Drug × Social interaction).
Saline (Sal), Nicotine (Nic), Isolation (Iso), and Social partner (Soc).
Abbreviations are described in the Methods (Immunoreactivity analysis) section and Fig. 2.
3.2.2. Experiment 2: exposure to social and drug unconditioned rewards
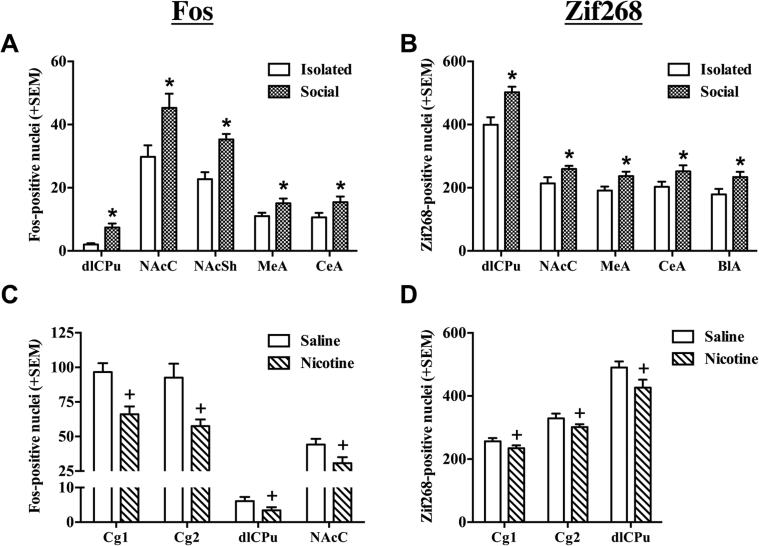
For rats that were sacrificed following the last US conditioning session in Experiment 2, both social condition and nicotine influenced Fos and Zif268 protein expression (see Fig. 4A–D, and Table 1, 2). ANOVAs of Fos expression revealed a significant main effect of Social Condition in the dlCPu (F(1,36) = 19.53, p < 0.001), NAcC (F(1,36) = 7.70, p < 0.01), NAcSh (F(1,36) = 20.03, p < 0.001), MeA (F(1,36) = 4.71, p < 0.05) and CeA (F(1,36) = 4.51, p < 0.05), where social conditioning increased Fos expression following the last US exposure, relative to isolate-conditioning, regardless of drug exposure (Fig. 4A). Only a non-significant trend towards a main effect of Social Condition was observed in the Cg1 (F(1,36) = 3.78, p = 0.06). We found a similar social-induced increase in Zif268 expression (Fig. 4B) in the dlCPu (F(1, 29) = 15.28, p < 0.001), NAcC (F(1, 29) = 4.70, p < 0.05), MeA (F(1, 27) = 5.50, p < 0.05), CeA (F(1, 27) = 3.81, p < 0.05, one-tailed), and BLA (F(1, 27) = 6.08, p < 0.05).
Fig. 4.
Effect of nicotine and social stimuli alone on IEG expression. Number of Fos- and Zif268-positive nuclei + SEM in regions exhibiting group differences among rats sacrificed 90-min after the last US conditioning session in the CS+ side of the apparatus. US conditions included either saline (Sal) or nicotine (Nic) injections followed by placement into the initially nonpreferred side of the CPP apparatus either alone (Iso) or with a social partner (Soc) in Experiment 2 (n = 7–10/group). Means of Fos- (A) and Zif268-positive (B) cells shown are collapsed across Drug condition. Asterisk (*) represents a main effect of Social Condition, where social pairings increased expression relative to isolation (ps < 0.05, ANOVA). Means of Fos- (C) and Zif268-positive (D) cells shown are collapsed across Social Condition. Plus sign (+) represents a main effect of Drug, where nicotine decreased expression relative to saline (ps < 0.05, ANOVA).
Table 2.
Mean (±SEM) of Zif268-positive nuclei for each region for Experiments 2.
| Brain Regionb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment 2 Groupa | Cg1 | Cg2 | dlCPu | NAcC | NAcSh | MeA | CeA | BLA | VTA |
| Sal + Iso | 252 ± 14 | 324 ± 21 | 466 ± 20 | 246 ± 28 | 166 ± 35 | 199 ± 19 | 188 ± 23 | 177 ± 28 | 4.2 ± 0.8 |
| Sal + Soc | 261 ± 15 | 333 ± 22 | 511 ± 31* | 254 ± 13* | 175 ± 21 | 228 ± 21* | 247 ± 39 | 264 ± 23* | 2.6 ± 0.5 |
| Nic + Iso | 220 ± 13+ | 296 ± 11+ | 341 ± 28+#† | 185 ± 23 | 147 ± 27 | 185 ± 17 | 214 ± 24 | 180 ± 23 | 2.2 ± 0.4# |
| Nic + Soc | 247 ± 10+ | 306 ± 13+ | 495 ± 21*+ | 263 ± 15* | 161 ± 17 | 245 ± 19* | 256 ± 14 | 213 ± 21* | 3.3 ± 0.5 |
Asterisk (*) indicates a main effect of Social Condition, p < 0.05; plus sign (+) indicates a main effect of Drug, p < 0.05; pound sign (#) indicates a decrease relative to Sal + Iso negative control group (ps ≤ 0.05, post-hoc independent samples t-test following a Drug × Social Condition interaction); dagger (†) indicates a decrease relative to Nic + Soc (ps < 0.05, post-hoc independent samples t-test following a Drug × Social interaction).
Saline (Sal), Nicotine (Nic), Isolation (Iso), and Social partner (Soc).
Abbreviations are described in the Methods (Immunoreactivity analysis) section and Fig. 2.
There was also a main effect of Drug Condition for Fos expression in the Cg1 (F(1,36) = 13.69, p < 0.001), Cg2 (F(1,36) = 9.56, p < 0.01), dlCPu (F(1,36) = 4.88, p < 0.05), and the NAcC (F(1,36) = 5.81, p < 0.05), where nicotine-conditioned groups exhibited decreased Fos expression relative to saline-conditioned groups, regardless of social condition (Fig. 4C). We observed a similar nicotine-induced decrease in Zif268 expression (Fig. 4D) in the Cg1 (F(1, 29) = 3.20, p < 0.05, one-tailed), Cg2 (F(1, 29) = 2.64, p ≤ 0.05, one-tailed) and the dlCPu (F(1, 29) = 7.66, p < 0.01).
3.2.3. Experiment 2: interactive effects of social and drug unconditioned rewards
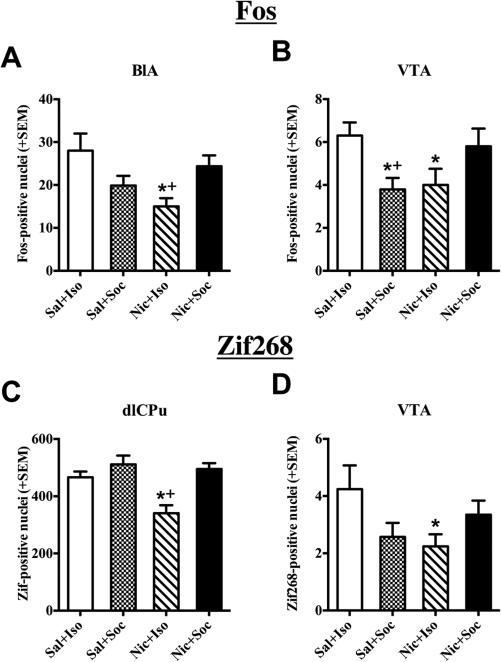
Significant Drug × Social Condition interactions were observed in rats from Experiment 2 for Fos expression (Fig. 5A, B) in the BLA (F(1,35) = 9.57, p < 0.01) and VTA (F(1, 34) = 9.65, p < 0.01). In the BLA, post-hoc tests for simple effects revealed decreased Fos expression in the Nic + Iso rats relative to the Sal + Iso and Nic + Soc rats (ps < 0.01). Similarly in the VTA, post-hoc tests for simple effects revealed decreased Fos protein expression in the Sal + Soc and Nic + Iso rats relative to the Sal + Iso rats (ps ≤ 0.05), as well as decreased Fos protein expression in the Sal + Soc relative to the Nic + Soc rats (p < 0.05). Similar interactions were found with Zif268 expression (Fig. 5C, D) in the VTA (F(1, 34) = 5.94, p < 0.05), where post-hoc tests for simple effects revealed that the Nic + Iso group had reduced Zif268 expression compared to the Sal + Iso group (p < 0.05). An additional interaction with Zif268 expression was found in the dlCPu (F(1, 29) = 4.61, p < 0.05), where post-hoc tests for simple effects revealed that the Nic + Iso group exhibited reduced Zif268 expression compared to the Sal + Iso and Nic + Soc groups (ps < 0.05).
Fig. 5.
Interactive effects of nicotine and social stimuli on IEG expression. Number of Fos- and Zif268-positive nuclei + SEM in regions exhibiting nicotine and social condition interaction effects among rats sacrificed 90-min after the last US conditioning session in the CS+ side of the apparatus. US conditions included either saline (Sal) or nicotine (Nic) injections followed by placement into the initially nonpreferred side of the CPP apparatus either alone (Iso) or with a social partner (Soc) in Experiment 2 (n = 7–10/group). Significant interactions were found in the BLA (A) and VTA (B) for Fos expression and in the dlCPu (C) and VTA (D) for Zif268 expression. Asterisk (*) represents a decrease relative to Sal + Iso negative controls (ps < 0.05, post-hoc independent samples t-test). Plus sign (+) represents a decrease relative to Nic + Soc group (ps ≤ 0.05, post-hoc independent samples t-test).
4. Discussion
The results demonstrate that the conditioned and unconditioned effects of nicotine and social rewards produce complex changes in immediate early gene expression in adolescent male rats. While we found few significant changes in response to the reward-conditioned environment, we found that direct exposure to social and nicotine rewards produces bi-directional activation of several reward-related brain regions, where social stimuli increased and nicotine decreased IEG expression. Furthermore, nicotine reduced IEG expression in several brain regions and this effect was reversed when nicotine was experienced in combination with a social partner. Collectively, these results suggest that low doses of nicotine may blunt activation of reward-related pathways, but social stimuli can consistently activate and even reverse the blunted activation of these pathways. These findings give insight into the neural circuits involved in the initial rewarding effects of smoking in the presence of social peers during adolescence.
Our CPP findings demonstrated that only 2 exposures to nicotine alone or in combination with a social partner produce robust CPP in adolescent male rats when US conditioning sessions occurred in close temporal proximity (i.e., over 2 consecutive days; Experiment 1); however, only the combination of nicotine paired with a social partner elicited robust CPP when the time between US conditioning sessions was extended (i.e., 2 days intervening; Experiment 2). The robust conditioning of nicotine observed in Experiment 1 was surprising because we previously found that the conditioning parameters used here were sub-threshold for establishing CPP with either nicotine or social reward alone [12]; however, one key difference across studies was the conditioning apparatus used. The present study used conditioning chambers adapted for use with smaller rodents that differed in olfactory, tactile, and visual cues from the chambers used in our previous study, and these changes likely altered the sensitivity for establishing CPP. CPP procedures are susceptible to ceiling effects, where CPP expression may appear equal even when the reward strength of the US varies as shown by using different conditioning parameters [12,65,68–70]. The extended time between US conditioning sessions in Experiment 2 compared to Experiment 1 likely produced weaker conditioning, such that once again the parameters were sub-threshold for establishing CPP with either nicotine or social reward alone.
Our neurochemical findings revealed that Fos protein expression patterns varied considerably when animals were expressing CPP (Experiment 1) versus experiencing the US (Experiment 2). Contrary to our predictions, the only effect observed following CPP expression testing was in the NAcC where rats that were socially conditioned exhibited less Fos relative to their isolated counterparts, regardless of whether they received nicotine or saline. These findings were surprising given that nicotine experienced with or without a social partner produced CPP (Fig. 3A) and previous research has shown an increase in Fos upon exposure to environmental cues associated with nicotine reward [71,72]. The lack of increased Fos in nicotine-conditioned animals in the present study may have been due to a ‘cancellation effect’ since rats were exposed to both the CS+ and CS− environments during the CPP test. In any case, the decrease in Fos in the NAcC of social-conditioned groups (Table 1) may have been due to violation of reward expectation. Previous studies have found that the reward circuitry, particularly the NAc, is heavily involved in processing incentive stimuli [73,74], incentive learning [75,76], and reward prediction errors (i.e., expectation of reward is violated) [77,78] including prediction errors associated with social reward [79–82]. Since exposure to conspecifics is a highly salient reward in adolescent rats, being alone in the previously social-paired side of the chamber on test day may have resulted in prediction-error effects leading to a decrease in Fos expression in the NAcC.
In contrast to the limited effects of environmental cues on Fos expression during the CPP test in Experiment 1, Fos expression in response to the last US exposure in Experiment 2 was altered in several of the regions analyzed. Three distinct patterns emerged, where (1) social-conditioned rats exhibited elevated IEG expression in the dorsolateral CPu (dlCPu), the nucleus accumbens core and shell (NAcC and NAcSh), and the medial and central amygdala (MeA, CeA) relative to isolated rats (Fig. 4A), (2) nicotine-conditioned rats exhibited less Fos in the anterior cingulate of the medial prefrontal cortex (Cg1, Cg2), dlCPu, and the NAcC relative to saline-conditioned rats (Fig. 4C), and (3) nicotine-conditioned and social-conditioned rats exhibited less Fos in the ventral tegmental area (VTA) and basolateral amygdala (BLA) than both saline-conditioned and nicotine + social-conditioned rats (Fig. 5A, B). These neurochemical interactions suggest that the synergistic interaction of nicotine and social rewards does not necessarily involve stronger activation of a common part of the corticolimbic circuitry. Given that this is contrary to our hypothesis, we chose to measure expression of another IEG, Zif268, and found that the results were largely the same as those observed for Fos (Figs. 4 and 5, Table 2).
The elevated Fos and Zif268 expression after social exposure in corticolimbic regions is consistent with previous reports demonstrating that these regions are involved in processing social information. For example, c-fos mRNA is increased in the dorsal and ventral striatum and the lateral amygdala after brief (i.e., 15 and 30-min) social exposure in juvenile rats [57,58]. In adolescent rats, a 60-min social exposure induced Fos protein expression in the basolateral and central amygdala, but this effect was not present in adult rats, suggesting that changes in the amygdala may be age-dependent [56]. The amygdala and striatum are likely involved in social play for non-human primates [83]. Similarly, the amygdala appears to be necessary for normal prosocial behavior in rodents [84–87] and exhibits changes in c-fos expression after play behavior [88].
Contrary to our predictions, we observed less Fos and Zif268 expression in the Cg1, Cg2, dlCPu, and NAcC in nicotine-conditioned rats relative to saline-conditioned rats in Experiment 2. These results are inconsistent with previous findings that have shown acute nicotine administration increases Fos protein and mRNA expression in the cingulate cortex [53–55,89], dorsal striatum [53–55], and ventral striatum, particularly NAcC [53–55,71,89,90]. However, one study reported decreased c-fos and zif268 expression in the frontal cortex, basolateral amygdala and the hippocampus of the mouse brain in response to a high dose of nicotine (1.0 mg/kg, i.p.) [91]. The reason for these discrepancies is unclear but may be due to the dose of nicotine used and/or age at the time of exposure. All but one of these studies utilized adult rats [53] and all of the reported studies administered a higher nicotine dose that was at least double (i.e., 0.21–0.5 mg/kg) the nicotine dose used in the present study, suggesting that Fos and Zif268 expression may be sensitive to age and dose effects.
The c-fos gene is transiently expressed as part of intracellular signaling in response to a variety of stimuli and its induction diminishes with repeated exposure to a given stimulus [92]. Since we administered nicotine twice in the present study, it is possible that the repeated exposure diminished nicotine-induced c-fos induction. However, this explanation seems unlikely because the ability of pharmacological stimuli to induce c-fos after repeated administration usually recovers within a few days, and therefore we spaced the 2 exposures in this experiment 72 h apart. Zif268, on the other hand, can be expressed at relatively high basal levels (Fig. 2C), where experimental manipulations can produce both increases and decreases in expression [93]. Since both Fos and Zif268 protein expression were reduced, the results suggest these regions exhibit reduced activation in response to nicotine. Another possibility is that nicotinic acetylcholine receptors (NAChRs) underwent rapid desensitization after the second nicotine exposure causing less activation of intracellular signaling, resulting in low levels of Fos and Zif268 expression compared to saline controls [94,95]. However, this too seems unlikely because we used a low dose of nicotine. Finally, social interaction can reduce expression of Fos and Zif268 that is normally induced by drugs of abuse. For example, when social conditioning competes with an already established preference for cocaine, decreases in FosB and Zif268 have been found in several regions including the accumbens, amygdala and VTA [96–98]. However, this is unlikely the reason for the decrease in Fos and Zif268 by nicotine since decreases were also observed in rats not exposed to a social partner. Despite the unexpected decrease in Fos and Zif268 expression after nicotine administration, these data appear to be orderly and the changes observed were region-specific rather than nonspecific across all brain regions; therefore, it is unlikely the changes observed are spurious.
Interestingly, the patterns of Fos and Zif268 expression in Experiment 2 were similar in the BLA, VTA, and dlCPu, where nicotine, and to a lesser extent social stimuli, reduced Fos and Zif268 expression relative to controls (i.e., Sal-Iso), and surprisingly rats conditioned with both nicotine and social rewards exhibited similar levels of expression as the controls. Social isolation is a robust stressor, which is known to activate the HPA-axis [99,100] and the BLA [101–103]. Thus, Fos and Zif268 expression in Sal + Iso controls may be indicative of isolation-induced stress reactivity rather than serving as a neutral baseline for comparison as intended. Moreover, controls underwent the same procedure at the same time as rats that received a social and/or nicotine US. Therefore, hearing rats playing in adjacent chambers may have been stressful for the controls. Indeed adolescent rats are prosocial [28,30,31] and highly motivated to seek-out and approach conspecifics [12,36,41,44]. In fact, social motivation increases the more socially-deprived a rat becomes [33,104]; therefore, the controls were likely in a state of high social motivation during CS+ conditioning sessions. This may have produced frustration stress due to the inability to interact with a partner, resulting in increased Fos and Zif268 in the BLA, VTA, and dlCPu. On the other hand, rats conditioned with both social and nicotine rewards were likely having a more intense rewarding experience relative to the rats conditioned with only one of these rewards, resulting in elevated Fos and Zif268 levels in the Nic + Soc group relative to Sal + Soc and Nic + Iso groups.
In conclusion, the results from Experiment 1 suggest that the nucleus accumbens core may be particularly sensitive to processes involved in incentive motivational effects of exposure to environmental stimuli previously associated with social rewards in adolescent male rats. Experiment 2 replicated our previous behavioral findings of a synergistic interaction between nicotine and social rewards in adolescent male rats [12]. However, the patterns of Fos and Zif268 expression observed in Experiment 2 contrasted markedly with our prediction that the combination of social and nicotine rewards would produce more robust activation relative to either reward given alone. While we did find that social conditioning, regardless of drug treatment, increased Fos and Zif268 expression in amygdala and striatal regions, nicotine with or without a social partner decreased expression in the anterior cingulate cortex and striatum. These patterns suggest that social and nicotine exposure uniquely alter intracellular signaling within cortical and limbic regions. Interestingly, we found that Fos and Zif268 expression was elevated in our nicotine + social and control groups relative to our single US groups (nicotine + isolated and saline + social) in the BLA, dlCPu, VTA. We suggest that different mechanisms are involved, where potential isolation stress effects may be responsible for the elevation of IEG expression in controls, whereas increased reward strength may be responsible for the elevation in the nicotine + social group. Taken together, these data are useful for formulating testable hypotheses regarding neural mechanisms of synergistic effects of social and nicotine reward exposure versus stress and anxiety associated with unmet need for social reward. The findings from the present study highlight the significance for understanding the influence of social context on nicotine rewarding effects.
HIGHLIGHTS.
Subthreshold social and nicotine reward presented in combination produced conditioned place preference.
Fos protein expression was reduced in the nucleus accumbens core following exposure to a social reward-associated environment.
Exposure to social reward increased, whereas nicotine decreased, Fos and Zif268 protein expression in several mesocorticolimbic brain regions.
Interactive expression effects of social and nicotine rewards were found in the basolateral amygdala, ventral tegmental area, and dorsolateral striatum.
Acknowledgments
This work was supported by NIDA grants R01DA11064 (J.L.N.), R21DA023123 (J.L.N.), F31DA30569 (R.M.B.), and F31DA033805 (N.A.P.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Services, U.S.D. o. H. a. H . The Health Consequences of Smoking- 50 Years of Progress. A Report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prvention, National Center for Chronic Disease Prevention and Health Promotion. Office on Smoking and Health; Atlanta: 2014. [Google Scholar]
- 2.Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. Am. J. Public Health. 1984;74(7):660–666. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am. J. Public Health. 1996;86(2):214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N. Engl. J. Med. 1991;325(13):968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- 5.Olds RS, Thombs DL. The relationship of adolescent perceptions of peer norms and parent involvement to cigarette and alcohol use. J. Sch. Health. 2001;71(6):223–228. doi: 10.1111/j.1746-1561.2001.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9(4):39–46. (Eng), 39–48(Fre) [PubMed] [Google Scholar]
- 7.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States : 1991–1993. Nicotin Tob. Res. 2000;2(3):263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 8.Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(Suppl. 1):S83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 9.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl.) 2004;174(3):389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 10.Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl.) 2006;186(2):201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 11.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol. Behav. 2002;77(1):107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 12.Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl.) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J. Pharmacol. Exp. Ther. 2007;322(1):399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 14.Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl.) 2008;198(2):201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- 15.Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl.) 2009;206(2):303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav. 2008;90(4):658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29(5):869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- 18.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 20.Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav. Brain Res. 2010;206(2):240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 22.O'Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl. 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann. N. Y. Acad. Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- 24.O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl.) 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 25.Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol. Biochem. Behav. 2006;85(3):648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol. Clin. Exp. Res. 2005;29(9):1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- 27.Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Dev. Psychobiol. 1978;11(3):213–225. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- 28.Panksepp J. The ontogeny of play in rats. Dev. Psychobiol. 1981;14(4):327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 29.Meaney MJ, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats (Rattus norvegicus) Anim. Learn. Behav. 1979;7:397–405. [Google Scholar]
- 30.Smith PK. Does play matter? Functional and evolutionary aspects of animal and human play. Behav. Brain Sci. 1982;5:139–184. [Google Scholar]
- 31.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Res. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 32.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999;34(2):129–138. [PubMed] [Google Scholar]
- 33.Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav. Brain Res. 1999;106(1–2):133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 34.Angermeier WF, Schaul LT, James WT. Social conditioning in rats. J. Comp. Physiol. Psychol. 1959;52(3):370–372. doi: 10.1037/h0042566. [DOI] [PubMed] [Google Scholar]
- 35.Evans MJ, Duvel A, Funk ML, Lehman B, Sparrow J, Watson NT, Neuringer A. Social reinforcement of operant behavior in rats: a methodological note. J. Exp. Anal. Behav. 1994;62(1):149–156. doi: 10.1901/jeab.1994.62-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim. Behav. 1981;29:259–270. February. [Google Scholar]
- 37.Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in Juvenile rats. Dev. Psychobiol. 1990;23(1):75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- 38.Werner CM, Anderson DF. Opportunity for interaction as reinforcement in a T-maze. Pers. Soc. Psychol. B. 1976;2(2):166–169. [Google Scholar]
- 39.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social-interaction in Juvenile rats. Physiol. Behav. 1992;51(4):667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 40.Crowder WF, Hutto CW. Operant place conditioning measures examined using 2 nondrug reinforcers. Pharmacol. Biochem. Behav. 1992;41(4):817–824. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- 41.Trezza V, Damsteegt R, Vanderschuren LJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur. Neuropsychopharmacol. 2009;19(9):659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 2013;65(1):255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl.) 2012;224(1):33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96(3):202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl.) 2012;224(1):81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp. Clin. Psychopharmacol. 2011 doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15(5):355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- 49.Jackson C. Initial and experimental stages of tobacco and alcohol use during late childhood: relation to peer, parent, and personal risk factors. Addict. Behav. 1997;22(5):685–698. doi: 10.1016/s0306-4603(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 50.Sussman S. Risk factors for and prevention of tobacco use. Pediatr. Blood Cancer. 2005;44(7):614–619. doi: 10.1002/pbc.20350. [DOI] [PubMed] [Google Scholar]
- 51.West P, Sweeting H, Ecob R. Family and friends’ influences on the uptake of regular smoking from mid-adolescence to early adulthood. Addiction. 1999;94(9):1397–1411. doi: 10.1046/j.1360-0443.1999.949139711.x. [DOI] [PubMed] [Google Scholar]
- 52.Geckova A, Stewart R, van Dijk JP, Orosova O, Groothhoff VW, Post D. Influence of socio-economic status, parents and peers on smoking behavior in adolescents. Eur. Addict. Res. 2005;11:204–209. doi: 10.1159/000086403. [DOI] [PubMed] [Google Scholar]
- 53.Schochet TL, Kelley AE, Landry CF. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience. 2005;135(1):285–297. doi: 10.1016/j.neuroscience.2005.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seppa T, Salminen O, Moed M, Ahtee L. Induction of Fos-immunostaining by nicotine and nicotinic receptor antagonists in rat brain. Neuropharmacology. 2001;41(4):486–495. doi: 10.1016/s0028-3908(01)00093-4. [DOI] [PubMed] [Google Scholar]
- 55.Salminen O, Seppa T, Gaddnas H, Ahtee L. The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J. Neurosci. 1999;19(18):8145–8151. doi: 10.1523/JNEUROSCI.19-18-08145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varlinskaya EI, Vogt BA, Spear LP. Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Dev. Psychobiol. 2013;55(7):684–697. doi: 10.1002/dev.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Kerkhof LW, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct. Funct. 2014;219(4):1181–1211. doi: 10.1007/s00429-013-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res. Bull. 2002;57(5):651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- 59.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastle RM, Kufahl PR, Turk MN, Weber SM, Pentkowski NS, Thiel KJ, Neisewander JL. Novel cues reinstate cocaine-seeking behavior and induce Fos protein expression as effectively as conditioned cues. Neuropsychopharmacology. 2012;37(9):2109–2120. doi: 10.1038/npp.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kufahl PR, Pentkowski NS, Heintzelman K, Neisewander JL. Cocaine-induced Fos expression is detectable in the frontal cortex and striatum of rats under isoflurane but not alpha-chloralose anesthesia: implications for FMRI. J. Neurosci. Methods. 2009;181(2):241–248. doi: 10.1016/j.jneumeth.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kufahl PR, Peartree NA, Heintzelman KL, Chung M, Neisewander JL. Region-specific effects of isoflurane anesthesia on Fos immunoreactivity in response to intravenous cocaine challenge in rats with a history of repeated cocaine administration. Brain Res. 2015;1594:256–266. doi: 10.1016/j.brainres.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63(10):823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.N.R.C. Committee, editor. Guide for the Care and Use of Laboratory Animals. 8 ed. National Academies Press (US); Washington, D.C: 2011. [Google Scholar]
- 65.Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav. 2012;105(3):749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moratalla R, Vickers EA, Robertson HA, Cochran BH, Graybiel AM. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. J. Neurosci. 1993;13(2):423–433. doi: 10.1523/JNEUROSCI.13-02-00423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paxinos G, Watson C, editors. The Rat Brain in Stereotaxic Coordinates. 4 ed. Academic Press; San Diego, CA: 1998. [Google Scholar]
- 68.Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl.) 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- 69.Bevins R. The reference-dose place conditioning procedure yields a graded dose-effect function. Int. J. Comp. Psychol. 2005;18:101–111. [Google Scholar]
- 70.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 71.Pascual MM, Pastor V, Bernabeu RO. Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology (Berl.) 2009;207(1):57–71. doi: 10.1007/s00213-009-1630-4. [DOI] [PubMed] [Google Scholar]
- 72.Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 73.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 74.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 75.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl.) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 76.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res. 2002;137(1–2):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 77.Schultz W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 78.Tobler PN, O'Doherty JP, Dolan RJ, Schultz W. Human neural learning depends on reward prediction errors in the blocking paradigm. J. Neurophysiol. 2006;95(1):301–310. doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, Voss HU, Ballon DJ, Casey BJ. Behavioral and neural properties of social reinforcement learning. J. Neurosci. 2011;31(37):13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn. Sci. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Poore JC, Pfeifer JH, Berkman ET, Inagaki TK, Welborn BL, Lieberman MD. Prediction-error in the context of real social relationships modulates reward system activity. Front. Hum. Neurosci. 2012;6:218. doi: 10.3389/fnhum.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: a comparative analysis using independent contrasts. J. Comp. Psychol. 2006;120(1):31–37. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- 84.Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav. Brain Res. 2002;136(2):571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 85.Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behaviour later in life; an animal model of neurodevelopmental psychopathological disorders. Behav. Brain Res. 2002;131(1–2):67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 86.Wolterink G, Daenen LE, Dubbeldam S, Gerrits MA, van Rijn R, Kruse CG, Van Der Heijden JA, Van Ree JM. Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. Eur. Neuropsychopharmacol. 2001;11(1):51–59. doi: 10.1016/s0924-977x(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 87.Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci. Biobehav. Rev. 1984;8(4):465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 88.Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156(2):247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 89.Mathieu-Kia AM, Pages C, Besson MJ. Inducibility of c-Fos protein in visuo-motor system and limbic structures after acute and repeated administration of nicotine in the rat. Synapse. 1998;29(4):343–354. doi: 10.1002/(sici)1098-2396(199808)29:4<343::aid-syn6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 90.Schilstrom B, De Villiers S, Malmerfelt A, Svensson TH, Nomikos GG. Nicotine-induced Fos expression in the nucleus accumbens and the medial prefrontal cortex of the rat: role of nicotinic and NMDA receptors in the ventral tegmental area. Synapse. 2000;36(4):314–321. doi: 10.1002/(SICI)1098-2396(20000615)36:4<314::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 91.Bachtell RK, Ryabinin AE. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neuroscience. 2001;103(4):941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- 92.Nestler EJ. Molecular neurobiology of addiction. Am. J. Addict. 2001;10(3):201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 93.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004;74(4):183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 94.Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28(7):371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not either/or: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011;16(2):273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- 97.Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front. Psychiatry. 2013;4:100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, Zernig G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs: social interaction. Front. Behav. Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73(3):261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 100.Serra M, Pisu MG, Mostallino MC, Sanna E, Biggio G. Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function. Brain Res. Rev. 2008;57(2):520–530. doi: 10.1016/j.brainresrev.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 101.Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14449–144454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. 2004;24(14):3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK. Response of the mu-opioid system to social rejection and acceptance. Mol. Psychiatry. 2013;18(11):1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]