ABSTRACT
The study of singlet oxygen in biological systems is challenging in many ways. Singlet oxygen is a relatively unstable ephemeral molecule, and its properties make it highly reactive with many biomolecules, making it difficult to quantify accurately. Several methods have been developed to study this elusive molecule, but most studies thus far have focused on those conditions that produce relatively large amounts of singlet oxygen. However, the need for more sensitive methods is required as one begins to explore the levels of singlet oxygen required in signaling and regulatory processes. Here we discuss the various methods used in the study of singlet oxygen, and outline their uses and limitations.
KEYWORDS: EPR spectroscopy, lipid peroxidation, reactive oxygen species, singlet oxygen, singlet oxygen sensor green, ultraweak photon emission
Singlet oxygen is a highly reactive form of Reactive Oxygen Species (ROS), which preferentially interacts with most biomolecules such as lipids, proteins, and DNA/RNA, with a preference for electron rich regions which imparts a form of selectivity to its interaction. Previous studies of singlet oxygen production under physiological conditions in plants stem from 3 main sources; by using photosensitizers such as Rose Bengal,1 by stimulation of photoinhibition through a combination of light stress and inhibitors of electron transport such as DCMU and DMBIB,2 and by using singlet oxygen generating mutant lines such as flu and Ch1.3,4 These produce copious amounts of singlet oxygen in the light. Intriguingly, singlet oxygen production was observed also in the dark, independent of chlorophyll, and was stimulated by wounding, dehydration and flagellin 22 elicitor treatments, in root tip cells of Arabidopsis.5 This raises the question about the possible sources of [dark] singlet oxygen and the need to measure low levels.
In our recent paper, we characterized the formation of singlet oxygen and cell death under dehydration stress in the dark in Arabidopsis, as well as by photodynamic means.6 In the course of this work, we investigated various methods that are presently being used to study singlet oxygen in biological systems. These measurements are challenging as the amount of singlet oxygen being produced in the dark is very low. By EPR spectroscopy it was estimated to be about 1 nmol gFW−1 min−1, which is much smaller than that observed in photoinhibited chloroplasts7 or in DCMU/high-light treated plants.8 In such circumstances, it is important to utilize methods that are sensitive enough to detect low levels of singlet oxygen, and also to improve the sensitivity of current methods. An indirect method uses qRT-PCR of transcripts activated by singlet oxygen. More direct methods include using probes that interact directly with singlet oxygen e.g. qualitative observation of fluorescent probe Singlet Oxygen Sensor Green (SOSG), or by quantitative EPR spectroscopy with spin traps that form stable radicals with singlet oxygen. This summary will highlight these methods and other approaches, to provide specific insights on their uses and limitations.
SOSG
SOSG reacts with singlet oxygen to produce SOSG endoperoxides (SOSG-EP). The formation of the endoperoxide exposes a fluorescein fluorophore. SOSG was characterized previously to be a sensitive and specific probe for singlet oxygen,9 being able to detect at least levels of singlet oxygen produced by nanomolar concentrations of the photosensitizer Rose Bengal,10 and based on the literature, appears to be the probe of choice when studying singlet oxygen. However, it has been observed that SOSG can be subjected to self-activation when exposed to specific wavelengths of light, indicating that careful use of this probe and proper controls are necessary to obtain meaningful results.11 The first issue lies with the direct conversion of the anthracene quencher moiety into the endoperoxide form when stimulated with strong laser light at 420–440 nm. This is important to consider when other fluorophores such as CFP are being studied within the same system, and the appropriate lasers and fluorophores should be applied accordingly. A second and more prevalent issue lies with the excitation of the fluorescein molecule of SOSG at 530 nm, which can transfer its energy to an oxygen molecule and generate singlet oxygen. Thus once SOSG is activated, a self-propagating singlet oxygen cycle may be initiated.12 The use of low laser excitation energy can serve to minimize the reaction but it means that SOSG should not be used to quantify singlet oxygen production, although qualitative measurements under controlled conditions are valid. Another issue is its intracellular localization. No ester derivatives are available that aid in sequestration of SOSG within the cell so that incubation times of at least 20 min at a relatively high concentration of 100 μM was found to be necessary. Once in the cell and activated, SOSG-EP can be detected in most organelles.5
EPR spectroscopy
EPR spectroscopy detects compounds with unpaired electrons. The direct measurement of singlet oxygen is difficult due to its extremely short half-life (∼4 µs in water). Specific spin traps were developed that react with singlet oxygen to form a stable nitroxide radical which can be measured using EPR.13 The theoretical limit of detection of EPR in a Bruker ELEXSYS E500 spectrometer has a rough concentration sensitivity limit of 4.0 × 10−12 mol. However, when working with biological samples, several key considerations have to be addressed. Firstly, the presence of water causes an attenuation of the EPR signal to a working limit of about 1 × 10−9 mol.14 In order to further improve the EPR signal to noise ratio, extraction methods have to be developed to separate the EPR responsive radical portion from its aqueous environment. Secondly, many biomolecules can interact with the spin traps. Hence, it is useful to check if modifications to the trap have occurred.
Thirdly, the amount of spin trap penetration into tissue is an important consideration, and one should note that not all spin traps function optimally at physiological pH. For example, some of the piperidine based spin traps, such as 2,2,6,6-tetramethylpiperidine (TEMP), and 2,2,6,6-tetramethylpiperidinol (4-hydroxy-TEMP), used frequently for singlet oxygen studies function optimally at pH 11, with sensitivity decreasing with pH.15 The sensitivity to pH of 4-hydroxy-TEMP is illustrated in Fig. 1. Thus, under these conditions at physiological pH 7 the probe works at about 10% of its potential rate. It is useful to account for this when selecting a spin trap to use, for example in cytosolic versus apoplastic or thylakoid membrane surface studies.16 Fourthly, the specificity and sensitivity of the spin trap plays a role in determining if a usable signal to noise ratio can be accomplished. The 4-hydroxy-TEMP spin trap was shown to produce an EPR signal of several folds higher than TEMP in photodynamic activation of rose bengal.10
Figure 1.
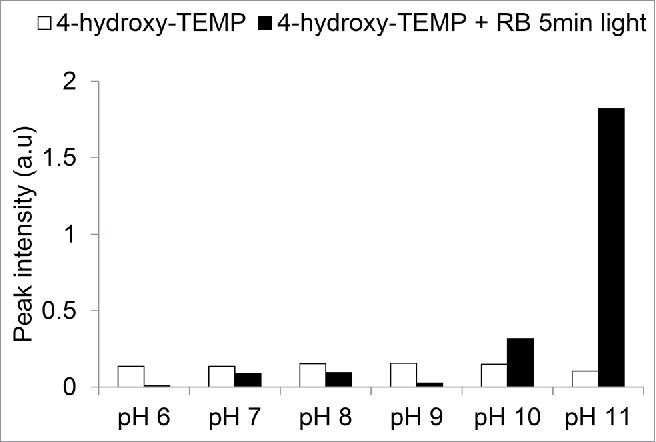
Sensitivity of the 4-hydroxy-TEMP spin trap to pH. Four-hydroxy-TEMP (50 mM) was prepared in buffers of varying pH: pH 6, (MES); pH 7, (Potassium phosphate); pH 8, (Tris-HCl); pH 9, (Glycine); pH 10 CAPS, pH 11, (water), with and without addition of 100 µM rose bengal and then exposed to light (1000 µE) for 5 min.
One common modification that can occur to spin traps in plant tissue is reductive or oxidative changes that render them [EPR silent].17,18,19 In our protocol, we utilized EDTA and N-ethyl maleimide (NEM) in the extraction buffer to curb various side reactions that could occur through enzymes, metal ions or sulfhydryl groups.6,17 In Fig. 2, we introduced the stable radical form of the 4-hydroxy-TEMP spin trap (4-hydroxy-TEMPO) to Arabidopsis seedlings, and showed that the addition of EDTA and NEM to the extraction buffer improved the signal to noise ratio. In the past decade, methods have been developed that have combined EPR spectroscopy with LC-MS techniques, which have proven to be powerful tools in studying and quantifying the formation of various spin trap-organic radical adducts. These methods are especially useful due to their great resolution of intracellular processes and their ability to measure EPR silent species.20,21,22. In summary, the amounts of singlet oxygen measured will depend on the type of spin trap used and the conditions of its use.
Figure 2.
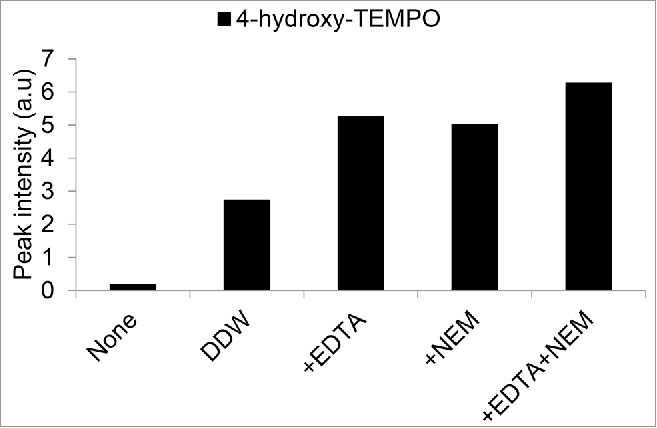
Effect of various extraction buffers on the EPR signal after incubating Arabidopsis seedlings with 4-hydroxy-TEMPO radical. Arabidopsis seedlings (2 week old) were incubated with 500 µM 4-hydroxy-TEMPO radical for 30 min, washed thoroughly with DDW, and then flash frozen. For extraction, Ethanol (75%) was supplemented with: DDW, 10 mM EDTA, 20 mM N-ethylmaleimide, or a combination of EDTA and NEM as indicated.
Lipid peroxidation
Lipid peroxidation is a downstream indirect effect of ROS formation, and lipid radicals have been suggested to play roles in cell signaling.23 As such, various methods of lipid peroxidation have been used as a proxy for measuring general ROS production. The simplest procedure is the Thiobarbituric (TBA) assay that uses 2-thiobarturic acid to form an adduct with Malondialdehyde (MDA), a downstream product of oxidized lipids. This adduct can then be measured via colorimetric or fluorometric means.24 The method is non-specific, as various ROS can cause lipid peroxidation. However, applying combined chromatographic and mass spectrometry techniques can serve to improve the resolution of MDA based analyses.25
High-performance liquid chromatography-electrospray ionization-tandem mass spectrometry allows for the discrimination between free radical (type I)- and singlet oxygen (1O2; type II)-mediated lipid peroxidation (LPO) signatures by using hydroxy fatty acids (HOTEs) as specific reporters of interaction with singlet oxygen.26 Enzymatic and non-enzymatic sources of lipid peroxidation can be resolved by this method.27 This method was used to show singlet oxygen production under various stress conditions including high light stress, methyl viologen treatment, drought,28 pathogen infection,29 and also with singlet oxygen producing flu and Ch1 plants.4,30 The measurements were particularly effective when applied to treatments that elicit a strong response and over the scale of hours or days. Indeed, under the photorespiratory stress conditions being tested, anti-oxidant deficient mutant plants such as catalase, xanthophyll and tocopherol were particularly effective in enhancing the response.4,26 The technique was also sensitive enough to measure the effects of wounding and high light stress, although in this case only total HOTE content was measured.31
Ultra-weak photon emission
A direct method to study singlet oxygen in vitro is to measure the weak photon emission directly emitted during the decay of singlet oxygen at 1270 nm.32,33 This method has been further developed to study singlet oxygen in various biological systems, including plants, which has provided a useful and specific tool to monitor singlet oxygen production.34 Most of these analyses focused on singlet oxygen production at the surfaces of cells35 or isolated organelles,36 or via the addition of photosensitizers.37,38 However, due to the exceptionally weak photon emission, it is difficult to detect signals that are emanating from within the cell, firstly simply due to light scattering by biological materials, which can reduce 1270 nm light penetration by 95%.35 This is further compounded by the extremely low quantum yield of singlet oxygen phosphorescence (Φp) in water which is close to 5 × 10−7, the short lifetime of singlet oxygen of ∼4 µs in water, as well as the myriad of biological molecules that can scavenge singlet oxygen.39 At the current state of the art, detection of endogenous singlet oxygen from chloroplasts in vivo via this technique was recently described as ‘a tall order’.40
Oxidation of other biomolecules can lead to the release of photons at other wavelengths.41 These emissions are: (1) triplet excited carbonyl in the near UVA and blue–green areas (350–550 nm), (2) singlet and triplet excited pigments in the green–red (550–750 nm) and red-near IR (750–1000 nm) areas, respectively and (3) collision of 2 1O2 (dimol emission) in the red (634 and 703 nm) and near IR (1270 nm) areas.42 Using a combination of different filters to monitor specific wavelengths of photon emission generated under various stress conditions, it was possible to characterize several properties of photon emission generated in both in vitro and in vivo systems.31,43 In further advances, the technique has been expanded to include visualization of spontaneous photon emission in microbial and animal cells, and also live animals and in humans.44,45 Hence, the technique requires attention to the types of ROS one is trying to measure, and the types of filters used. Importantly, the delayed fluorescence of chlorophyll, and the reaction of radical carbonyl groups with chlorophyll, can cause possible confounding factors in the data analysis and necessitates pre-incubation in the dark to allow for relaxation of the chlorophyll fluorescence.41,43
Real time quantitative PCR
Measuring the accumulation of specific transcripts by real time qPCR can serve as an indirect means to detect the ephemeral presence of singlet oxygen. By virtue of accumulated work using microarray or RNAseq experiments5,46,47 and Genevestigator databases, it has been possible to identify gene markers selective for specific ROS like singlet oxygen. Indeed, many abiotic and biotic stresses show correlation with transcripts that appear in the light-sensitive flu mutant as a result of photodynamic singlet oxygen production.5 This technique was combined with time course and concentration dependent studies to provide a set of specific marker genes. We showed that transcripts correlated with singlet oxygen, and not other ROS, such as superoxide and hydrogen peroxide, were induced under acute dehydration stress in the dark. The transcript induction levels were comparable to singlet oxygen produced photodynamically by rose bengal and acridine orange.6 Rose bengal was shown in vitro to have an approximately 2.5-fold higher singlet oxygen yield than acridine orange (Ito, 1978), and that difference appears to be correlated to the gene expression levels. However, one cannot reliably correlate the strength of transcript accumulation and singlet oxygen production between rose bengal and acridine orange as they accumulate in different subcellular areas; the cell periphery and vacuole, respectively .6 The use of real-time qPCR with the appropriate genes extends the sensitivity of the measurements.6 The observation that certain genes display a marked sensitivity to singlet oxygen that are estimated by our measurements to be nanomolar quantities, indicates that singlet oxygen seems to be a very potent signaling factor.
Conclusions
Singlet oxygen appears to affect the cell in a localization and dose dependent manner.48,49 When the amount of singlet oxygen is high enough to overload cell scavenging systems, the cell suffers cytotoxic damage and undergoes a rapid necrosis like cell death.50 At lower levels, singlet oxygen appears to trigger a programmed cell death type response.51 Much more remains to be understood about the role of singlet oxygen in the cell. In order to investigate the functions of this fascinating molecule at physiological concentrations, methods have to be developed and refined to provide reliable and accurate sources of information. Ideally, the improvements in specificity and sensitivity would be coupled with elucidation of the underlying biology.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Gutiérrez J, González-Pérez S, García-García F, Daly CT, Lorenzo Ó, Revuelta JL, McCabe PF, Arellano JB. Programmed cell death activated by rose bengal in arabidopsis thaliana cell suspension cultures requires functional chloroplasts. J Exp Bot 2014; 65:3081-3095; http://dx.doi.org/ 10.1093/jxb/eru151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot 2005; 56:337-46; PMID:15310815; http://dx.doi.org/ 10.1093/jxb/erh237 [DOI] [PubMed] [Google Scholar]
- 3.Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. FLU: A negative regulator of chlorophyll biosynthesis in arabidopsis thaliana. Proc Natl Acad Sci USA 2001; 98:12826-31; PMID:11606728; http://dx.doi.org/ 10.1073/pnas.221252798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramel F, Ksas B, Akkari E, Mialoundama AS, Monnet F, Krieger-Liszkay A, Ravanat JL, Mueller MJ, Bouvier F, Havaux M. Light-induced acclimation of the arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 2013; 25:1445-62; PMID:23590883; http://dx.doi.org/ 10.1105/tpc.113.109827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R. Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol 2014; 165:249-61; PMID:24599491; http://dx.doi.org/ 10.1104/pp.114.236380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh E, Carmieli R, Mor A, Fluhr R. Singlet oxygen induced membrane disruption and serpin-protease balance in vacuolar driven cell death in Arabidopsis thaliana. Plant Physiol 2016; http://dx.doi.org/ 10.1104/pp.15.02026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hideg E, Spetea C, Vass I. Singlet oxygen production in thylakoid membranes during photoinhibition as detected by EPR spectroscopy. Photosynth Res 1994; 39:191-9; PMID:24311071; http://dx.doi.org/ 10.1007/BF00029386 [DOI] [PubMed] [Google Scholar]
- 8.Fufezan C, Rutherford AW, Krieger-Liszkay A. Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett 2002; 532:407-10; PMID:12482601; http://dx.doi.org/ 10.1016/S0014-5793(02)03724-9 [DOI] [PubMed] [Google Scholar]
- 9.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J Exp Bot 2006; 57:1725-34; PMID:16595576; http://dx.doi.org/ 10.1093/jxb/erj181 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Ishiyama K, Ikai H, Kanno T, Sasaki K, Niwano Y, Kohno M. Reevaluation of analytical methods for photogenerated singlet oxygen. J Clin Biochem Nutr 2011; 49:87-95; PMID:21980223; http://dx.doi.org/ 10.3164/jcbn.10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Fujitsuka M, Majima T. Photochemistry of singlet oxygen sensor green. J Phys Chem B 2013; 117:13985-92; PMID:24111566; http://dx.doi.org/ 10.1021/jp406638g [DOI] [PubMed] [Google Scholar]
- 12.Ragas X, Jimenez-Banzo A, Sanchez-Garcia D, Batllori X, Nonell S. Singlet oxygen photosensitisation by the fluorescent probe singlet oxygen sensor green. Chem Commun 2009:2920-2; http://dx.doi.org/ 10.1039/b822776d [DOI] [PubMed] [Google Scholar]
- 13.Lion Y, Delmelle M, Van De Vorst A. New method of detecting singlet oxygen production. Nature 1976; 263:442-3; PMID:972689; http://dx.doi.org/ 10.1038/263442a0 [DOI] [PubMed] [Google Scholar]
- 14.Nesmelov YE, Gopinath A, Thomas DD. Aqueous sample in an EPR cavity: sensitivity considerations. J Magn Reson 2004; 167:138-46; PMID:14987608; http://dx.doi.org/ 10.1016/j.jmr.2003.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav DK, Pospísil P. Evidence on the formation of singlet oxygen in the donor side photoinhibition of photosystem II: EPR spin-trapping study. PLoS ONE 2012; 7:e45883; PMID:23049883; http://dx.doi.org/ 10.1371/journal.pone.0045883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moan J, Wold E. Detection of singlet oxygen production by ESR. Nature 1979; 279:450-1; PMID:16068192; http://dx.doi.org/ 10.1038/279450a0 [DOI] [PubMed] [Google Scholar]
- 17.Giotta GJ, Wang HH. Reduction of nitroxide free radicals by biological materials. Biochem Bioph Res Co 1972; 46:1576; http://dx.doi.org/ 10.1016/0006-291X(72)90788-7 [DOI] [PubMed] [Google Scholar]
- 18.Rosen GM, Finkelstein E. Use of spin traps in biological systems. Free Radic Biol Med 1985; 1:345-75; http://dx.doi.org/ 10.1016/8755-9668(85)90012-2 [DOI] [Google Scholar]
- 19.Lavi R, Sinyakov M, Samuni A, Shatz S, Friedmann H, Shainberg A, Breitbart H, Lubart R. ESR detection of 1O2 reveals enhanced redox activity in illuminated cell cultures. Free Radic Res 2004; 38:893-902; PMID:15621706; http://dx.doi.org/ 10.1080/1071576010001642646 [DOI] [PubMed] [Google Scholar]
- 20.Qian SY, Yue GH, Tomer KB, Mason RP. Identification of all classes of spin-trapped carbon-centered radicals in soybean lipoxygenase-dependent lipid peroxidations of omega-6 polyunsaturated fatty acids via LC/ESR, LC/MS, and tandem MS. Free Rad Biol Med 2003; 34:1017-28; PMID:12684086; http://dx.doi.org/ 10.1016/S0891-5849(03)00031-5 [DOI] [PubMed] [Google Scholar]
- 21.Guo Q, Qian SY, Mason RP. Separation and identification of DMPO adducts of oxygen-centered radicals formed from organic hydroperoxides by HPLC-ESR, ESI-MS and MS/MS. J Am Soc Mass Spectr 2003; 14:862-71; http://dx.doi.org/ 10.1016/S1044-0305(03)00336-2 [DOI] [PubMed] [Google Scholar]
- 22.Michail K, Siraki AG. Post-trapping derivatization of radical-derived EPR-silent adducts: application to free radicaldetection by HPLC/UV in chemical, biochemical, and biological systems and comparison with EPR spectroscopy. Anal Chem 2012; 84:6739-46; PMID:22724922; http://dx.doi.org/ 10.1021/ac301142c [DOI] [PubMed] [Google Scholar]
- 23.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014; 2014:31; http://dx.doi.org/ 10.1155/2014/360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999; 207:604-11; http://dx.doi.org/ 10.1007/s004250050524 [DOI] [PubMed] [Google Scholar]
- 25.Giera M, Lingeman H, Niessen WMA. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): a brief overview. Chromatographia 2012; 75:433-40; PMID:22593603; http://dx.doi.org/ 10.1007/s10337-012-2237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 2008; 148:960-8; http://dx.doi.org/ 10.1104/pp.108.125690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K, Herrfurth C, Feussner I, Apel K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 2012; 24:3026-39; PMID:22797473; http://dx.doi.org/ 10.1105/tpc.112.100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marok MA, Tarrago L, Ksas B, Henri P, Abrous-Belbachir O, Havaux M, Rey P. A drought-sensitive barley variety displays oxidative stress and strongly increased contents in low-molecular weight antioxidant compounds during water deficit compared to a tolerant variety. J Plant Physiol 2013; 170:633-45; PMID:23541087; http://dx.doi.org/ 10.1016/j.jplph.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Grun C, Berger S, Matthes D, Mueller MJ. Early accumulation of non-enzymatically synthesised oxylipins in Arabidopsis thaliana after infection with Pseudomonas syringae. Funct Plant Biol 2007; 34:65-71; http://dx.doi.org/ 10.1071/FP06205 [DOI] [PubMed] [Google Scholar]
- 30.Przybyla D, Gobel C, Imboden A, Hamberg M, Feussner I, Apel K. Enzymatic, but not non-enzymatic, 1O2 mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1 dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J 2008; 54:236-48; PMID:18182022; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03409.x [DOI] [PubMed] [Google Scholar]
- 31.Birtic S, Ksas B, Genty B, Mueller MJ, Triantaphylides C, Havaux M. Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J 2011; 67:1103-15; PMID:21595761; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04646.x [DOI] [PubMed] [Google Scholar]
- 32.Krasnovsky AA. Photoluminescence of singlet oxygen in pigment solutions. Photochem Photobiol 1979; 29:29-36; http://dx.doi.org/ 10.1111/j.1751-1097.1979.tb09255.x [DOI] [Google Scholar]
- 33.Khan AU, Kasha M. Direct spectroscopic observation of singlet oxygen emission at 1268 nm excited by sensitizing dyes of biological interest in liquid solution. Proc Natl Acad Sci USA 1979; 76:6047-9; PMID:16592729; http://dx.doi.org/ 10.1073/pnas.76.12.6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telfer A. Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-carotene. Plant Cell Physiol 2014; 55:1216-23; PMID:24566536; http://dx.doi.org/ 10.1093/pcp/pcu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanofsky JR, Sima PD. Singlet oxygen generation from the reaction of ozone with plant leaves. J Biol Chem 1995; 270:7850-2; PMID:7713876; http://dx.doi.org/ 10.1074/jbc.270.14.7850 [DOI] [PubMed] [Google Scholar]
- 36.Yadav DK, Kruk J, Sinha RK, Pospísil P. Singlet oxygen scavenging activity of plastoquinol in photosystem II of higher plants: Electron paramagnetic resonance spin-trapping study. Biochim Biophys Acta 2010; 1797:1807-11; PMID:20637718; http://dx.doi.org/ 10.1016/j.bbabio.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 37.Maisch T, Baier J, Franz B, Maier M, Landthaler M, Szeimies R-M, Bäumler W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA 2007; 104:7223-8; PMID:17431036; http://dx.doi.org/ 10.1073/pnas.0611328104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daub ME, Ehrenshaft M. The photoactivated cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu Rev Phytopathol 2000; 38:461-90; PMID:11701851; http://dx.doi.org/ 10.1146/annurev.phyto.38.1.461 [DOI] [PubMed] [Google Scholar]
- 39.Triantaphylides C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 2009; 14:219-28; PMID:19303348; http://dx.doi.org/ 10.1016/j.tplants.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 40.Li H, Melo TB, Arellano JB, Razi Naqvi K. Temporal profile of the singlet oxygen emission endogenously produced by photosystem II reaction centre in an aqueous buffer. Photosynth Res 2012; 112:75-9; PMID:22481218; http://dx.doi.org/ 10.1007/s11120-012-9739-4 [DOI] [PubMed] [Google Scholar]
- 41.Havaux M, Triantaphylides C, Genty B. Autoluminescence imaging: a non-invasive tool for mapping oxidative stress. Trends Plant Sci 2006; 11:480-4; PMID:16956784; http://dx.doi.org/ 10.1016/j.tplants.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 42.Pospisil P. Ultra-weak photon emission from living systems - from mechanism to application. J Photochem Photobiol B 2014; 139:1; PMID:25017820; http://dx.doi.org/ 10.1016/j.jphotobiol.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 43.Flor-Henry M, McCabe TC, Bruxelles GL, Roberts MR. Use of a highly sensitive two-dimensional luminescence imaging system to monitor endogenous bioluminescence in plant leaves. BMC Plant Biol 2004; 4:1-8; PMID:15005814; http://dx.doi.org/ 10.1186/1471-2229-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad A, Pospíšil P. Towards the two-dimensional imaging of spontaneous ultra-weak photon emission from microbial, plant and animal cells. Sci Rep 2013; 3:1211; PMID:23386970; http://dx.doi.org/ 10.1038/srep01211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ives JA, van Wijk EPA, Bat N, Crawford C, Walter A, Jonas WB, van Wijk R, van der Greef J. Ultraweak photon emission as a non-invasive health assessment: a systematic review. PLOS One 2014; 9:e87401; PMID:24586274; http://dx.doi.org/ 10.1371/journal.pone.0087401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Daniela Wagner, Éva Hideg, Cornelia Göbel, Ivo Feussner, et al.. Rapid induction of distinct stress responses after the release of singlet oxygen in arabidopsis. Plant Cell 2003; 15:2320-32; PMID:14508004; http://dx.doi.org/ 10.1105/tpc.014662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in arabidopsis. Plant Physiol 2006; 141:436-45; PMID:16603662; http://dx.doi.org/ 10.1104/pp.106.078717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006; 28:1091-101; PMID:17041898; http://dx.doi.org/ 10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- 49.Laloi C, Havaux M. Key players of singlet oxygen-induced cell death in plants. Front Plant Sci 2015; 6:39; PMID:25699067; http://dx.doi.org/ 10.3389/fpls.2015.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim C, Meskauskiene R, Apel K, Laloi C. No single way to understand singlet oxygen signalling in plants. EMBO Rep 2008; 9:435-9; PMID:18451767; http://dx.doi.org/ 10.1038/embor.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al.. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 2004; 306:1183-5; PMID:15539603; http://dx.doi.org/ 10.1126/science.1103178 [DOI] [PubMed] [Google Scholar]


