Abstract
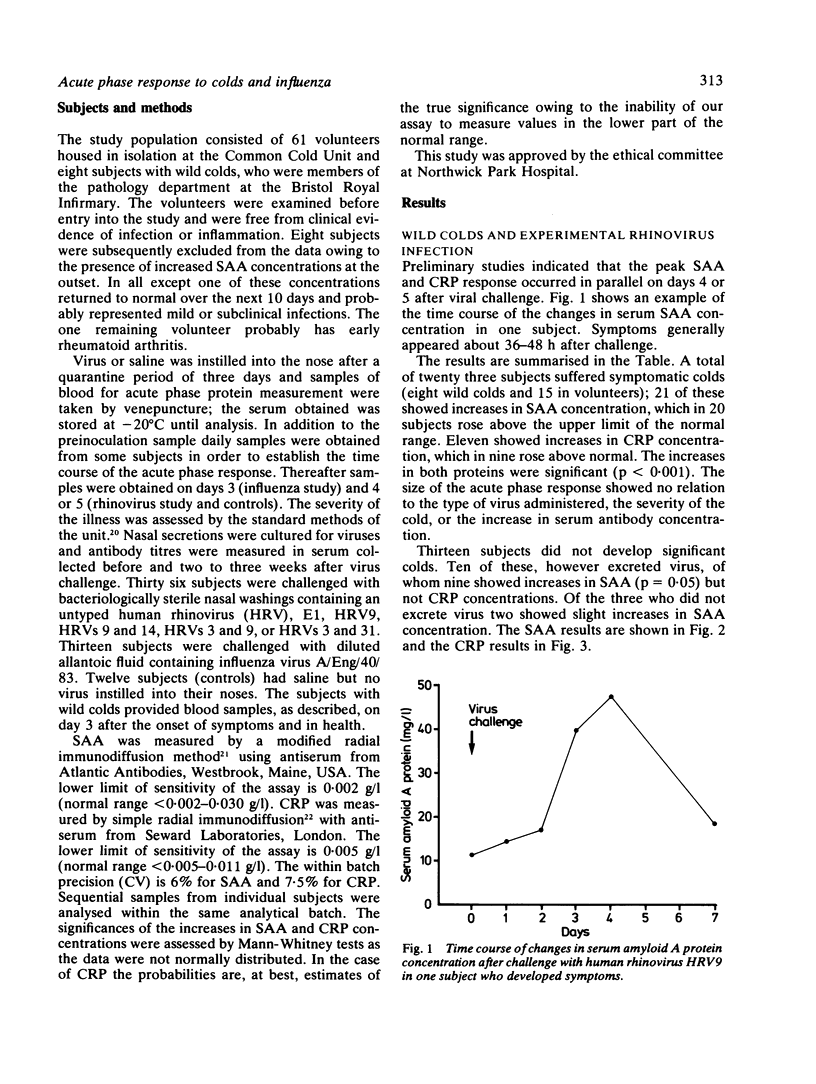
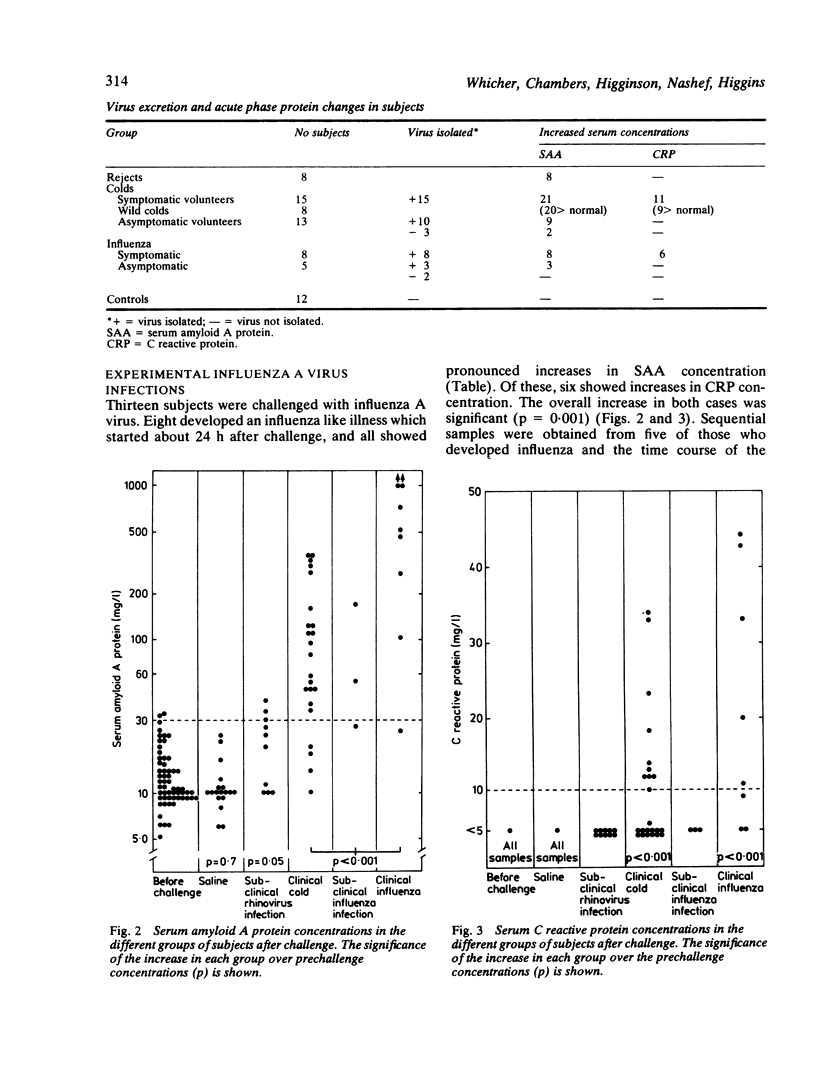
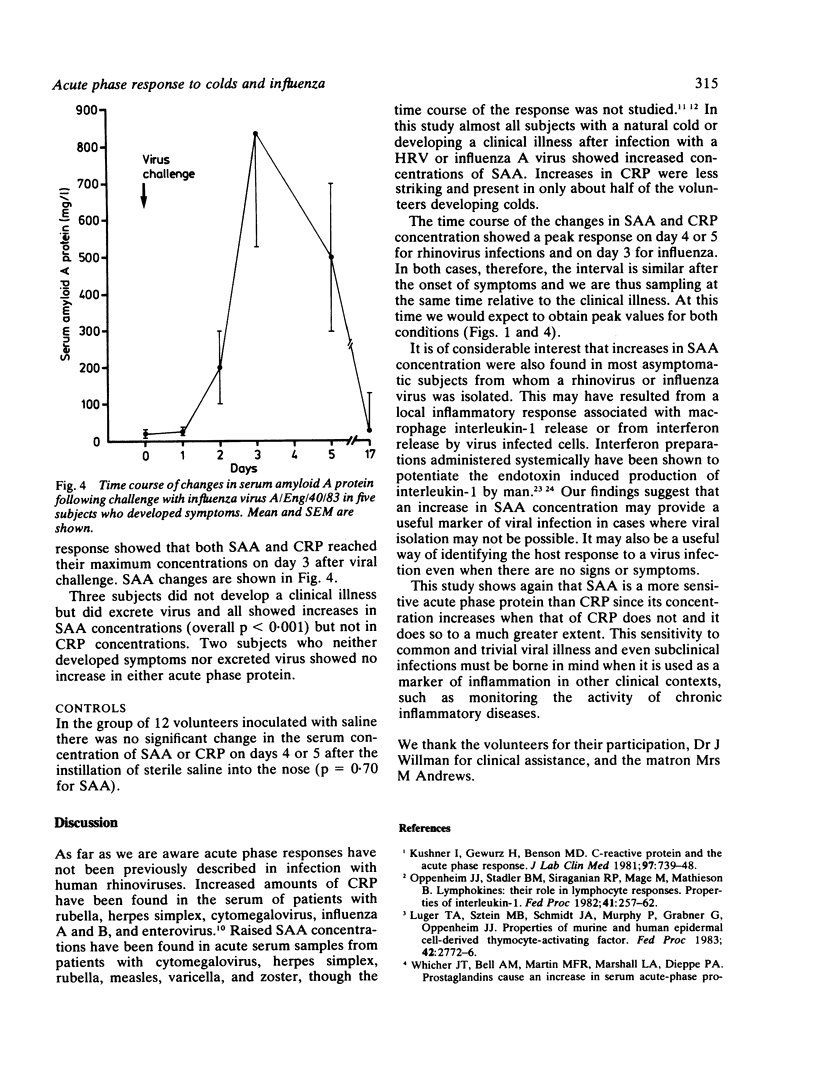
C reactive protein (CRP) and serum amyloid A protein (SAA) are sensitive and rapid acute phase reactants, and their measurement for monitoring inflammatory disease and assessing the prognosis in secondary amyloidosis is gaining widespread acceptance. The changes in these proteins in eight subjects suffering from natural colds, 15 subjects with experimentally induced colds (rhinoviruses E1, 3, 9, 14, or 31), and eight with experimentally induced influenza (A/Eng/40/83) were studied. SAA concentration increased in 21 of the 23 subjects with natural or experimental rhinovirus colds (mean increase 95 mg/l); CRP concentration increased in 11 (mean increase 11 mg/l). All subjects with influenza showed pronounced increases in SAA concentrations (mean increase 642 mg/l) while six showed increases in CRP concentration (mean increase 22 mg/l). All these increases were highly significant (p less than 0.001). Asymptomatic excretors of both rhinovirus and influenza virus showed significant increases in SAA concentration (p = 0.015 for rhinovirus and p less than 0.001 for influenza virus) but not in CRP concentration. No changes in SAA or CRP values were seen in 12 volunteers after challenge with saline. These observations suggest that caution is required in the interpretation of estimations of SAA concentration and that it may be too sensitive an acute phase protein for clinical use as its concentration may be raised in both trivial and asymptomatic viral infections.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenzana-Seisdedos F., Virelizier J. L. Interferons as macrophage-activating factors. II. Enhanced secretion of interleukin 1 by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1983 Jun;13(6):437–440. doi: 10.1002/eji.1830130602. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A., Martin T. R., Li J. J., McAdam K. P. Association of insulin pump therapy with raised serum amyloid A in type I diabetes mellitus. Lancet. 1984 Feb 25;1(8374):411–413. doi: 10.1016/s0140-6736(84)91750-1. [DOI] [PubMed] [Google Scholar]
- Chambers R. E., MacFarlane D. G., Whicher J. T., Dieppe P. A. Serum amyloid-A protein concentration in rheumatoid arthritis and its role in monitoring disease activity. Ann Rheum Dis. 1983 Dec;42(6):665–667. doi: 10.1136/ard.42.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Whicher J. T. Quantitative radial immunodiffusion assay for serum amyloid A protein. J Immunol Methods. 1983 Apr 15;59(1):95–103. doi: 10.1016/0022-1759(83)90149-7. [DOI] [PubMed] [Google Scholar]
- De Beer F. C., Mallya R. K., Fagan E. A., Lanham J. G., Hughes G. R., Pepys M. B. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet. 1982 Jul 31;2(8292):231–234. doi: 10.1016/s0140-6736(82)90321-x. [DOI] [PubMed] [Google Scholar]
- From the GMSC: Parallel importing: proposed action to protect patients deferred. Br Med J (Clin Res Ed) 1984 May 5;288(6427):1391–1392. [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Sztein M. B., Schmidt J. A., Murphy P., Grabner G., Oppenheim J. J. Properties of murine and human epidermal cell-derived thymocyte-activating factor. Fed Proc. 1983 Jul;42(10):2772–2776. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marhaug G., Permin H., Husby G. Amyloid-related serum protein (SAA) as an indicator of lung infection in cystic fibrosis. Acta Paediatr Scand. 1983 Nov;72(6):861–866. doi: 10.1111/j.1651-2227.1983.tb09831.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Enholm E., Teppo A. M. Is interferon an "inducer" of serum amyloid A? N Engl J Med. 1983 Oct 27;309(17):1060–1061. doi: 10.1056/NEJM198310273091715. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Ahonen J., von Willebrand E. Measurement of serum amyloid A protein concentrations as test of renal allograft rejection in patients with initially non-functioning grafts. Br Med J (Clin Res Ed) 1984 Feb 4;288(6414):360–361. doi: 10.1136/bmj.288.6414.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy P. L., Frank A. L., Ablow R. C., Masters S. J., Dolan T. F., Jr Value of the C-reactive protein test in the differentiation of bacterial and viral pneumonia. J Pediatr. 1978 Mar;92(3):454–456. doi: 10.1016/s0022-3476(78)80448-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Stadler B. M., Siraganian R. P., Mage M., Mathieson B. Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc. 1982 Feb;41(2):257–262. [PubMed] [Google Scholar]
- Pepys M. B. C-reactive protein fifty years on. Lancet. 1981 Mar 21;1(8221):653–657. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Measurement of serum amyloid A protein concentrations as test of renal allograft rejection. Br Med J (Clin Res Ed) 1984 Mar 17;288(6420):859–860. doi: 10.1136/bmj.288.6420.859-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes J. G., Cooper E. H. Comparison of serum amyloid A protein and C-reactive protein concentrations in cancer and non-malignant disease. J Clin Pathol. 1983 Jul;36(7):798–803. doi: 10.1136/jcp.36.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Shainkin-Kestenbaum R., Zimlichman S., Winikoff Y., Chaimovitz C., Pras M. Serum amyloid A levels in patients with infections due to cytomegalovirus, varicella-zoster virus, and herpes simplex virus. J Infect Dis. 1982 Sep;146(3):443–443. doi: 10.1093/infdis/146.3.443. [DOI] [PubMed] [Google Scholar]
- Saxstad J., Nilsson L. A., Hanson L. A. C-reactive protein in serum from infants as determined with immunodiffusion techniques. II. Infants with various infections. Acta Paediatr Scand. 1970 Nov;59(6):676–680. doi: 10.1111/j.1651-2227.1970.tb17704.x. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Zimlichman S., Winikoff Y., Pras M., Chaimovitz C., Sarov I. Serum amyloid A (SAA) in viral infection: rubella, measles and subacute sclerosing panencephalitis (SSPE). Clin Exp Immunol. 1982 Dec;50(3):503–506. [PMC free article] [PubMed] [Google Scholar]
- Whicher J. T., Bell A. M., Martin M. F., Marshall L. A., Dieppe P. A. Prostaglandins cause an increase in serum acute-phase proteins in man, which is diminished in systemic sclerosis. Clin Sci (Lond) 1984 Feb;66(2):165–171. doi: 10.1042/cs0660165. [DOI] [PubMed] [Google Scholar]


