SUMMARY
The transcription factor IRF-3 mediates cellular antiviral response by inducing the expression of interferon and other antiviral proteins. In RNA-virus infected cells, IRF-3’s transcriptional activation is triggered primarily by RIG-I-like receptors (RLR), which can also activate the RLR-induced IRF-3-mediated pathway of apoptosis (RIPA). Here, we have reported that the pathway of IRF-3 activation in RIPA was independent of and distinct from the known pathway of transcriptional activation of IRF-3. It required linear polyubiquitination of two specific lysine residues of IRF-3 by LUBAC, the linear polyubiquitinating enzyme complex, which bound IRF-3 in signal-dependent fashion. To evaluate the role of RIPA in viral pathogenesis, we engineered a genetically targeted mouse, which expressed a mutant IRF-3 that was RIPA-competent but transcriptionally inert; this single-action IRF-3 could protect mice from lethal viral infection. Our observations indicated that IRF-3-mediated apoptosis of virus-infected cells could be an effective antiviral mechanism, without expression of the interferon-stimulated genes.
INTRODUCTION
Virus infection triggers complex host responses, which determine the final outcome of the infection. Innate immune responses of the infected cell is the first line of host defense mechanism, which helps initiate and shape the subsequent adaptive immune responses, to eliminate the virus from the host. Various pattern recognition receptors (PRR) recognize viral components in the infected cell and trigger signaling pathways that lead to transcriptional induction of antiviral genes. Many RNA viruses, including Paramyxoviruses, and some DNA viruses activate the cytoplasmic RIG-I-like receptors (RLR) (Ramos and Gale, 2011); signaling by these receptors causes activation of transcription factors, such as IRF-3, NF-κB and AP1, which, in turn, induce the expression of many genes, including the interferon (IFN) and the IFN-stimulated genes (ISG).
The biochemistry of RLR-signaling has been extensively investigated; a large number of adaptors, enzymes and scaffolding proteins regulate this process both positively and negatively. Several components of this cascade are activated by phosphorylation or dephosphorylation and ubiquitination or deubiquitination. RLR activation requires its signal-dependent dephosphorylation, followed by K63-linked polyubiquitination of specific lysine residues. The two RLRs, RIG-I and MDA-5, exist as phosphorylated proteins in unstimulated cells; virus infection causes their dephosphorylation by the protein phosphatase PP1 to activate these receptors (Wies et al., 2013). RIG-I is further activated by ubiquitination of a specific lysine residue (K172); TRIM25, an E3 ligase, catalyzes the conjugation of K63-linked polyubiquitin chains on this lysine residue of RIG-I (Gack et al., 2007). Another E3 ligase, Riplet, catalyzes K63-linked polyubiquitin conjugation near the C-terminus of RIG-I (Oshiumi et al., 2010). Deubiquitination by USP15 counteracts TRIM25 activity, thereby limiting signaling by RIG-I (Pauli et al., 2014). RIG-I can also be activated by non-covalent interaction with unanchored K63-linked polyubiquitin chains (Zeng et al., 2010). The activated RIG-I associates with mitochondrial outer membrane-bound protein, IPS1, which recruits the TRAF family of E3 ligases. TRAF3, specifically required for IRF-3 activation, undergoes K63 polyubiquitination upon recruitment to the RIG-I and IPS-1 containing complex (Mao et al., 2010). The polyubiquitinated TRAF3 interacts with the Ser/Thr kinase, TBK1, which is activated by autophosphorylation and K63-linked polyubiquitination to function as a kinase for IRF-3 (Tu et al., 2013). DUBA deubiquitinates TRAF3 to down-regulate its interaction with TBK1 and consequent activation of IRF-3 (Kayagaki et al., 2007).
RLR-signaling activates IRF-3 as a transcription factor by TBK1-mediated phosphorylation of its specific Ser residues, thereby changing its conformation. Phosphorylated IRF-3 dimerizes, translocates from the cytoplasm to the nucleus and binds to the IFN-stimulated response elements (ISRE) in the promoters of the target genes to induce their transcription (Hiscott, 2007; Ikushima et al., 2013). To function as a transcription factor, IRF-3 requires the co-activator, β-catenin, which needs to be deacetylated by the cytoplasmic deacetylase, HDAC6, to act with IRF-3 (Chattopadhyay et al., 2013a). We have reported that IRF-3 can also be activated, by RLR signaling, as a pro-apoptotic factor, triggering the RLR-induced IRF-3 mediated Pathway of Apoptosis (RIPA). In RIPA, activated IRF-3 uses its BH3 domain to bind to the apoptotic protein, Bax, translocates to mitochondria and releases cytochrome C to the cytoplasm to cause apoptosis of virus infected cells (Chattopadhyay et al., 2010; Chattopadhyay and Sen, 2010; Chattopadhyay et al., 2011). Here, we report that the activation of IRF-3 in RIPA is achieved by linear polyubiquitination of its two specific Lys residues. The signaling proteins required for activating IRF-3 in the two pathways are distinct, although there are a few common components. In cell cultures, RIPA acts as an effective antiviral pathway, but its action is highly regulated (Chattopadhyay et al., 2011; White et al., 2011). In the early phase of infection by Sendai virus (SeV), although RIPA is activated, apoptosis is kept under control by the anti-apoptotic protein, XIAP, which itself is stabilized by viral activation of the PI3 kinase - AKT pathway. At the later phase of infection, XIAP is degraded and the brake on RIPA is released (White et al., 2011). In the absence of RIPA, infected cells do not die, but are persistently infected; ISG induction by the transcriptional action of IRF-3 is not sufficient to inhibit virus replication in these cells (Chattopadhyay et al., 2013b; Peters et al., 2008). Here, we demonstrate that RIPA protects mice from viral pathogenesis as well. For this purpose, we engineered a genetically targeted mouse that expressed an IRF-3 mutant that was transcriptionally inert, but active for RIPA; unlike Irf3−/− mice, the IRF-3 mutant-expressing mouse was protected from SeV pathogenesis. Our results show that the pro-apoptotic function of IRF-3 is sufficient to mediate an antiviral response in the absence of the role of this transcription factor in transcribing antiviral genes.
RESULTS
Ubiquitination of IRF-3 promotes RIPA
As shown in human cells, RLR activation by virus infection or dsRNA transfection triggered RIPA in mouse cells in an IRF-3-dependent manner (Figure 1A). When RLR signaling was activated in Irf3−/− MEFs, there was less apoptosis, as measured by Caspase 3 activity and PARP cleavage; however, ectopic expression of IRF-3 in these cells, restored their ability to mount RIPA (Figure 1A). Using MEFs from various genetically deleted mice, we investigated whether RIPA required additional signaling proteins, which are not required to activate IRF-3 as a transcription factor. We observed that TRAF-3 was required for both pathways (Chattopadhyay et al., 2010); in contrast, TRAF2 and TRAF6, which are not required for transcriptional activation of IRF-3 by RLR signaling, were essential for RIPA activation (Figure 1B). Because several TRAFs have ubiquitin E3 ligase activity, we inquired whether ubiquitination was the trigger for activating IRF-3 in RIPA. If so, altering the cellular concentrations of Ub or its conjugating enzymes should regulate RIPA. Because cells cannot tolerate total ablation of Ub expression, we resorted to its ectopic expression. Increased expression of exogenous Ub increased the cellular pools of both free Ub and polyubiquitinated proteins (Figure S1A). Ectopic expression of Ub (Figure 1C) clearly facilitated RIPA activation, as measured by PARP cleavage (Figure 1C). These results indicate that ubiquitination of IRF3 triggers its RIPA activity.
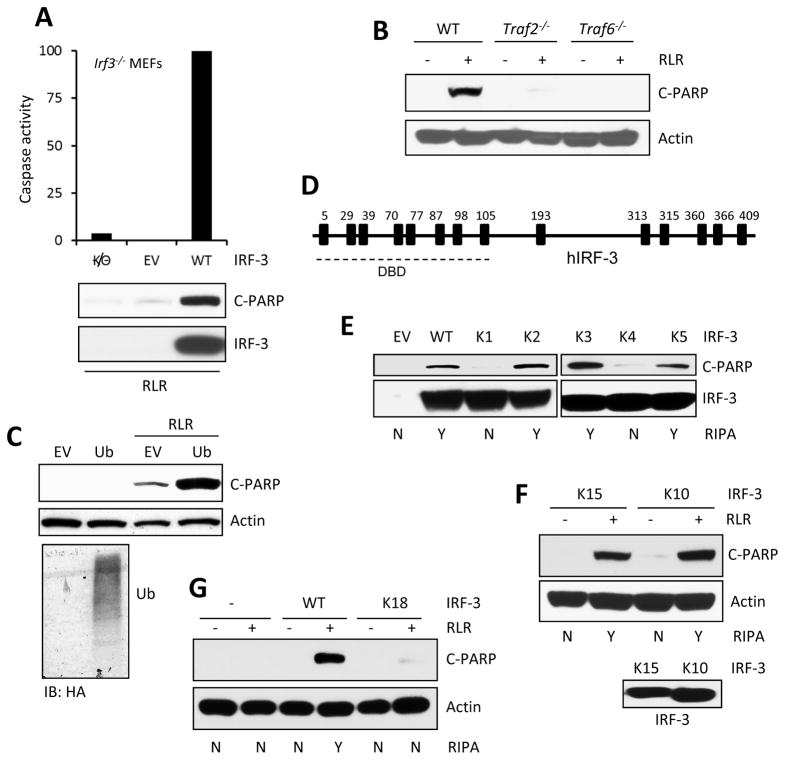
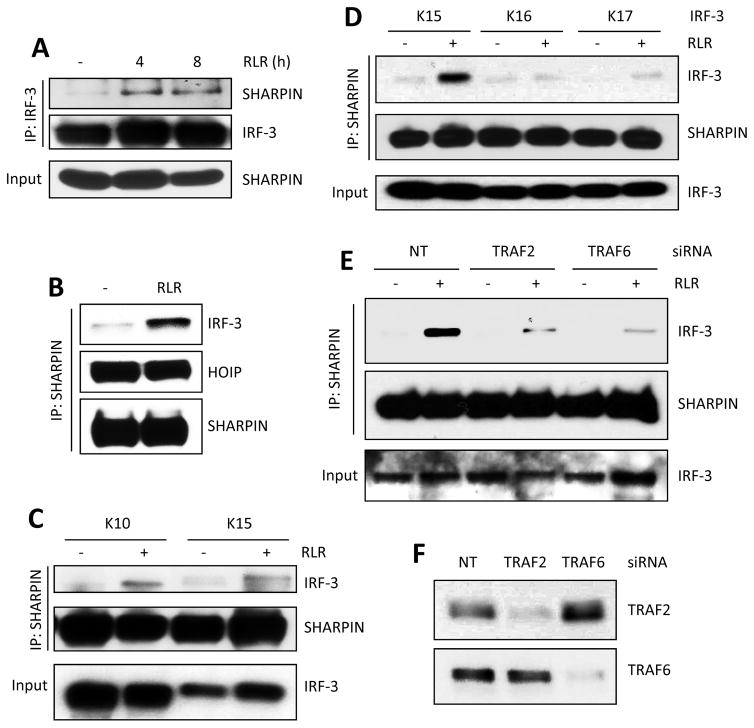
Figure 1. Ubiquitination of IRF-3 promotes RIPA, specific lysines in IRF-3 are required.
(A) RIPA was measured by caspase 3 activity or C-PARP concentrations in Irf3−/− MEFs expressing murine IRF-3 (WT) upon poly(I:C) transfection (RLR). (B) C-PARP concentrations in WT, Traf2−/− and Traf6−/− MEFs upon polyI:C transfection (RLR). (C) C-PARP concentrations in HT1080 cells transfected with WT Ub upon poly(I:C) transfection (RLR). The expression of ectopic Ub (HA) in HA-tagged Ub-transfected HT1080 cells (bottom panel). (D) Human IRF-3 and its lysine residues are shown, DBD: DNA binding domain. (E and F) WT or various lysine mutants of human IRF-3 (details in Table S1) were lentivirally expressed in HT1080.shIRF-3 cells; C-PARP was analyzed upon RLR stimulation (polyI:C transfection). (G) C-PARP w concentrations were measured in Irf3−/− MEFs expressing WT or K18 mutant of murine IRF-3 upon poly(I:C) transfection (RLR). EV, empty vector. The results presented here are representatives of at least three biological repeats. Please see Figure S1 for additional data related to Figure 1.
Identification of the Lys residues of IRF-3 that are needed for RIPA
Ub conjugation to proteins is achieved by isopeptide bond formation between Ub and Lys residues of the target protein. To identify the specific Lys residue(s) of human IRF-3, which are needed for its activation in RIPA, we mutated, singly or in combinations, the 14 Lys residues of IRF-3 to Arg residues (Figure 1D). Because the DNA-binding domain (DBD) of IRF-3 is not required for its action in RIPA (Chattopadhyay et al., 2010), we initially focused our attention to the Lys residues that are not located in that domain. The mutant proteins were individually expressed in HT1080.shIRF-3 cells in which IRF-3 expression had been ablated by targeting sequences present in the UTRs of the native mRNA. The IRF-3 mutant proteins were expressed to similar amounts; however, some mutants supported RIPA, others did not, thus providing leads to the identities of the essential Lys residues (The properties of the mutants are listed in Table S1). These analyses also provided further evidence for IRF-3 having two independent functions, as a transcription factor and a pro-apoptotic factor, because there were mutants that retained one activity but not the other. The mutants K1 and K4 were inactive in the apoptosis assay (Figure 1E) but as active as the WT protein in gene induction, as measured by Immuno blots of IFIT3, an IRF-3-inducible gene (Figure S1B). As expected, K1 and K4 mutants exhibited reduced cell death, as compared to WT and other mutants (Figure S1C). Conversely, the mutant K2 supported apoptosis, but not transcription, whereas mutants K3 and K5 retained both properties. Examination of the properties of additional mutants (Figure 1E, Table S1) indicated that Lys 193, Lys 313 and Lys 315 of IRF-3 were required for its apoptotic, but not transcriptional, activity; to strengthen this conclusion, we introduced additional mutations of Lys residues located in the DBD (Figures S1D–S1F). Further analyses revealed that, although Lys 193 was essential (mutant K4), between Lys 313 and Lys 315 (mutants K13 and K14), the presence of either one was sufficient for RIPA (Figure S1G). Finally, we showed that a mutant IRF-3 (K15), with only Lys 193 and Lys 313, was fully active in RIPA (Figure 1F); further mutation of either Lys residue (K16 and K17) destroyed this activity (Table S1). To test the universality of our conclusions, we generated a mouse IRF-3 mutant (K18), in which the three cognate Lys residues (188, 306, 308) had been mutated. As the test system, we used Irf3−/− MEF, which did not activate RIPA but the expression of WT IRF-3 restored RIPA activity to these cells (Figure 1A). When we compared the activities of equally expressed WT and K18 IRF-3, both induced the mouse Ifit3 strongly (Figure S1H); but only WT, not K18, triggered apoptotic cell death (Figures 1G, S1I). These results demonstrate that both human and mouse IRF-3 have similar characteristics for activation in RIPA.
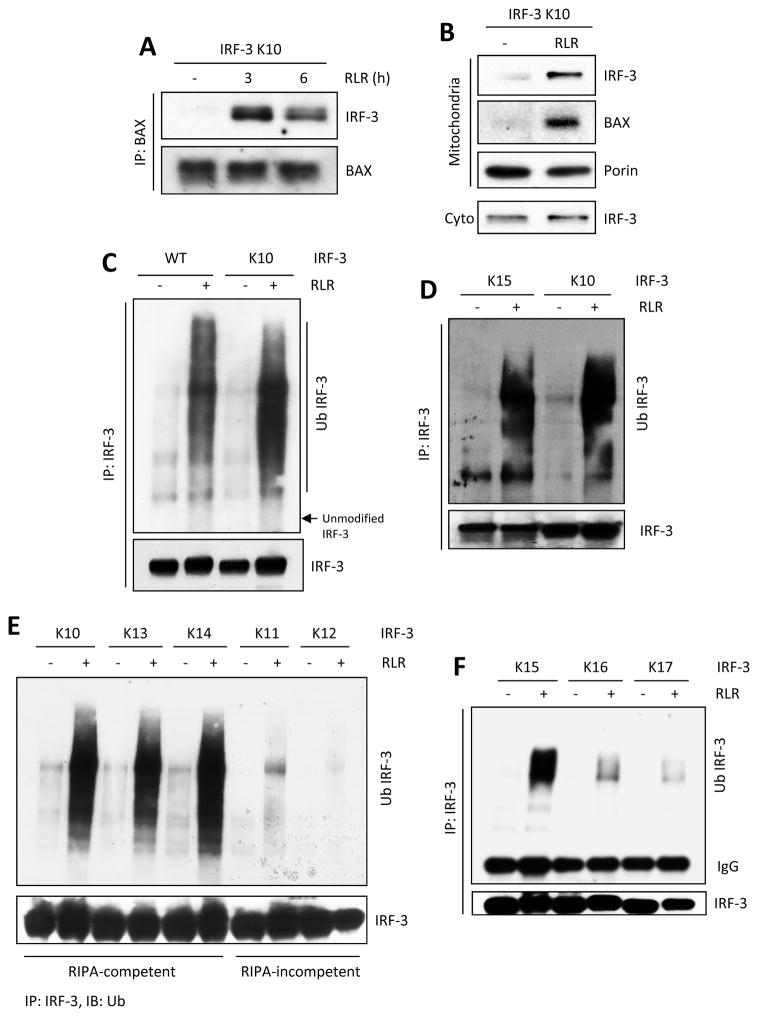
We further characterized the properties of different IRF-3 Lys mutants to identify the specific step, in the progression of RIPA, which required IRF-3 ubiquitination. In RIPA, IRF-3 interacts with BAX and the IRF-3 and BAX containing complex is translocated to the mitochondria (Chattopadhyay et al., 2010). We tested the critical IRF-3 mutants for these properties. We isolated mitochondria from RLR-stimulated and unstimulated cells and ensured its purity (Figure S2A). Similar to the WT protein (Chattopadhyay et al., 2010), the RIPA-competent K10 mutant interacted with Bax in a signal dependent fashion (Figure 2A) and both IRF-3 and Bax translocated to mitochondria (Figure 2B). The RIPA-competent K3 and K15 mutants, but not the RIPA-incompetent K1 mutant, translocated to mitochondria upon RLR stimulation (Figures S2B and S2C). The K10 mutant was ubiquitinated upon RLR activation to a degree similar to the WT protein (Figure 2C) indicating that, under our experimental conditions, the three Lys residues present in K10 were probably the only targets of ubiquitination in WT IRF-3 as well. The K15 mutant, with only two Lys residues, had very similar ubiquitination profile (Figure 2D). When ubiquitination of other mutants were compared, there was a perfect correlation between their RIPA-competency and ubiquitination (Figures 2E and 2F). These results strongly indicate that ubiquitination of Lys 193 and either Lys 313 or Lys 315, is a prerequisite for activating RIPA.
Figure 2. Minimal lysine mutant of IRF-3 is functional in various steps of RIPA and is ubiquitinated on specific lysines.
(A) IRF-3 and BAX interaction was analyzed by co-IP assay in IRF-3 K10 mutant expressing cells, upon polyI:C transfection (RLR). (B) Translocation of IRF-3 and BAX to mitochondrial fractions in K10-expressing cells, upon polyI:C transfection (RLR). (C–F) Total ubiquitination of IRF-3 was analyzed for WT or various lysine mutants of IRF-3 in SeV-infected (RLR) cells. The results presented here are representatives of at least three biological repeats. Please see Figure S2 for additional data related to Figure 2.
Unbranched ubiquitin linkage of IRF-3 promotes RIPA
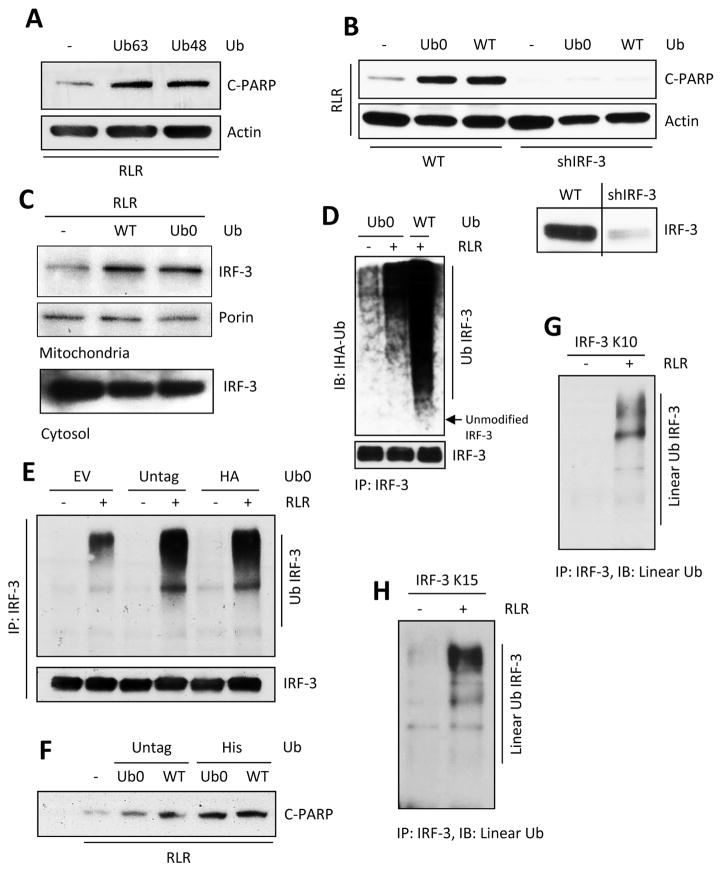
Polyubiquitin chains are built by attaching an ubiquitin moiety to the ubiquitin already linked to the target protein. Branched polyubiquitin chains are built by isopeptide bond formation between Lys 63 or Lys 48 of the attached Ub and the carboxyl terminus of the incoming Ub. In contrast, linear polyubiquitination requires head to tail peptide bond formation between two adjacent Ub moieties; consequently, no Lys residue of Ub is needed for this process. To determine the nature of the polyubiquitin chain linked to IRF-3 in RIPA, we ectopically expressed different Lys mutants of Ub and measured the degrees of augmentation of RIPA. Both Ub63 mutant of Ub, which had only one Lys at residue 63 and the Ub48 mutant having only one Lys at residue 48, could augment RIPA strongly (Figure 3A) indicating that RIPA required neither the K48- nor the K63- linked branched polyubiquitination of IRF-3, thus favoring the possibility that it is modified by linear polyubiquitination. Indeed, a lysine-less Ub mutant (Ub0) was as efficient as WT Ub in promoting RIPA (Figure S3A). We confirmed that the observed apoptosis was RIPA-mediated; silencing of IRF-3 eliminated the poly ADP ribose polymerase (PARP)-cleavage augmentation by Ub0 or WT Ub (Figure 3B). As expected, the Ub0-linked IRF-3 translocated to mitochondria (Figure 3C) and was polyubiquitinated (Figures 3D and S3B). The ectopically expressed Ub, in the above experiments, had His or HA epitope tags at their amino termini which were used for the peptide linkages of linear polyubiquitination. To rule out any possible artifact caused by the epitope tags, we tested the effects of expressing natural untagged Ub0; it was as effective as tagged Ub in modifying IRF-3 (Figure 3E) and promoting RIPA (Figure 3F). These results strongly indicated that linear polyubiquitination of IRF-3 was required for RIPA. To ensure that this phenomenon was natural and did not need ectopic expression of Ub, we took advantage of an antibody that specifically recognizes only linear poly Ub chains (Matsumoto et al., 2012). In cells expressing the K10 mutant (Figure 3G), or the K15 mutant (Figure 3H) of IRF-3, RLR activation caused linear polyubiquitination of IRF-3 using the endogenous pool of Ub. Using this assay, we also confirmed that the RIPA-incompetent IRF-3 mutants, K1, K4, K16 and K17 had reduced linear ubiquitination (Figures S3C and S3D). Moreover, endogenous IRF-3 was modified by linear ubiquitination upon RLR-stimulation (Figure S3E). These results indicate that linear ubiquitination of IRF-3 is required for RIPA.
Figure 3. Unbranched, linear ubiquitination of IRF-3 triggers RIPA.
(A) C-PARP was analyzed in HT1080 cells transfected with K63 (Ub63) or K48 (Ub48) mutants of Ub upon polyI:C transfection (RLR). (B) HT1080 (Wt) or HT1080.shIRF-3 cells were transfected with WT or K0 (Ub0) mutant of Ub and C-PARP was analyzed upon polyI:C transfection (RLR); lower panel: IRF-3 expression in HT1080 (Wt) and shIRF-3 cells. (C) Mitochondrial translocation of IRF-3 upon polyI:C transfection (RLR) in WT or K0 (Ub0) Ub transfected cells. (D) Total ubiquitination of IRF-3 in K0 (Ub0) or Wt Ub expressing cells upon polyI:C transfection (RLR). (E and F) Total ubiquitination of IRF-3 (E) and the amounts of C-PARP (F) were analyzed in various Ub transfected cells upon RLR stimulation (E, SeV infection, F, polyI:C transfection). (G and H) Linear ubiquitination of IRF-3 was analyzed for the lysine mutants (as indicated) of IRF-3 upon SeV infection (RLR), using linear ubiquitin specific antibody (Genentech). The results presented here are representatives of at least three biological repeats. Please see Figure S3 for additional data related to Figure 3.
LUBAC mediates IRF-3 linear polyubiquitination and RIPA
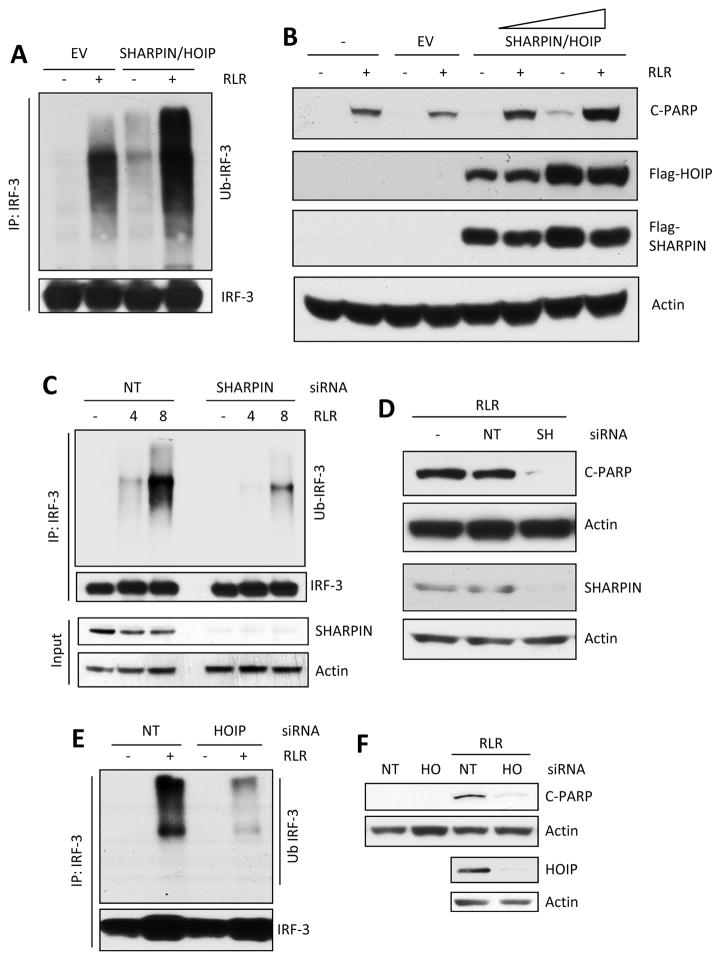
The addition of Ub to proteins requires the successive actions of three enzymes E1, E2 and E3 enzymes; the E3 ligases, which number in hundreds, impart the specificity of the target proteins. Many E3 ligases, including different TRAF proteins and TRIM proteins, are known to associate with the signaling RLR complex; however, they all promote branched polyubiquitination and therefore are unlikely candidates for promoting linear polyubiquitination of IRF-3. The most likely candidate is the LUBAC complex, comprising of three proteins, SHARPIN, HOIP and HOIL-1. LUBAC promotes linear polyubiquitination and is known to be recruited to the complex containing RLR and MAVS during signaling; hence we tested for the possible involvement of LUBAC in RIPA. Ectopic expression of SHARPIN and HOIP enhanced the RLR-activated IRF-3 polyubiquitination (Figure 4A) and the consequent RIPA, which increased with increasing expression of SHARPIN and HOIP (Figures 4B). Conversely, silencing the expression of SHARPIN (Figure 4D) reduced both polyubiquitination of IRF-3 (Figure 4C) and RIPA (Figures 4D and S4). Similar effects were observed (Figures 4E, 4F and S4) when HOIP expression was silenced (Figure 4F). These results indicated that LUBAC was required for RIPA.
Figure 4. LUBAC is involved in ubiquitination of IRF-3 and RIPA.
(A and B) IRF-3 ubiquitination (A, RLR, SeV infection) and RIPA (B, RLR, polyI:C transfection) were analyzed in IRF-3 K10 mutant expressing cells co-transfected with SHARPIN and HOIP plasmids (EV, empty vector). (C and D) IRF-3 ubiquitination (C, RLR, SeV infection) and RIPA (D, RLR, polyI:C transfection) were analyzed in IRF-3 K10 mutant expressing cells transfected with non-targeting (NT) or SHARPIN (SH) siRNA (Thermo Scientific). Silencing of SHARPIN was analyzed by Immuno blot. (E and F) IRF-3 ubiquitination (E, RLR, SeV infection) and RIPA (F, RLR, polyI:C transfection) were analyzed in IRF-3 K10 mutant expressing cells transfected with non-targeting (NT) or HOIP (HO) siRNA (Thermo Scientific). Silencing of HOIP was analyzed by Immuno blot. The results presented here are representatives of at least three biological repeats. Please see Figure S4 for additional data related to Figure 4.
To examine a possible physical interaction between IRF-3 and LUBAC, we performed co-immunoprecipitation assays. IRF-3 was bound to SHARPIN, but only after RLR activation (Figure 5A). Conversely, both HOIP and IRF-3 were co-precipitated with SHARPIN; HOIP was bound to it constitutively but IRF-3 was recruited only after RLR activation (Figure 5B). To test whether the IRF-3 Lys mutants that were active in RIPA could associate with LUBAC, we tested the K10 and the K15 mutants. From stimulated cells, K15, having only two Lys residues at 193 and 313, co-precipitated with SHARPIN, as strongly as K10 (Figure 5C). However, inactive K16, having only Lys 313, and inactive K17, having only Lys 193, did not co-precipitate with SHARPIN (Figure 5D). These results indicate that IRF-3 interaction with LUBAC requires both Lys 313 (or 315) and Lys 193. Further analyses revealed that both TRAF2 and TRAF6 were required for this interaction. If the expression of either TRAF was silenced (Figure 5F), the binding of IRF-3 to SHARPIN was greatly diminished (Figure 5E). This observation provides a biochemical basis for the observed need of TRAF2 and TRAF6 in RIPA (see Figure 1B).
Figure 5. IRF-3 and LUBAC form a complex and this is dependent on TRAFs and specific lysine residues in IRF-3.
(A) Co-IP of IRF-3 and SHARPIN in RLR (SeV infection)-stimulated cells at the indicated time. (B–D) SHARPIN and HOIP were co-transfected in IRF-3 mutant (as indicated) expressing cells, and co-IP of IRF-3 and SHARPIN was analyzed upon RLR stimulation (SeV infection). (E) IRF-3 K10-expressing cells were transfected with a non-targeting (NT), TRAF2 or TRAF6 specific siRNA (Thermo Scientific) and co-IP of IRF-3 and SHARPIN was analyzed upon RLR stimulation (SeV). (F) Silencing efficiency of the TRAF2 and TRAF6 specific siRNAs. The results presented here are representatives of at least three biological repeats.
RIPA protects cells and mice from virus infection in the absence of antiviral gene expression
IRF-3 is known to be an important component of host’s innate immune defense against virus infection and its antiviral role is thought to be mediated entirely by its ability to induce interferon (IFN) and other antiviral proteins. The discovery of an alternative in vitro function of IRF-3 raised the question of whether RIPA regulates viral pathogenesis well. To address this question, we used a RIPA-competent, but transcriptionally inert, mutant of IRF-3 first in tissue culture cells and then in mice. Replacement of two Ser residues with Ala near the C-terminal of murine IRF-3, Ser 388 and Ser 390, generated the S1 mutant (Table S1). When expressed in Irf3−/− MEF, the S1 mutant could not induce genes as measured by the abundance of Ifit1, Ifit3 and Ifnb1 mRNAs (Figure S5A–S5C) and Ifit3 protein (Figure 6A, lower panel). However, it could activate RIPA as measured by PARP cleavage (Figure 6A, upper panel). As expected, like the WT protein, the S1 mutant translocated to mitochondria (Figure 6B); however, only the WT protein translocated to the nucleus as well (Figure 6C). The RIPA-active, S1 mutant interacted with SHARPIN in a signal-dependent fashion, indicating that its apoptotic function is mediated by RIPA (Figure 6D). Both WT and S1 IRF-3 strongly inhibited the replication of two strains of Sendai virus, Strain 52 (Figure 6E) and Cantell (Figure S5D). The antiviral activity of S1 IRF-3 was RIPA-dependent; ablation of BAX expression (Figure 6F) or inhibition of caspase activities (Figure 6G), in S1-expressing MEFs, ameliorated the antiviral effect, as measured by enhanced viral protein expression (Figures 6F and 6G). The human IRF-3 K10 mutant, which was active for RIPA, but transcriptionally inert, blocked virus replication, when expressed in human HT1080.shIRF-3 cells (Figure S5E). Similarly, the mouse IRF-3 K18 mutant, which was active in transcription, but not RIPA, also blocked virus replication (S5F).
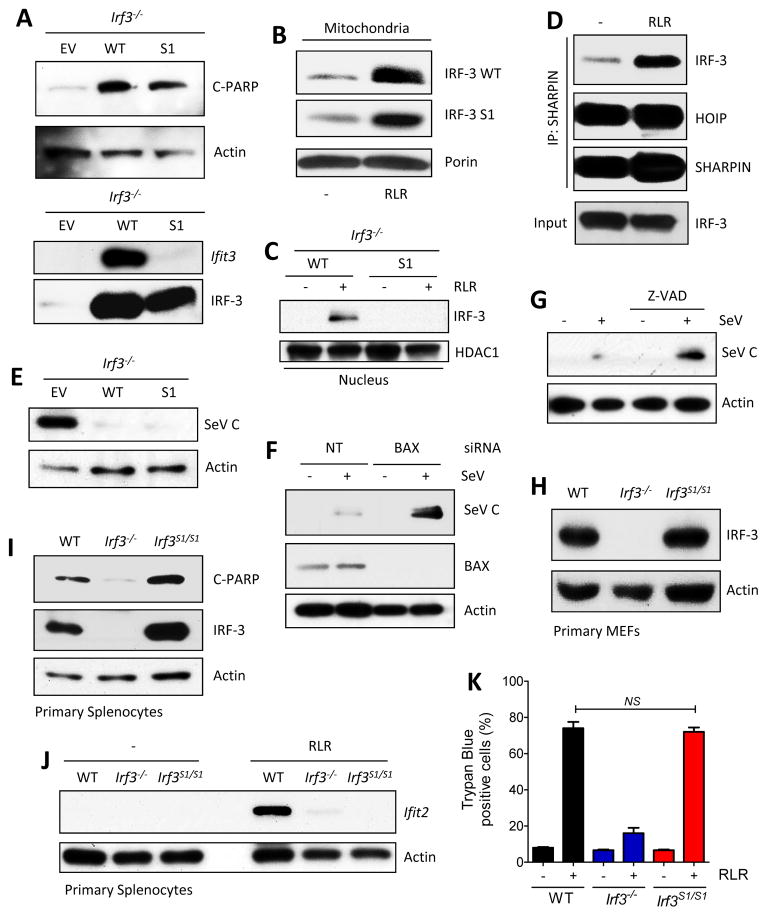
Figure 6. RIPA and transcriptional activities of IRF-3 S1 mutant in vitro and ex vivo.
(A) RIPA (C-PARP, top panel) and transcriptional (Ifit3, bottom panel) activities of IRF-3 in RLR (polyI:C transfected) stimulated Irf3−/− MEFs expressing WT or S1 mutant (SS388/90AA) of murine IRF-3. (B and C) RLR-induced translocation of IRF-3 (WT or S1 mutant, expressed in Irf3−/− MEFs) to mitochondria or nucleus; porin and HDAC1 are markers of mitochondrial and nuclear extracts. (D) SHARPIN and HOIP were co-transfected in S1-expressing Irf3−/− MEFs, and co-IP of IRF-3 and SHARPIN was analyzed upon RLR stimulation (SeV infection). (E) Expression of SeV C protein in Irf3−/− MEFs expressing WT or S1 mutant of IRF-3 upon SeV (52 strain) infection. (F) SeV C protein expression in Irf3−/− infected MEFs expressing S1 mutant after transfection of non-targeting or BAX-specific siRNA. (G) SeV C protein expression in Irf3−/− MEFs, expressing S1 mutant, after treatment with caspase inhibitor (Z-VAD). (H) IRF-3 expression in primary MEFs isolated from WT, Irf3−/− and S1 mice analyzed by Immunoblot. (I) Primary splenocytes from mice (genotypes indicated) were infected with SeV (RLR) and analyzed for Ifit2 induction by Immuno blot. (J) Primary splenocytes isolated from the mice (genotypes indicated) were analyzed for RIPA (C-PARP) upon RLR stimulation (polyI:C transfection). (K) Trypan blue-positive dead cells in RLR-stimulated (polyI:C transfected) WT, Irf3−/− and S1 MEFs. The results presented here are representatives of at least three biological repeats. Please see Figure S5 for additional data related to Figure 6.
For assessing RIPA’s role in blocking viral pathogenesis in vivo, we generated a genetically targeted mouse, called S1 mouse, harboring only the S1 mutant gene of IRF-3 (Figures S6A and S6B). IRF-3 was expressed, in cells of the S1 mouse, at an amount similar to that in WT mouse (Figure 6H). RLR stimulation of S1 cells ex vivo caused RIPA activation (Figure 6I) but no gene induction (Figure 6J). As expected, RIPA activation in S1 cells was both Bax- and caspase-dependent (Figures S6C and S6D). The S1 mutant triggered similar degree of cell death, induced by RIPA, as the WT IRF-3 (Figure 6K). Similarly, activation of TLR3 by dsRNA and TLR4 by LPS in primary immune cells, isolated from mice, showed that Ifit2, an IRF-3-inducible gene was induced only in cells from WT mice, but not Irf3−/− or S1 mice (Figure S6E and S6F). These results assured us that the genetically targeted mouse, expressing S1 IRF-3, was well suited for challenge with a pathogenic virus.
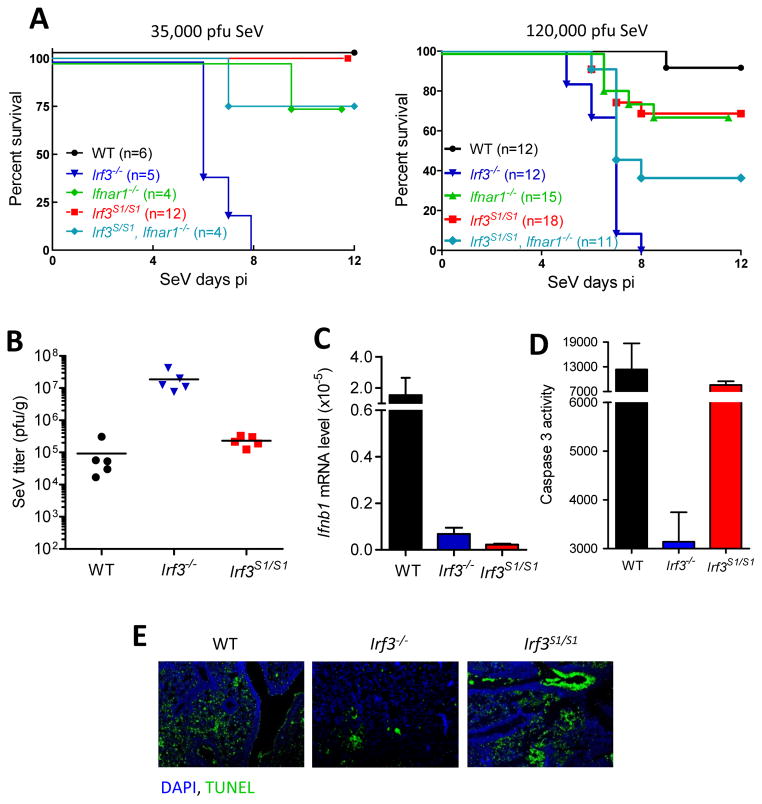
Because SeV has been used by us for activating RIPA in most of our in vitro experiments, we chose to challenge the IRF-3 S1 mice with intranasal infection of SeV 52 strain at two doses (Figure 7A). At both doses, all Irf3−/− mice died. In contrast, all WT and S1 mice survived at the low dose; but at the higher dose, they were mildly susceptible. The mortality of Irf3−/− mice was accompanied by severe irreversible weight loss whereas the WT and S1 mice, although suffered significant loss of body weight, recovered from it similarly (Figures S6G and S6H). We characterized the pathogenesis further in mice infected with the higher dose of SeV. There were about 100 fold higher virus titers in the lungs of infected Irf3−/− mice, as compared to those in the lungs of infected WT and S1 mice (Figure 7B). As expected, IFN-β was strongly induced in the infected lungs of WT mice but not in those of Irf3−/− and S1 mice (Figure 7C). However, unlike the lungs of the Irf3−/− mice, the lungs of the WT and the S1 mice showed strong signs of apoptotic action as measured by caspase 3 activation (Figure 7D), PARP cleavage (Figure S6I) and TUNEL staining (Figure 7E).
Figure 7. Genetically targeted Irf3 S1 mice exhibit reduced viral load in lungs and are protected from viral pathogenesis.
(A) Mice (genotypes indicated) were intranasally infected with SeV (two different pfu per mouse, as indicated) and monitored for morbidity for the indicated time. B to E is from mice infected with the higher dose of virus. (B) Lungs isolated from SeV-infected mice (genotypes indicated) were analyzed for infectious viral titer by plaque assay. (C) Lung mRNAs from SeV-infected mice were analyzed for IFN-β induction by qRT-PCR. (D) Lung homogenates from SeV-infected mice (genotypes indicated) were analyzed for caspase-3 activity. (E) TUNEL staining from SeV-infected lung sections of the indicated mice. The results presented here are representatives of at least three biological repeats. Please see Figure S6 for additional data related to Figure 7.
Our results clearly established that, in addition to the well-known IFN-mediated antiviral effect of IRF-3, the RIPA-mediated apoptotic effect also contributes substantially to the overall protection from viral pathogenesis. The above conclusion was further supported by testing the susceptibility of mice with additional genotypes. As reported before (Wetzel et al., 2014), unlike the highly susceptible Irf3−/− mice, the Ifnar1−/− mice, in which no type I IFN can function, were only partially susceptible to SeV infection, both at the low and the high doses of the virus (Figure 7A); presumably these mice were protected by RIPA and not by other IRF-3 induced proteins, besides IFN. To definitively prove this point, we generated a strain of mouse, Irf3S1/S1, Ifnar1−/−, expressing the S1 mutant of IRF-3 but no IFNAR1. In these mice, IRF-3 cannot induce any genes, and IFNs, even if induced by other IRFs, cannot function; however, RIPA is intact. Compared to the Irf3−/− mice, the Irf3S1/S1, Ifnar1−/− mice were highly protected from the lethal effects at both doses of SeV infection (compare Irf3S1/S1, Ifnar1−/− and Irf3−/−, Figure 7A). These results demonstrate that IRF-3-mediated RIPA activity can impart strong protection against viral pathogenesis.
DISCUSSION
As a transcription factor, IRF-3 induces the synthesis of type I IFN which blocks viral replication and pathogenesis; here, we have validated RIPA as another arm of IRF-3-mediated antiviral innate immune response of the host. RIPA uses IRF-3 and several other known components of the RLR signaling pathway that leads to IFN induction, but not IFN itself or any IFN-stimulated antiviral genes; moreover, it is an antiviral response pathway that does not require new cellular gene expression (Chattopadhyay et al., 2010). In the process of investigating how RIPA is activated, we have uncovered a mechanism of IRF-3 activation that is distinct from the mechanism of its activation as a transcription factor. Because the two activation pathways require the presence of different residues of IRF-3, it was possible to design the S1 mutant of IRF-3 which is active in RIPA but not as a transcription factor. In IRF-3 S1 expressing mouse, RLR activation of RIPA caused apoptosis of the infected cells, viral burden was reduced without IFN induction and the infected mice were protected from viral pathogenesis.
Unlike the use of phosphorylation for activating IRF-3 as a transcription factor, in RIPA, IRF-3 is activated by ubiquitination. Ubiquitination of many other proteins of the RLR signaling pathway has been reported (Gack et al., 2007; Liu et al., 2013; Maelfait and Beyaert, 2012; Tu et al., 2013; Ye et al., 2014); the nature of the ubiquitination linkages on most of these proteins is either K48-linked branches, leading to proteolytic degradation of the target protein, or K63-linked branches leading to its activation in signal transduction (Nakhaei et al., 2009; Zeng et al., 2009). Although degradative ubiquitination of IRF-3 has been described before (Chen et al., 2007; Yu and Hayward, 2010; Zhang et al., 2008), our analysis of RIPA provided an example of activating ubiquitination of IRF-3. But surprisingly, activation was achieved, not by K63-linked polyubiquitination, but by linear polyubiquitination of specific Lys residues of IRF-3. This modification allowed IRF-3 to interact with Bax and trigger RIPA. The linear polyubiquitination of IRF-3 is carried out by the LUBAC complex of enzymes comprising of SHARPIN, HOIP and HOIL-proteins (Tokunaga et al., 2009). LUBAC is also used by RLR signaling to ubiquitinate NEMO, a regulatory subunit of the IKK complex which phosphorylates IκB; NEMO interacts with TBK1 as well and regulates IRF-3’s transcriptional activation (Belgnaoui et al., 2012). Two TRAF proteins, TRAF2 and TRAF6, were selectively required for activating RIPA; in their absence there was reduced interaction between LUBAC and IRF-3. It is not yet clear whether these TRAFs were functioning as E3 ubiquitin ligases or as adaptors in RIPA. If they were acting as enzymes, their ubiquitinating target could be one of the two essential lysine residues making the other one available for ubiquitination by LUBAC. The two IRF-3 signaling pathways share several common proteins; for example, IPS-1, TRAF3 and TBK1 are required for activating IRF-3 in both pathways. IPS-1 provides the platform for assembly of the signaling complex and TRAF3 recruits TBK1 to it. For the activation of IRF-3 as a transcription factor, TBK1 phosphorylates specific Ser residues of the protein (Fitzgerald et al., 2003; Mori et al., 2004; Servant et al., 2003). Because these Ser residues are dispensable in RIPA, the exact function of TBK1 in activating IRF-3 is currently unclear. In RIPA, it may be phosphorylating other Ser residues of IRF-3 or those in the downstream signaling proteins.
Like the S1 mutant, the K2, K10, K11 and K12 mutants of IRF-3 were also deficient in transcriptional activity, indicating that ubiquitination of specific Lys residues of IRF-3 may be required for that pathway as well. The mutational analysis of IRF-3’s Lys residues produced several mutants that were selectively active; for example, K4, a single-Lys mutant, was inactive in RIPA but active in transcription. Some of the Lys mutations also stabilized IRF3 indicating they might be targets of degradative K48-branched polyubiquitination (data not shown). Thus, it is not unlikely that ubiquitination of different Lys residues determines the different fates of IRF-3; its activation in RIPA, its activation as a transcription factor or its degradation by the proteasomal pathway.
To establish that RIPA has antiviral activity in cell culture, we used SeV-Cantell or SeV52 strains, both of which activate RLR signaling. To ascertain which of the two activities of IRF-3, the transcriptional or the apoptotic activity, is responsible for inhibiting virus replication, we used two mutants of IRF-3, S1 and K10, which are active in RIPA but not in transcription. When expressed in Irf3−/− MEF or in IRF-3 silenced HT1080 cells, both IRF-3 mutants inhibited SeV replication. For evaluating the role of RIPA in vivo, we engineered a genetically targeted mouse expressing only the S1 mutant of IRF-3 and used SeV52, which infects the respiratory tract of mouse to cause morbidity and mortality in Irf3−/− mice. As expected, ex vivo, in primary immune cells from the KI mouse, IRF-3 could not be transcriptionally activated and the same was true in vivo in the lungs of infected mice. In contrast, the apoptotic activity of IRF-3 S1 was as strong as WT IRF-3. More importantly, it had strong antiviral effects and inhibited pathogenesis. These results firmly establish RIPA as a bona fide antiviral pathway that uses IRF-3 as a pro-apoptotic factor activated by RLR signaling. We believe that in WT mice both activities of IRF-3 contribute toward preventing viral pathogenesis (Chattopadhyay et al., 2013a); their relative contributions are, however, difficult to determine because in the absence of one in a mutant mouse, the other probably compensates for it. This is clear from the results obtained using a RIPA-incompetent K18 mutant, which blocked SeV replication using the transcriptional branch of IRF-3. The relative contributions, in the natural situations, of the two functions will also depend on the nature of the infecting virus. Moreover, many viruses activate IRF-3 through TLR activation, which does not trigger RIPA, in some cell types whereas in other cell types they use RLR. Our results strongly indicate that the RIPA-branch of IRF-3 is protective against SeV infection, even in the absence of all ISGs, induced by IRF-3 or IFN-signaling; it is also likely that the strong resistance of Ifnar1−/− mice is provided by the RIPA-branch of IRF-3. RIPA-like activities of IRF-3 have been reported for other disease models. Human monocytes do not allow productive infection by HTLV1 by triggering rapid apoptosis using a RIPA-like pathway that requires STING, IRF-3 and Bax (Sze et al., 2013). Hepatocyte cell death is a major cause of liver injury in alcoholic liver disease (ALD). Using a mouse model, a recent study indicates that alcohol triggers ER stress, causing apoptosis mediated by STING, IRF-3 and Bax. (Petrasek et al., 2013). Thus, it appears that the physiological role of RIPA may not be restricted to fighting viral infection only. Our S1 and Irf3S1/S1, Ifnar1−/− mice will be quite valuable to test the role of RIPA in other pathogenesis conditions. Overall, our study demonstrates that IRF-3 has two independent roles in antiviral immunity: it induces ISG expression and triggers RIPA. Moreover, our observations indicate that RIPA can be an effective mechanism, even in the absence of the IFN system, for protecting organisms from viral infection.
EXPERIMENTAL PROCEDURES
Cells and Reagents
Human HT1080, HT1080.shIRF-3 cells, and WT, Traf2−/−, Traf6−/−, Irf3−/− mouse embryonic fibroblasts (MEFs) have been previously described (Chattopadhyay et al., 2010; Peters et al., 2008). All the cell lines were maintained in DMEM supplemented with 10% FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin; HT1080.shIRF-3 cells were maintained in the presence of G418 (400 μg/ml). Mouse primary cells (MEFs, splenocytes and BMDMs) from the appropriate genotype were isolated using standard procedures and used as indicated in the figure legends. Antibodies against human IFIT3, murine Ifit2 and Ifit3 (Terenzi et al., 2007), and SeV C protein were raised at the Hybridoma Core, Lerner Research Institute. Antibodies against phospho-S396-IRF-3, cleaved PARP, SHARPIN, HOIP were from Cell Signaling; antibodies against ubiquitin, IRF-3, BAX were from Santa Cruz, anti-V5 antibody was from Invitrogen, anti-actin antibody was from Sigma-Aldrich, anti-Porin antibody was from Calbiochem, and linear ubiquitin antibody was kindly provided by Genentech. WT and mutant ubiquitin plasmids were obtained from Addgene, SHARPIN and HOIP expression plasmids were kindly provided by Jae Jung (University of Southern California). Poly(I:C) was obtained from GE Healthcare, Lipopolysaccharides (LPS) was obtained from Sigma and Lipofectamine 2000 was obtained from Invitrogen. Poly(I:C) transfection was performed using Lipofectamine 2000 according to manufacturer’s instructions.
Generation and expression of IRF-3 mutants, testing RIPA and transcriptional pathways
Wt human and mouse IRF-3 were sub-cloned into pLVX-IRES-puromycin (Clontech) vector. The IRF-3 mutants (lysine to arginine and serine/threonine to alanine) were generated by Megaprimer PCR methods and all mutations were confirmed by sequencing. The WT or mutants of IRF-3 were expressed lentivirally in HT1080.shIRF-3 cells or Irf3−/− MEFs using standard transduction protocol and the transduced cells were selected with Puromycin (1 μg/ml). After one round of puromycin selection, the pool of cells was used for functional analyses (ubiquitination of IRF-3, IRF-3-induced genes, co-immunoprecipitations and RIPA) by RLR stimulations (transfecting polyI:C with Lipofectamine 2000 or SeV infection at moi:10). Gene induction (by qRT-PCR and Western analyses) was measured after 6h and RIPA (C-PARP) was analyzed after 16h, of RLR stimulation. For ubiquitination, the cells were RLR-stimulated as indicated in figure legends. The details of SeV infection has been reported previously (Chattopadhyay et al., 2010; Peters et al., 2008).
Immunoprecipitation, Immunoblot analysis and ubiquitination assay
For co-immunoprecipitation and Immunoblot analyses, the cells were lysed in 50 mM Tris buffer, pH 7.4 containing 150 mM of NaCl, 0.1% Triton X-100, 1 mM sodium orthovanadate, 10 mM of sodium fluoride, 10 mM of β-glycerophosphate, 5 mM sodium pyrophosphate and protease inhibitors (Roche) by keeping on ice for 30 min followed by centrifugation to clear the lysates. Equal amounts of total proteins from the cell lysates were analyzed by SDS-PAGE and Western blot. For ubiquitination assays, the RLR-stimulated cells were lysed in 50 mM Tris buffer, pH 7.4, 150 mM NaCl, 0.2% Triton X-100, 0.5% SDS, 1 mM sodium orthovanadate, 10 mM of sodium fluoride, 10 mM of β-glycerophosphate, 5 mM sodium pyrophosphate, protease inhibitors (Roche), PhosSTOP (Roche) and MG132. The cell lysates, containing equal amounts of total proteins, were incubated at 95°C for 5 minutes with occasional vortexing and applied to brief sonication, diluted with lysis buffer, followed by centrifugation at 14,000 rpm to clear the lysates. The protein extracts were used for immunoprecipitation using V5-agarose (Sigma) after pre-clearing with mouse IgG agarose (Sigma) beads. After immunoprecipitation, the beads were washed rigorously with lysis buffer containing 300 mM NaCl, leaving on a rotator for 15 min and repeating this step for two additional times. The bound proteins were eluted from the beads by boiling with 2X SDS buffer for 3 minutes and the eluates were analyzed on a 10% SDS-PAGE.
siRNA experiments
The siRNAs against human SHARPIN, HOIP, TRAF2, TRAF6 were obtained from Thermo Scientific and transfected using DharmaFECT 4 reagent using manufacturer’s instructions. After 48h of siRNA transfection, the cells were used for functional analyses (ubiquitination, co-immunoprecipitation and RIPA). A non-targeting siRNA (Thermo Scientific, D-001810-10-05) was used as a control and was transfected using the same protocol as above.
Isolation of sub-cellular fractions
Mitochondrial and cytosolic fractions from the RLR-stimulated cells were isolated as described previously using the Mitochondria Isolation Kit (Pierce Biotechnology) following the manufacturer’s instructions (Chattopadhyay et al., 2010). Isolated mitochondrial fractions were washed with PBS and extracted in lysis buffer for Western analysis. Mitochondrial fractions were assessed to ensure purity by Western analyses of a cytoplasmic protein, tubulin.
Mice and virus infection
All mice used in this study were of C57BL/6 genetic background and both sexes. The Irf3−/− mice were obtained from RIKEN Bio Resource Center, Japan. The genetically targeted Irf3S1/S1 (S1) mice were custom-generated by Taconic Farms, Inc using the design of the target allele as described in Figure S6A. For virus infections, the desired pfu of SeV (indicated in figure legends) in 35ul of endotoxin-free PBS were intransally administered to isofluorane-anesthetized 8-10 week old mice. The mice were monitored daily for their body weight loss and disease symptoms. All the animal procedures were performed according to the protocols approved by the institutional animal care and use committee.
Caspase activity
Caspase-3/7 activity of the cell lysates and the lung homogenates were performed using previously described procedures (Chattopadhyay et al., 2010). Briefly, the cell lysates or the lung homogenates were used for measuring caspase activity using the Apo-ONE™ Homogeneous Caspase-3/7 assay according to protocols provided by the manufacturer (Promega, Madison, WI). The Caspase activity of WT IRF-3-expressing cells was arbitrarily set as 100 and all other values were normalized to this (Figure 1A).
RNA isolation and qRT-PCR analyses
For qRT-PCR analyses, RNA was isolated from the stimulated cells using Roche RNA isolation kit and reverse transcription with random hexamers were performed according to the manufacturer’s instructions. For isolating lung-associated RNA, lungs were isolated by snap-freezing in liquid nitrogen and RNA was extracted using TRIzol reagent (Invitrogen). DNase I treatment (DNAfree, Applied Biosystems/Ambion) and reverse transcription with random hexamers (ImProm-II, Promega) were performed according to the manufacturer’s instructions. For realtime PCR, 0.5 ng of RNA was used in 384 well-format realtime PCRs in a Roche LightCycler 480 II using Applied Biosystem’s SYBR Green PCR core reagents. The expression of the induced mRNAs was normalized to 18S rRNA for each sample. The primer sequences have been described before (Fensterl et al., 2012).
Virus titration
For quantification of infectious SeV in lungs, mice were anesthetized with ketamine (100 mg/kg) and xylazine (5mg/kg) and blood was removed from organs by cardiac perfusion with 10 ml of PBS. The lungs were snap-frozen in liquid nitrogen, weighed, pestle/tube-homogenized (Kimble/Kontes) in 1 ml of PBS per lung, and virus was titered in 10-fold serial dilutions on LLCMK2 cells by plaque assay as described before (Chattopadhyay et al., 2011). Results are expressed as plaque-forming units (pfu) per gram of tissue.
TUNEL staining of lung sections
For detection of apoptotic cells in the SeV-infected lung sections, the DeadEnd fluormetric TUNEL system (Promega) was used according to manufacturer’s instructions. All objects were then mounted with VectaShield (with DAPI, Vector Labs, Burlingame, CA), and examined with a Leica DRM fluorescence microscope.
Supplementary Material
Acknowledgments
This work was supported by American Heart Association Scientist Development Grant 15SDG25090212 (SC) and the National Institutes of Health grants AI073303 (GCS) and CA062220 (GCS). We would like to thank Jae Jung (University of Southern California) for kindly providing the LUBAC expression plasmids and Genentech for the linear ubiquitin-specific antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belgnaoui SM, Paz S, Samuel S, Goulet ML, Sun Q, Kikkert M, Iwai K, Dikic I, Hiscott J, Lin R. Linear ubiquitination of NEMO negatively regulates the interferon antiviral response through disruption of the MAVS-TRAF3 complex. Cell host & microbe. 2012;12:211–222. doi: 10.1016/j.chom.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Wetzel JL, Sen GC. Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators. MBio. 2013a;4:e00636. doi: 10.1128/mBio.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Yamashita M, Sen GC. Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus. Journal of virology. 2013b;87:16–24. doi: 10.1128/JVI.01853-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, Williams BR, Sen GC. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Sen GC. IRF-3 and Bax: a deadly affair. Cell Cycle. 2010;9:2479–2480. doi: 10.4161/cc.9.13.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Yamashita M, Zhang Y, Sen GC. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. Journal of virology. 2011;85:3708–3716. doi: 10.1128/JVI.02133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Rijnbrand R, Jangra RK, Devaraj SG, Qu L, Ma Y, Lemon SM, Li K. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology. 2007;366:277–292. doi: 10.1016/j.virol.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS pathogens. 2012;8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nature immunology. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. The Journal of biological chemistry. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Ikushima H, Negishi H, Taniguchi T. The IRF family transcription factors at the interface of innate and adaptive immune responses. Cold Spring Harbor symposia on quantitative biology. 2013;78:105–116. doi: 10.1101/sqb.2013.78.020321. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O’Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–1632. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, Sun L, Chen ZJ. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J, Beyaert R. Emerging role of ubiquitination in antiviral RIG-I signaling. Microbiology and molecular biology reviews : MMBR. 2012;76:33–45. doi: 10.1128/MMBR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao AP, Li S, Zhong B, Li Y, Yan J, Li Q, Teng C, Shu HB. Virus-triggered ubiquitination of TRAF3/6 by cIAP1/2 is essential for induction of interferon-beta (IFN-beta) and cellular antiviral response. The Journal of biological chemistry. 2010;285:9470–9476. doi: 10.1074/jbc.M109.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto ML, Dong KC, Yu C, Phu L, Gao X, Hannoush RN, Hymowitz SG, Kirkpatrick DS, Dixit VM, Kelley RF. Engineering and structural characterization of a linear polyubiquitin-specific antibody. Journal of molecular biology. 2012;418:134–144. doi: 10.1016/j.jmb.2011.12.053. [DOI] [PubMed] [Google Scholar]
- Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. The Journal of biological chemistry. 2004;279:9698–9702. doi: 10.1074/jbc.M310616200. [DOI] [PubMed] [Google Scholar]
- Nakhaei P, Mesplede T, Solis M, Sun Q, Zhao T, Yang L, Chuang TH, Ware CF, Lin R, Hiscott J. The E3 ubiquitin ligase Triad3A negatively regulates the RIGI/MAVS signaling pathway by targeting TRAF3 for degradation. PLoS pathogens. 2009;5:e1000650. doi: 10.1371/journal.ppat.1000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H, Miyashita M, Inoue N, Okabe M, Matsumoto M, Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell host & microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Pauli EK, Chan YK, Davis ME, Gableske S, Wang MK, Feister KF, Gack MU. The Ubiquitin-Specific Protease USP15 Promotes RIG-I-Mediated Antiviral Signaling by Deubiquitylating TRIM25. Sci Signal. 2014;7:ra3. doi: 10.1126/scisignal.2004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Chattopadhyay S, Sen GC. IRF-3 activation by Sendai virus infection is required for cellular apoptosis and avoidance of persistence. Journal of virology. 2008;82:3500–3508. doi: 10.1128/JVI.02536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA, Szabo G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos HJ, Gale M., Jr RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Current opinion in virology. 2011;1:167–176. doi: 10.1016/j.coviro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant MJ, Grandvaux N, tenOever BR, Duguay D, Lin R, Hiscott J. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. The Journal of biological chemistry. 2003;278:9441–9447. doi: 10.1074/jbc.M209851200. [DOI] [PubMed] [Google Scholar]
- Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell host & microbe. 2013;14:422–434. doi: 10.1016/j.chom.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Terenzi F, White C, Pal S, Williams BR, Sen GC. Tissue-specific and inducer-specific differential induction of ISG56 and ISG54 in mice. Journal of virology. 2007;81:8656–8665. doi: 10.1128/JVI.00322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nature cell biology. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Tu D, Zhu Z, Zhou AY, Yun CH, Lee KE, Toms AV, Li Y, Dunn GP, Chan E, Thai T, et al. Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell reports. 2013;3:747–758. doi: 10.1016/j.celrep.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel JL, Fensterl V, Sen GC. Sendai virus pathogenesis in mice is prevented by Ifit2 and exacerbated by interferon. Journal of virology. 2014;88:13593–13601. doi: 10.1128/JVI.02201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Chattopadhyay S, Sen GC. Phosphatidylinositol 3-kinase signaling delays sendai virus-induced apoptosis by preventing XIAP degradation. Journal of virology. 2011;85:5224–5227. doi: 10.1128/JVI.00053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JS, Kim N, Lee KJ, Nam YR, Lee U, Joo CH. Lysine 63-linked TANK-binding kinase 1 ubiquitination by mindbomb E3 ubiquitin protein ligase 2 is mediated by mitochondrial antiviral signaling protein. Journal of virology. 2014 doi: 10.1128/JVI.02037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Hayward GS. The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity. 2010;33:863–877. doi: 10.1016/j.immuni.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Xu M, Liu S, Sun L, Chen ZJ. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Molecular cell. 2009;36:315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Tian Y, Wang RP, Gao D, Zhang Y, Diao FC, Chen DY, Zhai ZH, Shu HB. Negative feedback regulation of cellular antiviral signaling by RBCK1-mediated degradation of IRF3. Cell research. 2008;18:1096–1104. doi: 10.1038/cr.2008.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.