Supplemental Digital Content is available in the text.
Keywords: gadolinium, mitral valve, mitral valve annulus, mitral valve prolapse, papillary muscles
Abstract
Background—
Arrhythmic mitral valve prolapse (MVP) is characterized by myxomatous leaflets and left ventricular (LV) fibrosis of papillary muscles and inferobasal wall. We searched for morphofunctional abnormalities of the mitral valve that could explain a regional mechanical myocardial stretch.
Methods and Results—
Thirty-six (27 female patients; median age: 44 years) arrhythmic MVP patients with LV late gadolinium enhancement on cardiac magnetic resonance and no or trivial mitral regurgitation, and 16 (6 female patients; median age: 40 years) MVP patients without LV late gadolinium enhancement were investigated by morphofunctional cardiac magnetic resonance. Mitral annulus disjunction (median: 4.8 versus 1.8 mm; P<0.001), end-systolic mitral annular diameters (median: 41.2 versus 31.5; P=0.004) and end-diastolic mitral annular diameters (median: 35.5 versus 31.5; P=0.042), prevalence of posterior systolic curling (34 [94%] versus 3 [19%]; P<0.001), and basal to mid LV wall thickness ratio >1.5 (22 [61%] versus 4 [25%]; P=0.016) were higher in MVP patients with late gadolinium enhancement than in those without. A linear correlation was found between mitral annulus disjunction and curling (R=0.85). A higher prevalence of auscultatory midsystolic click (26 [72%] versus 6 [38%]; P=0.018) was also noted. Histology of the mitral annulus showed a longer mitral annulus disjunction in 50 sudden death patients with MVP and LV fibrosis than in 20 patients without MVP (median: 3 versus 1.5 mm; P<0.001).
Conclusions—
Mitral annulus disjunction is a constant feature of arrhythmic MVP with LV fibrosis. The excessive mobility of the leaflets caused by posterior systolic curling accounts for a mechanical stretch of the inferobasal wall and papillary muscles, eventually leading to myocardial hypertrophy and scarring. These mitral annulus abnormalities, together with auscultatory midsystolic click, may identify MVP patients who would need arrhythmic risk stratification.
Arrhythmic mitral valve prolapse (MVP) is characterized by left ventricular (LV) fibrosis at the level of papillary muscles (PMs) and inferobasal wall.1 We provided evidence of this structural substrate for electric instability both by histopathology in sudden cardiac death (SCD) patients with myxomatous mitral valve (MV) and by late gadolinium enhancement (LGE) on contrast-enhanced cardiac magnetic resonance (CE-CMR) in arrhythmic MVP patients, with the morphology of arrhythmias well correlating with the site of scarring.1 Thus, we hypothesized a localized mechanical stretch on the LV myocardium by the prolapsing leaflets producing replacement-type fibrosis.
See Editorial by Lancellotti and Garbi
Myxomatous MV is characterized by leaflet thickening and redundancy, interchordal hooding, chordal elongation, and annular dilatation because of accumulation of proteoglycans.2 In 1986, Hutchins et al3 first advanced a role of the mitral annular disjunction (MAD), defined as a separation between left atrial (LA) wall at the level of MV junction and the LV free wall, in disease pathogenesis. According to their hypothesis, this peculiar anatomy of the mitral annulus could trigger a mechanical stress on the leaflets, leading to myxomatous degeneration, as a consequence of an excessive mobility of MV apparatus.
Although MAD has been evaluated in MVP patients with severe valve regurgitation,4 its role in arrhythmic MVP remains to be elucidated.
Because MVP is a relatively common echocardiographic finding and only a small proportion of MVP patients is characterized by ventricular arrhythmias, our aim was to assess whether morphological and functional characteristics of the MV apparatus could explain the propensity in some patients to develop a regional LV fibrosis at risk of electric instability.
Methods
Study Populations
The study included consecutive arrhythmic patients (either right bundle branch block or polymorphic ventricular arrhythmias) referred to our Cardiology Clinic from January 2010 to July 2014 with echocardiographic diagnosis of MVP and who underwent CE-CMR for the identification of LGE as previously defined. Complex ventricular arrhythmias consisted of ventricular fibrillation and ventricular tachycardia, either nonsustained or sustained.1
MVP patients with LGE on CE-CMR constituted our study population, whereas those without LGE on CE-CMR, enrolled in the same period, served as control group. Patients with an echocardiographic diagnosis of MVP but without history of arrhythmias did not undergo CE-CMR.
Exclusion criteria were moderate-to-severe mitral regurgitation, tricuspid dysplasia or regurgitation, cardiomyopathies or congenital heart abnormalities, hemodynamic unstable conditions, and contraindication to CE-CMR. The institutional review board approved the study, and all patients gave informed consent.
Among 60 MVP patients with mild or trivial regurgitation, 8 were excluded for either contraindication to CMR (n=6) or poor image quality (n=2).
The pathology arm of the study included SCD patients with MVP and LV fibrosis.1 Hearts from sex- and age-matched patients who died suddenly as a result of extracardiac causes served as controls.
Protocols of Investigation
Clinical Study
All patients underwent cardiovascular evaluation that included history, careful physical examination, 12-lead ECG, 2-dimensional transthoracic echocardiography, 12-lead 24-hour Holter monitoring, and CE-CMR. Cardiac auscultation was performed in all patients, paying particular attention to the detection of midsystolic click and late systolic murmur, progressively anticipated in systole by postural changes.5–7 CMR was performed on a 1.5-T scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). All patients underwent a detailed CE-CMR study protocol as previously described.1,8
On CMR, a series of morphofunctional parameters were carefully evaluated. MAD was defined as a separation between the LA-valve junction and the atrial aspect of the LV free wall.3 According to this definition, the length of MAD (or elongated mitral annulus) was measured from the LA wall–posterior MV leaflet junction to the top of the LV inferobasal wall during end systole4,9 (Figure 1A; Data Supplement). In particular, the upper limit of MAD was defined at the level of P2 scallop insertion to the LA wall, whereas the lower limit was defined at the level of LA connection with LV myocardium.4
Figure 1.
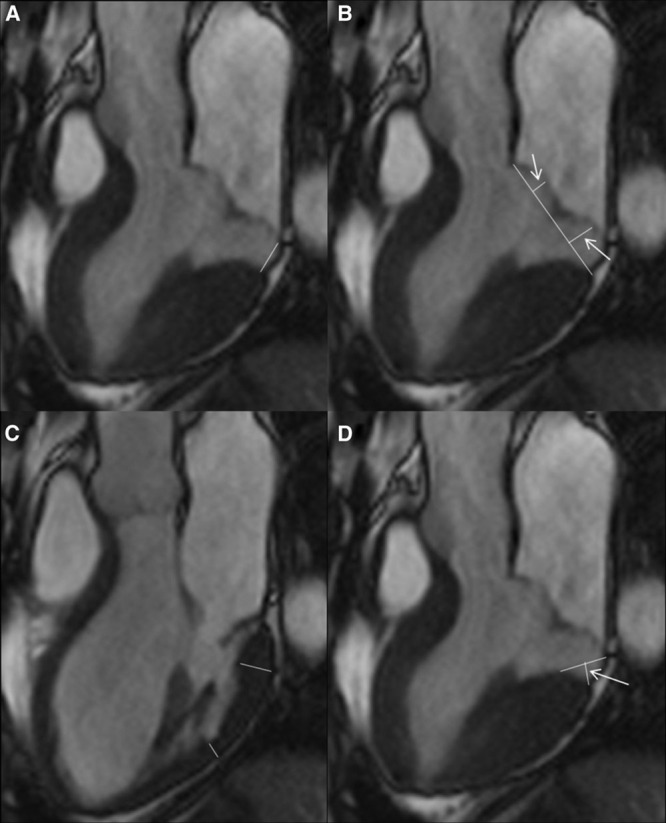
Cardiac magnetic resonance measures in patients with mitral valve prolapse. A, On 3-chamber, long-axis view, the length of mitral annulus disjunction (MAD; continuous white line) is measured from the left atrial (LA) wall–posterior MV leaflet junction to the top of the left ventricular (LV) inferobasal wall during end systole. B, On the same systolic frame, the prolapsed distance is measured as the maximum distance of the leaflet beyond the mitral annulus (white arrows). C, The LV thickness of basal and mid segments of the inferolateral wall is measured in the same long-axis view on diastole. D, The quantitative assessment of curling (white arrow) is provided by tracing a line between the top of LV inferobasal wall and the LA wall–posterior MV leaflet junction, and from this line, a perpendicular line to the lower limit of the mitral annulus during end systole.
All these measurements were calculated from the 3-chamber view for the LV outflow tract long-axis view, equivalent to the transthoracic parasternal long-axis view, obtained by an image plane perpendicular to the mitral annular major axis centered at the aortic outflow track10, and from 4-chamber view11 (Figure 1).
According to previous studies on MVP,9–12 additional morphofunctional parameters were evaluated: (1) ventricular volumes; (2) lengths of MV leaflets; (3) prolapsed distance (measured as the maximum prolapsed distance during peak systole beyond the mitral annulus) (Figure 1B); (4) diastolic maximum MV leaflet thickness; (5) mitral annular diameter during end systole and end diastole; and (6) ratio of basal to midventricular end-diastolic wall thickness (Figure 1C). The last parameter was expressed both as continuous variable (millimeters) and as a cutoff defined on the basis of median value.
Finally, the presence of the so-called curling, defined as an unusual systolic motion of the posterior mitral ring on the adjacent myocardium,13 was also evaluated (Data Supplement). When present, a quantitative assessment of curling was provided, by tracing a line between the top of LV inferobasal wall and the LA wall–posterior MV leaflet junction, and from this line a perpendicular line to the lower limit of the mitral annulus was traced and expressed in millimeters (Figure 1D). Curling was defined as severe when the value was higher than the median value.
All these morphofunctional parameters were independently assessed by 2 experienced observers (M.P.M. and B.G.) who were blinded to clinical data.
Pathological Anatomy Study
Formalin-fixed hearts from patients who died suddenly with myxomatous MV degeneration and LV replacement-type fibrosis at the level of PM and inferobasal wall were examined to assess the presence of MAD, as previously defined.3 To this aim, longitudinal full thickness samples of the left atrioventricular junction along the lateral and posterior walls, including the LA wall, the LV wall, and the attachment of the posterior MV leaflet, were obtained for histology. Five-micrometer-thick sections were stained with hematoxylin–eosin, Weigert–van Gieson, and Heidenhain trichrome. Morphometric analysis was performed with an Image-Pro Plus program (version 4.0. Media Cybernetics) to measure the MAD at ×2 magnification expressed both in micrometers and millimeters. Quantitative analysis was performed by 2 blinded expert pathologists (C.B. and S.R.).
Statistical Analysis
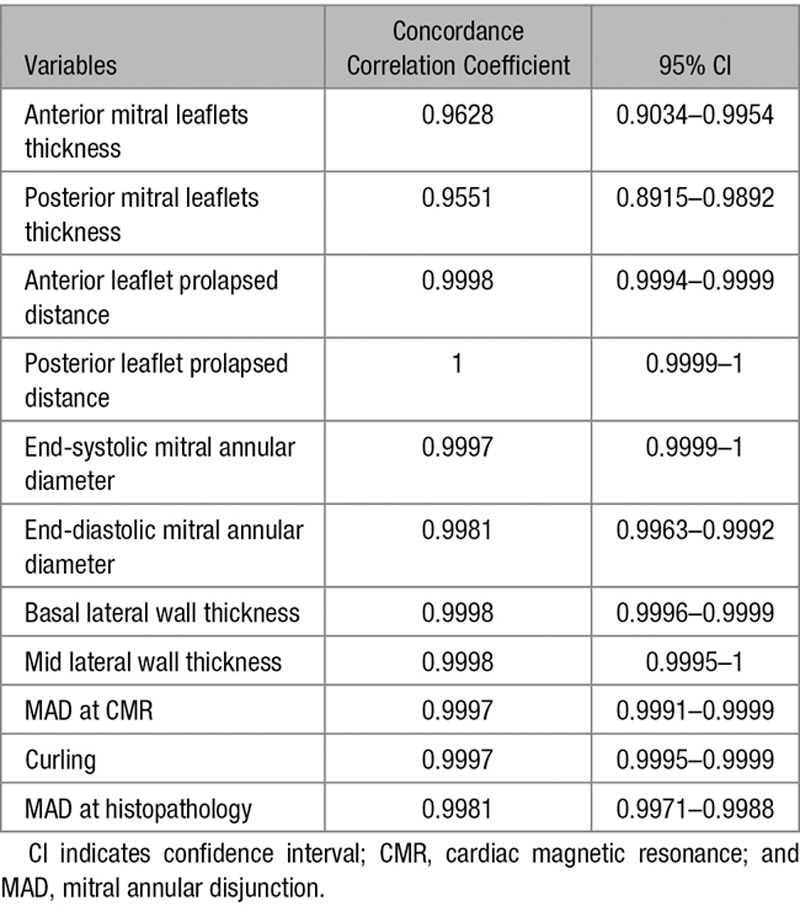
Results were expressed as median and quartiles because data were not normally distributed. Normal distribution was assessed with the Shapiro–Wilk test. Categorical data were expressed as number and percentage of population. In case of categorical variable, differences between groups were evaluated by the χ2 test or Fisher exact test as appropriate. Wilcoxon rank-sum test was used for quantitative variables. The interobserver agreement for CMR and histopathology measurements of MAD were evaluated with the Lin14 Concordance Correlation Coefficient and the 95% confidence interval calculated with the bootstrap method considering 2000 resamplings. The linear correlation was expressed with Spearman correlation coefficient. In all the analyses, a value of P<0.05 was considered statistically significant. Statistics were analyzed with SPSS version 19 (SPSS, Inc, Chicago, IL) and SAS 9.4 (SAS Institute, Inc, Cary, NC).
Results
MVP Patients With LGE
The baseline clinical and CMR findings of the 36 (27 female patients; median age: 44 years) arrhythmic MVP patients with LV fibrosis identified as LGE (median LV LGE 1.5%) are summarized in Table 1. The control group consisted of MVP without LGE (n=16; 6 female patients; median age: 40 years).
Table 1.
Clinical and CMR Characteristics
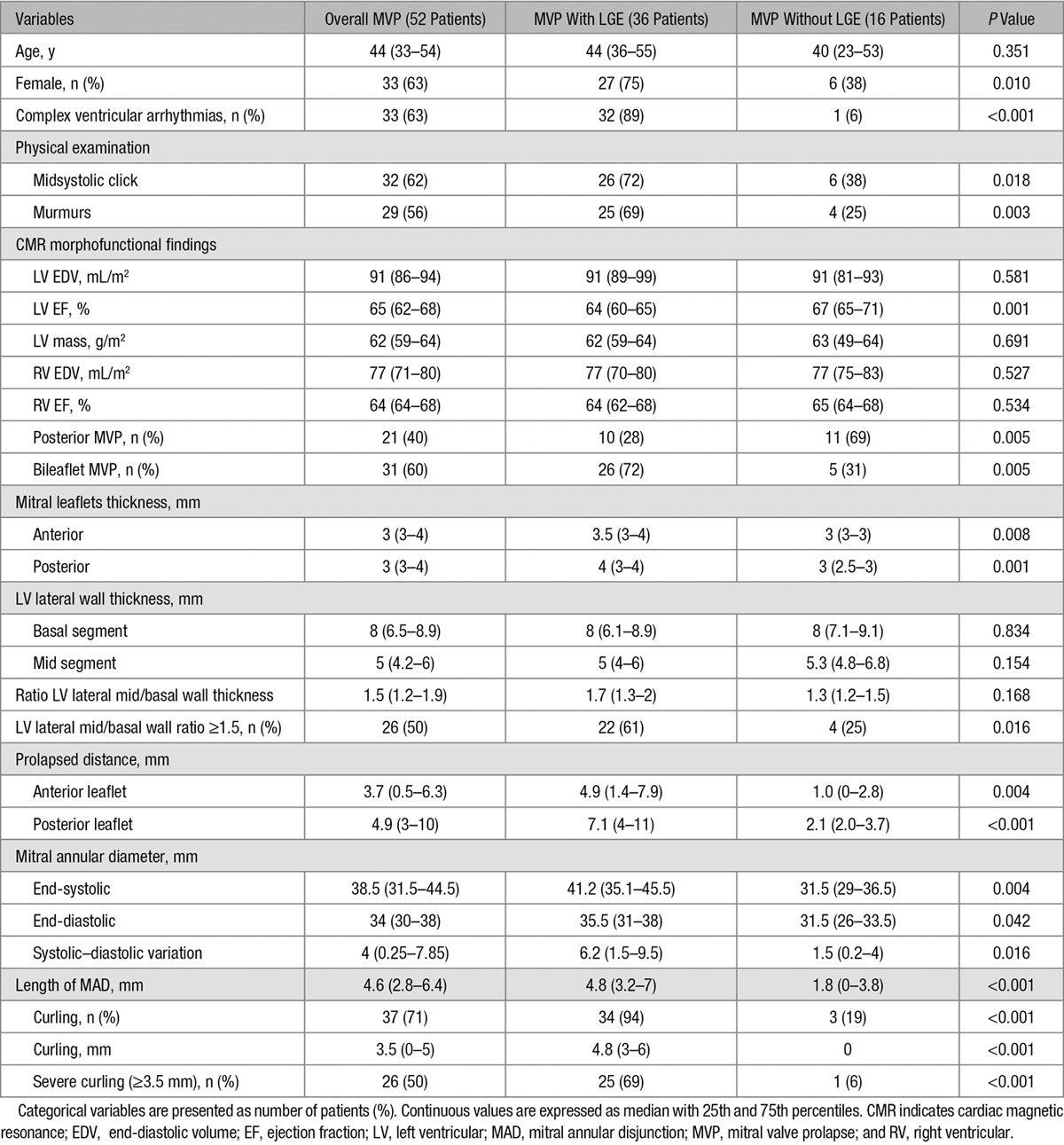
A higher prevalence of midsystolic click (72% versus 38%; P=0.018), late systolic murmur (69% versus 25%; P=0.003), complex ventricular arrhythmias originating from the LV (32/36 [89%] versus 1/16 [6%]; P<0.001), and bileaflet MVP (26/36 [72%] versus 5/16 [31%]; P=0.005) was found in MVP patients with LV LGE as compared with those without.
Moreover, MVP patients with LGE had a longer MAD (median: 4.8 versus 1.8 mm; P<0.001) and higher prevalence of curling (34 [94%] versus 3 [19%]; P<0.001). A representative case of a patient with curling and LV fibrosis is shown in Figure 2. The median value of the depth of curling was 3.5 mm in the overall population, with a statistically significant higher value in MVP patients with LGE (4.8 versus 0 mm; P<0.001). Using the median value as a cutoff for severe curling, it was more prevalent in MVP patients with LGE (25% versus 1%; P<0.001). A linear correlation was found between MAD and curling (R=0.85; Figure 3) and between MAD and extent of LGE (R=0.61; Figure 4).
Figure 2.
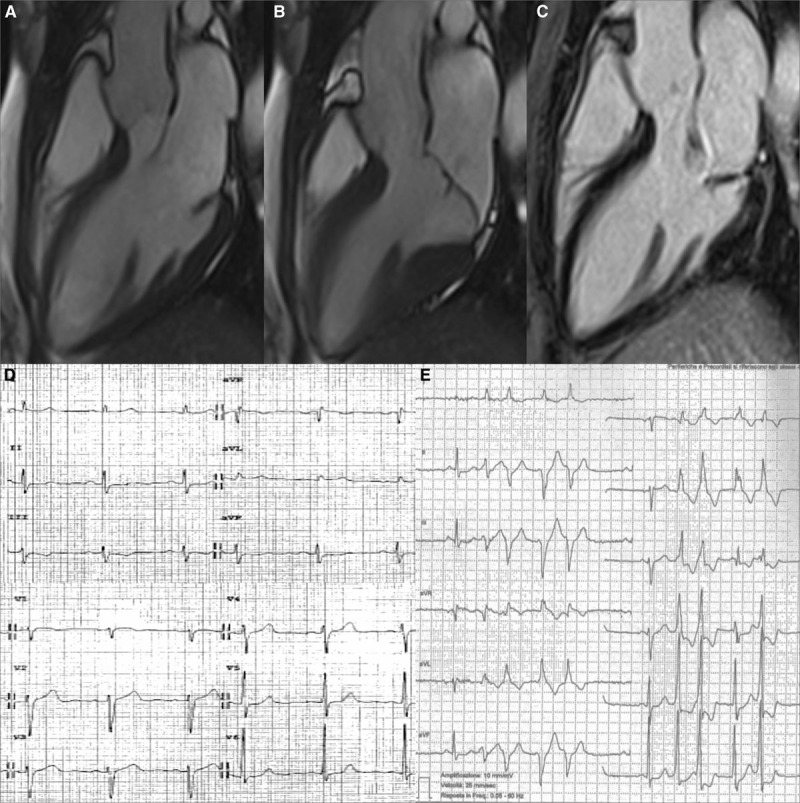
Representative case of arrhythmic mitral valve prolapse with mitral annular disjunction, curling, and late gadolinium enhancement (LGE). A 36-y-old woman with mitral valve prolapse and complex ventricular arrhythmias. On cine cardiac magnetic resonance (CMR) 3-chamber, long-axis view (diastolic frame A, systolic frame B), a mitral annulus disjunction is detectable; on contrast-enhanced CMR, a midmural LGE in the LV inferobasal region under posterior valve leaflet is visible (C). The 12-lead ECG (D) shows a negative T wave in III-aVF. Nonsustained ventricular tachycardia with right bundle branch block morphology originating from the LV inferobasal wall near the mitral annulus is also recorded in the 24-h Holter ECG (E).
Figure 3.
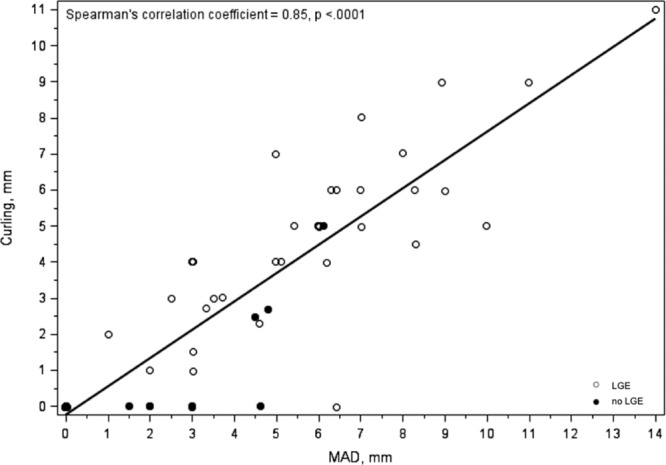
Relationship between length of mitral annular disjunction and curling in vivo. A significant correlation (R=0.85) between the depth of curling and length of mitral annulus disjunction (MAD; both expressed as mm) on cardiac magnetic resonance is observed. LGE indicates late gadolinium enhancement.
Figure 4.

Relationship between length of mitral annular disjunction and amount of late gadolinium enhancement in vivo. A significant correlation (R=0.61) between the length of mitral annulus disjunction (MAD; expressed as mm) and the amount of late gadolinium enhancement (LGE; expressed as percentage of left ventricular [LV] mass) on cardiac magnetic resonance is observed.
Patients with LV LGE showed an increased leaflet thickness both for anterior (median: 3.5 versus 3 mm; P=0.008) and posterior leaflet (median: 4 versus 3 mm; P=0.001) and a more pronounced prolapsed distance (anterior prolapsed distance median value: 4.9 versus 1 mm; P=0.004 and posterior prolapsed distance median value: 7.1 versus 2.1 mm; P<0.001). The median value of ratio between basal and mid portion of lateral wall thickness was 1.5 in the overall population, with no differences between the 2 groups. By applying the median value of 1.5 as a cutoff for increased ratio, it was more prevalent in the LGE MVP group (22 [61%] versus 4 [25%]; P=0.016). Both end-systolic (median: 41.2 versus 31.5; P=0.004) and end-diastolic mitral annular diameters (median: 35.5 versus 31.5; P=0.042) differed between the 2 groups with a paradoxical increase of the mitral annulus diameter during systole versus diastole in the LGE group. The values of interobserver agreement are shown in Table 2. Patients with bileaflet MVP (n=31) had a longer MAD (4.8 versus 2.5; P<0.001), higher prevalence of curling (28 [90%] versus 9 [43%]; P<0.001) and of LGE (26 [84%] versus 10 [48%]; P=0.005) as compared with patients with single (posterior) leaflet MVP (n=21).
Table 2.
The Inter- and Intraobserver Agreement for CMR and Histopathology Measurements
MVP Patients With Midsystolic Click
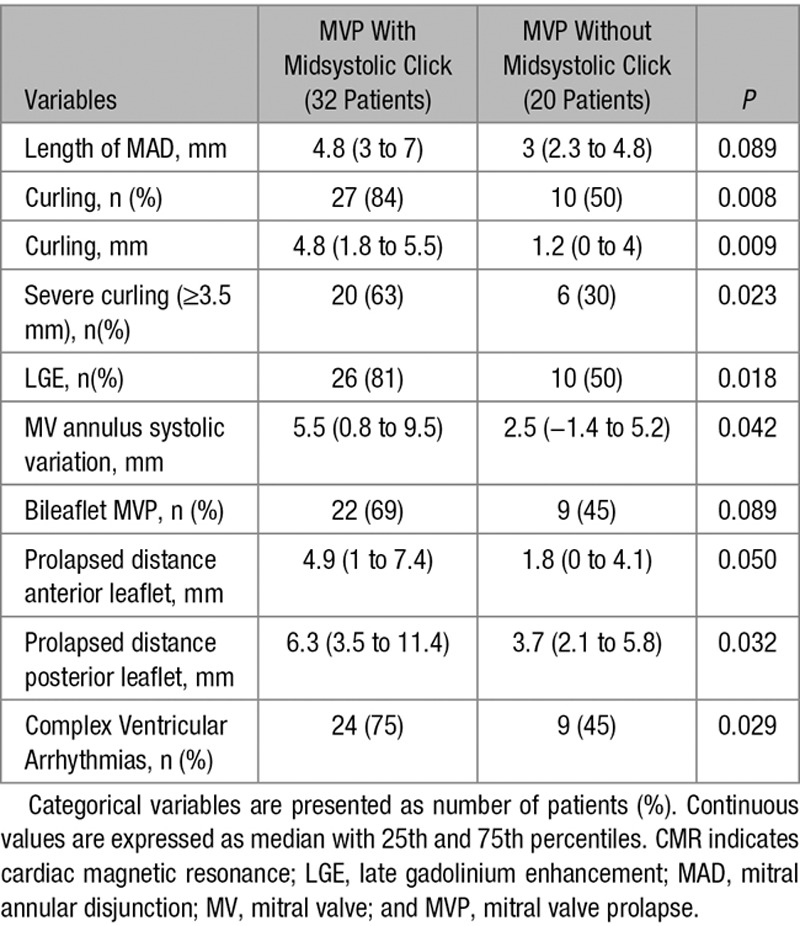
On the basis of auscultatory findings, 32 MVP patients had a midsystolic click. Patients with midsystolic click showed an elongated MAD (median 4.8 versus 3 mm; P=0.1), curling (84% versus 50%; P=0.008), severe curling (≥4 mm in 63% versus 30%; P=0.023), LGE (81% versus 50%; P=0.018), and complex ventricular arrhythmias (75% versus 45%; P=0.029) as compared with those without click (Table 3).
Table 3.
CMR Findings According to Midsystolic Click on Auscultation
MVP Patients With Curling
The 37 MVP with curling had a higher prevalence of negative T waves (32% versus 13%; P=0.3), LGE (92% versus 13%; P<0.001), and amount of LGE (1.6% of LV mass versus 0; P=0.001) as compared with those without curling.
MVP SCD Patients With LV Fibrosis
MV leaflets were redundant, thick, and elongated, with either isolated posterior (n=18, 36%) or bileaflet (n=32, 64%) involvement in a consecutive series of 50 SCD Patients (30 female patients; median age 36 years) with MVP and LV fibrosis. SCD patients with MVP had a longer MAD as compared with a series of 20 (14 female patients; median age: 34 years) SCD hearts without MVP (median: 3 versus 1.5 mm; P≤0.001; Figure 5). The values of interobserver agreement are shown in Table 2.
Figure 5.
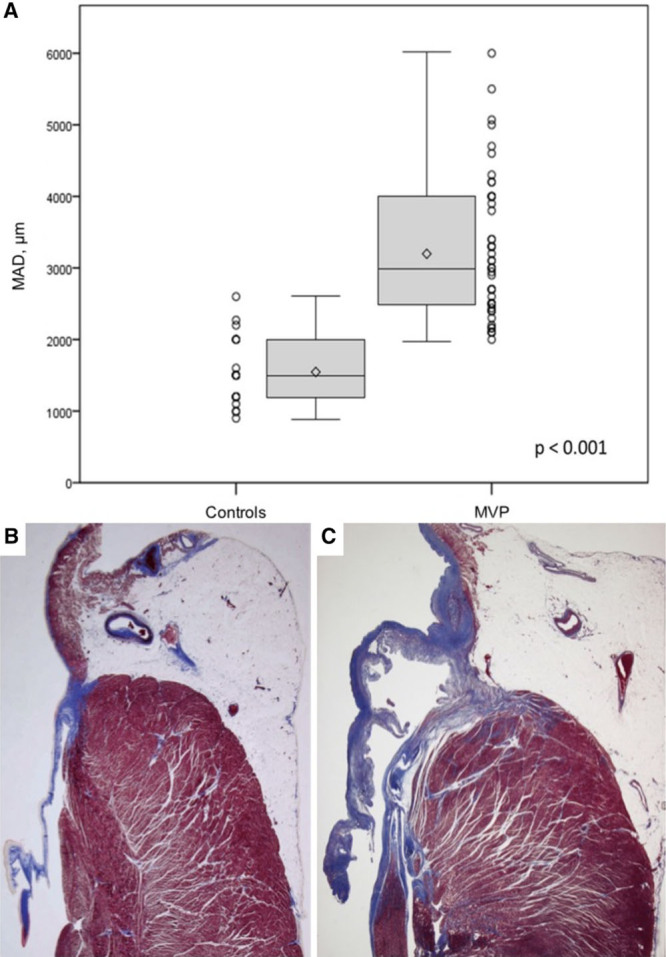
Length of mitral annular disjunction (MAD) in sudden cardiac death (SCD) patients: controls vs mitral valve prolapse (MVP) patients. The length of MAD (measured as micrometers) in SCD patients with MVP is significantly higher than in controls (A). Representative histology of the mitral annulus showing the absence of MAD in a SCD control (B) as compared with an elongated MAD in a SCD patients with MVP (C).
Discussion
We have recently demonstrated that arrhythmic MVP is characterized by myxomatous MV and LV fibrosis at the level of PM and inferobasal wall, by assessing a consecutive series of patients who either died suddenly or presented with complex ventricular arrhythmias.1 The present study clearly shows that MAD and systolic curling of the posterior MV leaflet are associated with LV fibrosis, accounting for the excessive mobility of the MV apparatus and systolic stretch of the myocardium closely linked to the valve. The distribution of LV fibrosis, as demonstrated by histopathology and CE-CMR, was in fact in keeping with a mechanical injury of the myocardium caused by the continuous traction of the prolapsing leaflets and elongated chordae.
Mitral Annulus Disjunction and MVP
The mitral annulus is a component of the MV apparatus and consists of a cord-like ring of collagen and elastic fibers distributed along the atrioventricular junction and giving support to the MV leaflets. It is conventionally divided into anterior and posterior portions, although the real annulus is the one that serves as hinge point for the mural posterior leaflet of the MV. The concept of MAD was originally introduced by Bharati et al15 and then systematically investigated by Hutchins et al3 in the 1980s, referring to an anatomic variation of the fibrous mitral annulus. In the latter pathological study of 900 hearts from adult autopsies, 25 (3%) had a morphologically typical floppy MV and 23 of them (92%) showed a MAD. This was defined as a variation in the attachment of the posterior MV leaflet, that accounts for a wide separation between the LA wall–MV junction and the LV attachment.3 In other words, the posterior annulus seemed stretched curtain-like, as compared with the normal cord-like structure.16 In the same series, MAD was rare in hearts without floppy MV (42 cases, 5%). Because these patients were significantly younger than those with a floppy MV, the authors suggested that this anatomic variation could play a role in the pathogenesis of myxomatous valve degeneration, by means of increased mechanical stress induced by the excessive mobility of the MV apparatus.3
To the best of our knowledge, this structural abnormality of the mitral annuls remained mostly forgotten and just a matter of speculation for pathologists up to 2005, when Eriksson et al4 observed MAD by direct surgical inspection in some patients undergoing MV repair with advanced myxomatous disease. By applying a standardized transesophageal echocardiography protocol, the same authors found a 98% of prevalence of MAD in a surgical series of MVP patients with severe mitral regurgitation. Furthermore, a positive correlation between the severity of MAD and the number of diseased MV scallops was demonstrated, supporting the concept of an association between MAD and the severity of MVP. These authors also emphasized that impact of MAD on mitral annular function. In fact, in advanced MV disease, they did not observe any significant diastolic–systolic change in annular diameter, suggesting that not only mitral annulus geometry but also function is altered with loss of systolic contraction. On the basis of these findings, and the possible increased failure risk of MV repair because of an unrecognized MAD, Eriksson et al4 modified their surgical repair technique to optimize long-term results.
Later on, Carmo et al9 for the first time demonstrated that MAD is easily detectable and measurable also by routine transthoracic echocardiography. Using this technique in patients with myxomatous MV, including different degrees of mitral regurgitation, they found a MAD prevalence of 55%, more common in women (63% versus 38%) and often associated with chest pain.9 In this study, an association between MAD and mitral annulus dysfunction was also evidenced, with a paradoxical systolic increase of the mitral annulus diameter. Noteworthy, although sustained ventricular arrhythmias were not detected, an increased frequency of premature ventricular beats and nonsustained ventricular tachycardia in patients with MAD versus those without was reported: the wider the magnitude of MAD, the higher the incidence of nonsustained ventricular tachycardia.
In our study, we provide the evidence that MAD is associated with arrhythmic MVP, a theory previously only supposed.3,9 In our series, a quantifiable MAD was always detectable in both arrhythmic MVP patients with LV LGE (median: 4.8 mm) and SCD cases with LV fibrosis (median: 3 mm), mostly located close to the annulus in the inferobasal wall. The slight difference between CMR and histopathology MAD values is most likely the consequence of artifacts because of partial volume effects, as previously described in other echocardiographic studies.16,17 Moreover, the higher values reported by Eriksson et al4 (10±3 mm) can be explained by their peculiar study population that included patients with severe mitral regurgitation, that, on the contrary, were excluded in our series. Interestingly, Carmo et al9, by studying MVP patients with the entire spectrum of mitral regurgitation, demonstrated an intermediate value of MAD of 7.4±8.7 mm.
Systolic Curling of the Mitral Annulus and MVP
The motion of the mitral annulus is passive and determined by the contraction and relaxation of adjacent atrial and ventricular musculature. As consequence, in normal condition, the posterior mitral ring and its adjacent myocardium move downward and anteriorly in systole, in synchrony with the remainder of the LV.16
Gilbert et al13 in 1976 provided the first echocardiographic demonstration of a peculiar functional abnormality of the mitral annulus in MVP patients, describing an unusual systolic curling of the posterior mitral ring on the adjacent myocardium, so that the systolic movement of the ring was primarily downward with little, if any, anterior motion, thus resulting in a curled appearance when visualized in real-time motion. The authors concluded that the cause of curling in MVP was uncertain because, differently from previous reports, they did not visualize LV motion abnormalities either by echocardiography or angiography.
In our series of arrhythmic MVP patients, we demonstrate for the first time that curling of the mitral annulus is associated with MAD and accounts for annular hypermobility. Furthermore, a linear correlation has been found between the length of MAD and the severity of curling. These data create a link between the hypothesis made by Hutchins et al3 and early angiographic observations of contractility abnormalities in MVP patients characterized by an arrhythmic profile, thus advancing the hypothesis of MVP as a cardiomyopathic condition.18–20
Basal LV Hypertrophy and MVP
A relative increase of the LV mass, which does not seem to be caused by mitral regurgitation, has been recognized in MVP since many years, suggesting that myocardial involvement is an integral part of arrhythmic MVP.21 On the basis of few observations of MVP patients with asymmetrical LV hypertrophy isolated to the inferobasal wall, Maron et al22 even advanced the hypothesis of a novel form of hypertrophic cardiomyopathy. More recently, Zia et al11 performed a CMR study demonstrating the existence of a relative concentric basal LV hypertrophy, focal and localized to the base in MVP patients as compared with controls. Moreover, they found a significantly increased ratio of basal to midventricular end-diastolic wall thickness mostly in the lateral wall, compared with all other segments. A positive correlation between the degree of relative basal LV hypertrophy and the excursion of the MV annulus, defined as the distance between the position of the annulus in end diastole and end systole, was also detected.
All these findings are in keeping with a locally increased myocardial function adjacent to the prolapsed MV leaflet, eventually leading to focal hypertrophy of the LV base. The abnormal contractility of this LV region accounts in part for the so-called systolic curling motion. Noteworthy, original angiographic studies evidenced a bulging on the inferobasal wall, because of the abnormal contractility of the basal portion compared with the mid one, as to resemble the so-called ballerina-foot pattern.18,23–28
In our arrhythmic MVP patients, we also found a relative hypertrophy of inferobasal wall, as compared with the adjacent mid portion. Moreover, a midmural LGE in the LV inferobasal wall was demonstrated in 72% of cases, all with MAD and curling. On the contrary, in the study by Zia et al,11 no fibrosis as LGE was detectable in the context of basal hypertrophy. This discrepancy may be explained by the selection of MVP population, because we included MVP patients without mitral regurgitation, but with LGE and arrhythmic profile, differently from Zia et al,11 who included retrospective patients with MVP and various degrees of valve regurgitation.
Arrhythmic MVP: From Mitral Annulus Disjunction to LV Inferobasal Stretch
Although we demonstrated the role of LV fibrosis as a substrate of electric instability in arrhythmic MVP, a systematic investigation of morphofunctional abnormalities of the MV apparatus, capable to explain why a subgroup of MVP patients develop LV fibrosis, was still missing. The present study clearly demonstrates that, in arrhythmic MVP patients, the MV is characterized by MAD, systolic curling, and myxomatous leaflets thickening.
On the basis of our previous observations in arrhythmic MVP1 and the results reported in this study, the following cascade of events can be hypothesized. MAD is the cause of systolic curling motion; this morphofunctional alteration represents the basis for paradoxical increase of the MV annulus diameter during systole, myxomatous degeneration of MV leaflets, and myocardial stretch in the LV inferobasal segment. Our data in the subset of patients with arrhythmic MVP confirm, and further extend, the previous observation made by Hutchins et al.3
This impairment in the mitral annulus contractility not only influences the geometry and function of mitral ring and adjacent LV segments with relative hypertrophy but also involves a complex force balance of all MV components including PMs. As a result of the abnormal contractility produced by MAD, the mechanical stretch is directly transmitted to PMs that can also show LGE in the stretched areas. The regional area of hypercontraction, originally hypothesized and defined of unknown cause by Nutter et al23, has a clear anatomic basis in the association of MAD with systolic curling. This association can increase wall stress in the inferobasal wall and PM, as evidenced by hypertrophy and replacement-type fibrosis, both documented at postmortem by histology and confirmed in vivo as LGE by CE-CMR in our previous study. The frequent observation of T-wave abnormalities on inferolateral leads at 12-lead ECG possibly suggests a disturbed repolarization of the area with abnormal contractility, as previously advanced.1,6,29
The genesis of malignant arrhythmias in MVP probably recognizes the combination of the substrate (myocardial fibrosis) and the trigger (mechanical stretch) eliciting premature ventricular beats.1,30 Noteworthy, Vaidya et al31 reported a series of cases where the surgical correction of bileaflet MVP was associated with a reduction in malignant arrhythmia and appropriate implantable cardioverter defibrillator shocks. In other words, mitral valve repair could relieve the mechanical stretch of the myocardium, thus leading to a reduction, but no disappearance, of ventricular arrhythmias, as to confirm a key role of abnormal mechanical forces.
When comparing bileaflet and single-leaflet MVP patients, the former have longer MAD and higher prevalence of curling and LGE. The fact that most of our arrhythmic patients had a bileaflet MVP is probably because of the entry criteria, since only patients with mild or absent mitral regurgitation were enrolled to exclude a confounding effect of hemodynamic impairment. In fact, it is likely that a retained coaptation of the leaflets does occur when both leaflets are involved.
Midsystolic Click, Arrhythmias, and MVP
In the past, following the original description of Barlow and Bosman32 and Barlow et al,33 many studies addressed the syndrome of midsystolic click, late systolic murmur, and ballooning posterior mitral leaflet as a cause of distressing chest pain and life-threatening arrhythmias.6,18,27,34–36 More recently, because of the progressive disappearance of auscultation in the clinical setting,37 the role of midsystolic click as a marker of malignant MVP was overlooked.
In our series, most of MVP patients with LGE had a midsystolic click on auscultation. Noteworthy, by reclassifying the population on the basis of presence of midsystolic click, those with click had curling, LGE, and arrhythmias more frequently. In other words, MVP patients presenting with midsystolic click on auscultation, MAD, curling, and mitral annular abnormal contractility are those who need further evaluation for arrhythmic risk stratification through CE-CMR. On the basis of our data, this auscultatory finding is likely because of the tension produced on the MV apparatus by the abnormal systolic curling.7
In light of these considerations, it is not surprisingly that in the early era of MVP, its diagnosis, being based on classical auscultatory findings, allowed the identification of patients with high prevalence of severe ventricular arrhythmias at risk of SCD.6,18,27,33–36 Arrhythmic MVP should be kept clearly distinct from the echocardiographic MVP, which is defined simply as single-leaflet or bileaflet prolapse of at least 2 mm beyond the long-axis annular plane. Although the latter is a relatively common condition with a benign behavior,38–41 no data are available on the prevalence of the nosographic entity of arrhythmic MVP and further studies are needed.
Conclusions
An association between MAD, curling, and LV fibrosis has been first detected by CMR in MVP patients with an arrhythmic malignant profile and then confirmed by histology in SCD patients with MVP. This unique annular morphology may account for the excessive mobility of the leaflets, as visualized by systolic curling and clinically suggested by auscultatory midsystolic click, and for the stretch-related myocardial remodeling of the inferobasal wall. Further studies are needed to confirm this hypothesis.
Acknowledgments
We would like to acknowledge the skillful technical assistance of Anna Saracino and Daniele Iannazzone for histology and Marco Pizzigolotto for illustrations.
Sources of Funding
This work was supported by the Registry for Cardio-Cerebro-Vascular Pathology, Veneto Region, Venice, Italy; Target Projects 331/12, RP 2014-00000394, Regional Health System, Venice, Italy; and TRANSAC Strategic Research Grant CPDA133979/13, University of Padua, Italy.
Disclosures
None.
Supplementary Material
Footnotes
Drs Perazzolo Marra and Basso contributed equally to this work.
The Data Supplement is available at http://circimaging.ahajournals.org/lookup/suppl/doi:10.1161/CIRCIMAGING.116.005030/-/DC1.
CLINICAL PERSPECTIVE
We aimed to assess whether morphological and functional characteristics of the mitral valve (MV) apparatus could explain the propensity in some patients to develop a regional left ventricular fibrosis. The present study shows that mitral annulus disjunction and systolic curling of the posterior MV leaflet are associated with basal–mid left ventricular relative hypertrophy and fibrosis and account for the excessive mobility of the MV apparatus and systolic stretch of the myocardium closely linked to the valve. Thus, the nosographic entity of arrhythmic MV prolapse should be kept clearly distinct from the echocardiographic MV prolapse, which is defined simply as single-leaflet or bileaflet prolapse of at least 2 mm beyond the long-axis annular plane. Moreover, on the basis of our findings, MV prolapse patients presenting with midsystolic click on auscultation, MV leaflets thickening, and annulus abnormalities, ie, mitral annulus disjunction and curling, are those who need further evaluation for arrhythmic risk stratification through contrast-enhanced cardiac magnetic resonance. Prospective studies are warranted to assess the role of targeted catheter ablation and surgical repair in selected MV prolapse patients with a high arrhythmic burden.
References
- 1.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, Rigato I, Migliore F, Pilichou K, Bertaglia E, Cacciavillani L, Bauce B, Corrado D, Thiene G, Iliceto S. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40:468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med. 1986;314:535–540. doi: 10.1056/NEJM198602273140902. doi: 10.1056/NEJM198602273140902. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson MJ, Bitkover CY, Omran AS, David TE, Ivanov J, Ali MJ, Woo A, Siu SC, Rakowski H. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr. 2005;18:1014–1022. doi: 10.1016/j.echo.2005.06.013. doi: 10.1016/j.echo.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Weis AJ, Salcedo EE, Stewart WJ, Lever HM, Klein AL, Thomas JD. Anatomic explanation of mobile systolic clicks: implications for the clinical and echocardiographic diagnosis of mitral valve prolapse. Am Heart J. 1995;129:314–320. doi: 10.1016/0002-8703(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 6.Rizzon P, Biasco G, Brindicci G, Mauro F. Familial syndrome of midsystolic click and late systolic murmur. Br Heart J. 1973;35:245–259. doi: 10.1136/hrt.35.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathey DG, Decoodt PR, Allen HN, Swan HJ. The determinants of onset of mitral valve prolapse in the systolic click-late systolic murmur syndrome. Circulation. 1976;53:872–878. doi: 10.1161/01.cir.53.5.872. [DOI] [PubMed] [Google Scholar]
- 8.Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, Vettor G, Tona F, Tarantini G, Cacciavillani L, Corbetti F, Giorgi B, Miotto D, Thiene G, Basso C, Iliceto S, Corrado D. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856–863. doi: 10.1016/j.hrthm.2014.01.014. doi: 10.1016/j.hrthm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53. doi: 10.1186/1476-7120-8-53. doi: 10.1186/1476-7120-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Zia MI, Valenti V, Cherston C, Criscito M, Uretsky S, Wolff S. Relation of mitral valve prolapse to basal left ventricular hypertrophy as determined by cardiac magnetic resonance imaging. Am J Cardiol. 2012;109:1321–1325. doi: 10.1016/j.amjcard.2011.12.029. doi: 10.1016/j.amjcard.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Peters DC, Kissinger KV, Goddu B, Yeon SB, Manning WJ, Nezafat R. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve prolapse. Am J Cardiol. 2010;106:243–248. doi: 10.1016/j.amjcard.2010.02.035. doi: 10.1016/j.amjcard.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert BW, Schatz RA, VonRamm OT, Behar VS, Kisslo JA. Mitral valve prolapse. Two-dimensional echocardiographic and angiographic correlation. Circulation. 1976;54:716–723. doi: 10.1161/01.cir.54.5.716. [DOI] [PubMed] [Google Scholar]
- 14.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 15.Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M. The conduction system in mitral valve prolapse syndrome with sudden death. Am Heart J. 1981;101:667–670. doi: 10.1016/0002-8703(81)90235-0. [DOI] [PubMed] [Google Scholar]
- 16.Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 2012;164:163–176. doi: 10.1016/j.ahj.2012.05.014. doi: 10.1016/j.ahj.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Mérot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Pucéat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH Leducq Mitral Transatlantic Network. Mitral valve disease–morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. doi: 10.1038/nrcardio.2015.161. doi: 10.1038/nrcardio.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulotta SJ, Gulco L, Padmanabhan V, Miller S. The syndrome of systolic click, murmur, and mitral valve prolapse–a cardiomyopathy? Circulation. 1974;49:717–728. doi: 10.1161/01.cir.49.4.717. [DOI] [PubMed] [Google Scholar]
- 19.Scampardonis G, Yang SS, Maranhão V, Goldberg H, Gooch AS. Left ventricular abnormalities in prolapsed mitral leaflet syndrome. Review of eighty-seven cases. Circulation. 1973;48:287–297. doi: 10.1161/01.cir.48.2.287. [DOI] [PubMed] [Google Scholar]
- 20.Crawford MH, O’Rourke RA. Mitral valve prolapse: a cardiomyopathic state? Prog Cardiovasc Dis. 1984;27:133–139. doi: 10.1016/0033-0620(84)90023-9. [DOI] [PubMed] [Google Scholar]
- 21.Haikal M, Alpert MA, Whiting RB, Kelly D. Increased left ventricular mass in idiopathic mitral valve prolapse. Chest. 1982;82:329–333. doi: 10.1378/chest.82.3.329. [DOI] [PubMed] [Google Scholar]
- 22.Maron BJ, Sherrid MV, Haas TS, Lindberg J, Kitner C, Lesser JR. Novel hypertrophic cardiomyopathy phenotype: segmental hypertrophy isolated to the posterobasal left ventricular free wall. Am J Cardiol. 2010;106:750–752. doi: 10.1016/j.amjcard.2010.04.037. doi: 10.1016/j.amjcard.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 23.Nutter DO, Wickliffe C, Gilbert CA, Moody C, King SB., 3rd The pathophysiology of idiopathic mitral valve prolapse. Circulation. 1975;52:297–305. doi: 10.1161/01.cir.52.2.297. [DOI] [PubMed] [Google Scholar]
- 24.Ehlers KH, Engle MA, Levin AR, Grossman H, Fleming RJ. Left ventricular abnormality with late mitral insufficiency and abnormal electrocardiogram. Am J Cardiol. 1970;26:333–340. doi: 10.1016/0002-9149(70)90726-5. [DOI] [PubMed] [Google Scholar]
- 25.Liedtke AJ, Gault JH, Leaman DM, Blumenthal MS. Geometry of left ventricular contraction in the systolic click syndrome. Characterization of a segmental myocardial abnormality. Circulation. 1973;47:27–35. doi: 10.1161/01.cir.47.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Farry JP, Simon AL, Ross AM, Cohen LS, Wolfson S. Quantitative angiographic assessment of the mitral annulus in the prolapsing leaflet syndrome. Circulation. 1975;52(suppl II):11–12. [Google Scholar]
- 27.Jeresaty RM. The syndrome associated with mid-systolic click and-or late systolic murmur. Analysis of 32 cases. Chest. 1971;59:643–647. doi: 10.1378/chest.59.6.643. [DOI] [PubMed] [Google Scholar]
- 28.Gooch AS, Vicencio F, Maranhao V, Goldberg H. Arrhythmias and left ventricular asynergy in the prolapsing mitral leaflet syndrome. Am J Cardiol. 1972;29:611–620. doi: 10.1016/0002-9149(72)90161-0. [DOI] [PubMed] [Google Scholar]
- 29.Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 30.Noseworthy PA, Asirvatham SJ. The knot that binds mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:551–552. doi: 10.1161/CIRCULATIONAHA.115.017979. doi: 10.1161/CIRCULATIONAHA.115.017979. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya VR, DeSimone CV, Damle N, Naksuk N, Syed FF, Ackerman MJ, Ponamgi SP, Nkomo VT, Suri RM, Noseworthy PA, Asirvatham SJ. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46:137–143. doi: 10.1007/s10840-015-0090-5. doi: 10.1007/s10840-015-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J. 1966;71:166–178. doi: 10.1016/0002-8703(66)90179-7. [DOI] [PubMed] [Google Scholar]
- 33.Barlow JB, Bosman CK, Pocock WA, Marchand P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br Heart J. 1968;30:203–218. doi: 10.1136/hrt.30.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shappell SD, Marshall CE, Brown RE, Bruce TA. Sudden death and the familial occurrence of mid-systolic click, late systolic murmur syndrome. Circulation. 1973;48:1128–1134. doi: 10.1161/01.cir.48.5.1128. [DOI] [PubMed] [Google Scholar]
- 35.Jeresaty RM. Mitral valve prolapse–Click syndrome. Prog Cardiovasc Dis. 1973;15:623–652. doi: 10.1016/s0033-0620(73)80026-x. [DOI] [PubMed] [Google Scholar]
- 36.Jeresaty RM. Sudden death in the mitral valve prolapse-click syndrome. Am J Cardiol. 1976;37:317–318. doi: 10.1016/0002-9149(76)90328-3. [DOI] [PubMed] [Google Scholar]
- 37.Adolph RJ. In defense of the stethoscope. Chest. 1998;114:1235–1237. doi: 10.1378/chest.114.5.1235. [DOI] [PubMed] [Google Scholar]
- 38.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 39.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 40.Savage DD, Garrison RJ, Devereux RB, Castelli WP, Anderson SJ, Levy D, McNamara PM, Stokes J, 3rd, Kannel WB, Feinleib M. Mitral valve prolapse in the general population. 1. Epidemiologic features: the Framingham Study. Am Heart J. 1983;106:571–576. doi: 10.1016/0002-8703(83)90704-4. [DOI] [PubMed] [Google Scholar]
- 41.Marks AR, Choong CY, Sanfilippo AJ, Ferré M, Weyman AE. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med. 1989;320:1031–1036. doi: 10.1056/NEJM198904203201602. doi: 10.1056/NEJM198904203201602. [DOI] [PubMed] [Google Scholar]


