Introduction
The epidermal growth factor receptor (EGFR) family plays a critical role in the development and maintenance of the heart. ErbB2 and ErbB4 null mutant mice display impaired development of trabeculae and the embryos die before embryonic day 11, whereas the conditional deletion of ErbB2 in the adult heart leads to dilated cardiomyopathy and an increased sensitivity to anthracyclines [1–3]. Clinical trials with the immunotherapeutic agent trastuzumab (Herceptin®) support the hypothesis of a critical role of the ErbB2 receptor in the heart. Inhibition of human ErbB2 (HER2) with trastuzumab, given either in combination with or after chemotherapy, prolongs survival in women with HER2 positive metastasizing breast cancer [4] and improves disease free survival in the adjuvant setting [5, 6]. However, alone and in combination with chemotherapeutic agents like doxorubicin (Doxo), trastuzumab therapy was associated with cardiac dysfunction [4]. We showed, that in adult cardiomyocytes treatment with anti-ErbB2 antibodies changes MAPK-signaling and increases Doxo- or paclitaxel-induced myofibrillar disarray, which may explain contractile dysfunction seen in patients [7].
Based on these previous observations, we hypothesized that ErbB2-inhibition with tyrosine kinase inhibitors would induce myofibrillar disarray and contractile dysfunction in cardiomyocytes. We found, that a ERBB1/ErbB2 tyrosine kinase inhibitor induces similar changes as antibodies to ErbB2. Since the inhibition of ErbB2-receptors in cancer cells promotes cell death, we expected to find similar effects in myocytes. We did not find induction of cell death in cardiomyocytes either with a single EGFR- or with the combined EGFR/ErbB2 tyrosine kinase inhibitor. Based on these observations, we predict that dual EGFR/ErbB2 tyrosine kinase inhibitors have a similar profile of cardiac side effects as anti-ErbB2 agents such as trastuzumab.
Material and Methods
Antibodies used
Myomesin mouse monoclonal antibody clone B-4 (a kind gift from J.C. Perriard, ETH-Zurich, Switzerland); p-Erk1/2 mouse monoclonal antibody clone E-4 (Santa Cruz Biotechnology); total Erk1/2 CT rabbit polyclonal antibody, article 06-182 (Milipore); p-Akt/PKB rabbit polyclonal antibody Ser-473 (Cell Signaling Technology); total Akt rabbit polyclonal antibody (Cell Signaling Technology); p-GATA4 rabbit polyclonal ab5245 (Abcam); ErbB1 (EGFR) rabbit polyclonal antibody 1005 (Santa Cruz Biotechnology); ErbB2 rabbit polyclonal antibody C-18 and ErbB4 rabbit polyclonal antibody C-18 (Santa Cruz Biotechnology); p-tyrosine mouse monoclonal antibody PY-20 (Santa Cruz Biotechnology).
Isolation of adult rat ventricular cardiomyocytes
Adult (250–300 g) male Wistar rats from an in-house breeding facility were used. Isolation of calcium-tolerant adult rat ventricular myocytes (ARVM) was done according to previously published methods [8]. Cardiomyocytes were plated onto laminin-coated (Invitrogen) dishes (Nunc, VWR International) and maintained in medium containing 10% fetal calf serum (FCS) (PAA laboratories), cytosine 1-β-D-arabinofuranoside 10 µM, creatine monohydrate (Sigma) 20 mM, penicillin 100 IU/mL and streptomycin 100 mg/mL (Invitrogen) in MEM199, for the entire culture period (10–12 days). Medium was changed every 3 days and directly before treatment with pharmacologic agents. This investigation conforms with the “Principle of laboratory animal care” published by the US National Institutes of Health (NIH Publication No. 86-23, revised 1996). All experiments involving animals were approved by the review board for animal experimentation of the state veterinary office, Bern, Switzerland (license no 17/06).
Immunofluorescence microscopy
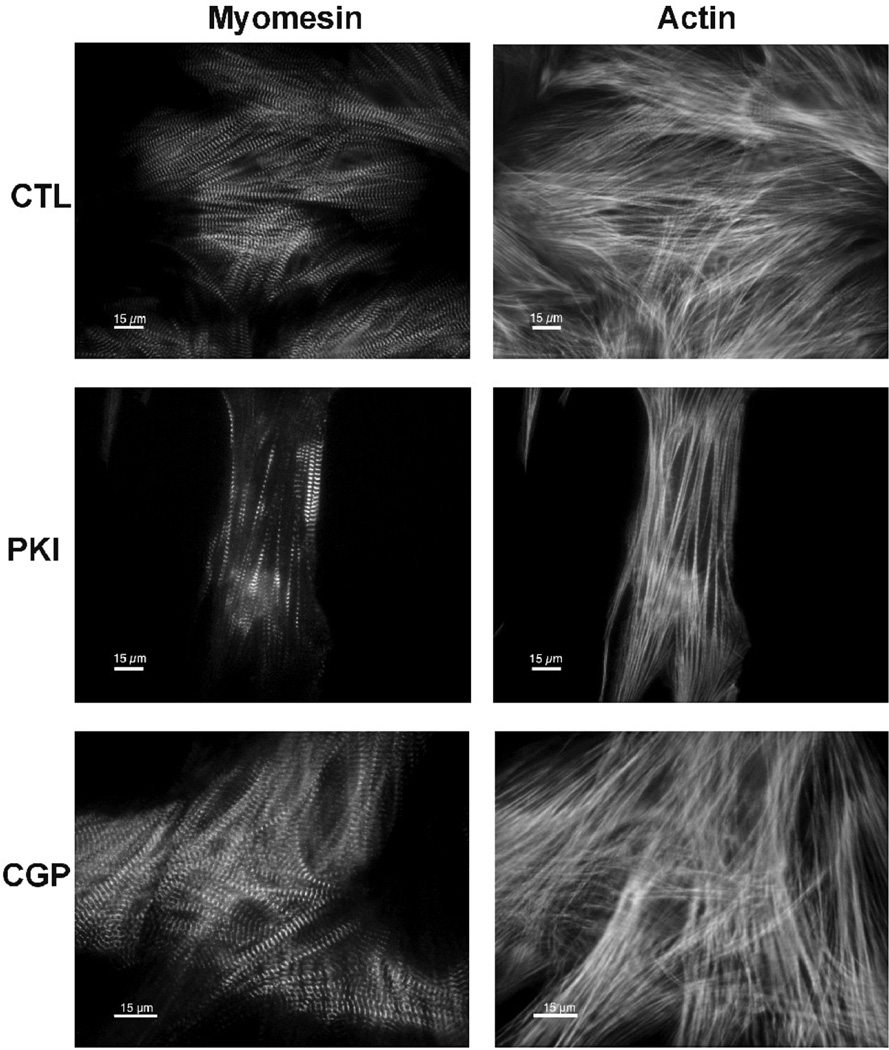
Immunostaining was done according to previously published methods [9]. Secondary antibodies and dyes were: goat-anti mouse coupled to Alexa Fluor-488 (Invitrogen) and phalloidin coupled to Alexa Fluor-532 (Invitrogen). The morphology of the cytoskeleton was then analyzed using an inverted fluorescence microscope LEITZ DM IL (Leica) equipped with a 40x objective (1.3 oil, Olympus). To quantify the effect of treatments on myofibril structure, an investigator blinded to treatments counted cells with at least 10% of myofibrillar structural damage or reduction of sarcomere area, according to previously published methods [10]. Pictures shown in Fig. 2 were taken using an inverted microscope (Nikon Eclipse TE2000-U) equipped with a 60x oil immersion objective and digital camera (DXM1200F).
FIG. 2.
Myofibrillar structural damage induced by tyrosine kinase inhibitors. Cells were treated for 48 hours and then immunostained for the M-line protein myomesin (left column) and stained with phalloidin to visualize F-actin (right column) (all scale bars 15 µm). CTL: Untreated cardiomyocytes. PKI: PKI 1µM for 48h. CGP: CGP 1µM for 48h.
Cell death assays
TUNEL assay was performed according to the manufacturers instructions (“In situ cell death detection kit AP”, Roche Applied Science), with the exception of prolonged permeabilization steps. Incubation with DNAse-I (Sigma) was used as positive control for DNA nick labeling. In order to avoid interference of red-fluorescent nuclei in the counting of TUNEL-positive cells by fluorescence microscopy after Doxo-treatment, conversion to an alkaline-phosphatase based detection system was performed (BCIP/NBT substrate, Sigma). LDH release test was performed according to the manufacturer (“Cytotoxicity detection kit”, Roche Applied Science). Results were normalized assuming CTL 0% of LDH release and cultures treated with 0.2 % triton-X for 10 minutes as 100%. Color intensity was measured using a Safire microplate reader (Tecan). The reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is thought to mainly occur in the mitochondria through the action of succinate dehydrogenase, therefore providing a measure of mitochondrial function. After incubation in MTT for 2 hours cells were washed two times with PBS and lysed to release formazan from active cardiomyocytes (lysis: 0.6% glacial acetic acid and 10% SDS in DMSO) and lysates were analyzed in a Safire microplate reader (Tecan).
Phosphorylation of Erk1/2, Akt/PKB, ErbB2, ErbB4, and Gata4
For the detection of p-Erk1/2 and p-Akt cells were cultured in medium with 10% serum for 10 days, washed with cold PBS and lysed using modified RIPA lysis buffer as previously described [7]. P-Erk1/2, p-Akt, and p-Gata-4 were detected with phospho-specific antibodies as mentioned above. Activation of ErbB2 and ErbB4 receptor tyrosine kinase was detected by immunoprecipitation followed by western blotting for phospho-tyrosine as previously described [11]. Nitrocellulose membranes were incubated with secondary antibodies conjugated with horseradish peroxidase (Pierce). Chemiluminescent signals were produced using the Supersignal West Dura substrate (Pierce) and detected on Hyperfilm (Amersham Biosciences). To test the blocking effect of PKI166 (PKI) or CGP059326 (CGP) on ErbB2 and ErbB4 phosphorylation, ARVM were treated with 1 µM PKI or with 1µM CGP for 3 hours and then stimulated with the extracellular domain of neuregulin-1β (NRG) (sodium-azide free, NeoMarkers) for 10 minutes. ErbB2 and ErbB4 were then immunoprecipitated and tyrosine phosphorylation was assessed by Western blot.
Contractility and calcium transients
Glass coverslips with myocytes cultured for 10 days were incubated in Tyrode’s buffer containing 1 µM of membrane-permeant fura-2-AM (Invitrogen) for 10 minutes and were rinsed with Tyrode’s buffer containing 500 µM of probenecid to prevent leakage of fura-2. Temperature stability of the chamber assembly and superfused buffer was maintained by a dual-channel temperature controller (Warner Instrument Corp). Contraction and calcium transients of long-term cultured ARVMs were measured by a video camera taking images at 240 Hz (Myocam, IonOptix) and using a photomultiplier tube for detecting fura-2 fluorescence as described for freshly-isolated cardiomyocytes [8]. For the real-time measurement of contractility, the region of interest was set to those places of the cells where an unidirectional striation of sarcomeres was discernible in phase contrast video images. All sarcomere length and calcium measurements were equally filtered by a lowpass Butterworth algorithm in IonWizard (IonOptix, Milton MA, USA).
Statistical analysis
All data were expressed as mean ± SEM. Data were analyzed with the One-Way ANOVA statistical test and the Bonferroni post-test was used to compare single treatments. Graph layouts were created with the program Prism 4 (Graphpad).
Results
PKI166 inhibits both EGFR and ErbB2, but not ErbB4
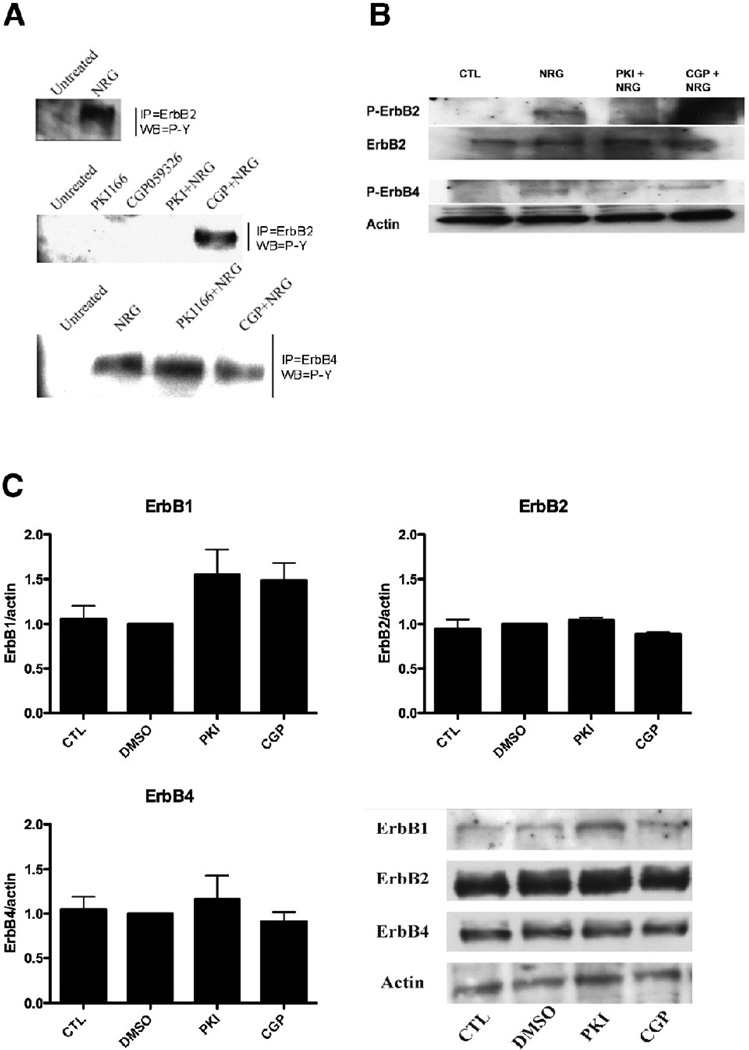
Tyrosine kinase inhibitors are small molecules that compete with ATP for the binding site on the cytoplasmic region of the receptors [12]. We tested by immunoprecipitation and western blot in adult rat myocytes the ability of tyrosine kinase inhibitors to inhibit ErbB2 or ErbB4 phosphorylation upon stimulation by NRG. In control cells, NRG activated both ErbB2 and ErbB4 receptors; PKI but not CGP inhibited NRG-induced phosphorylation of ErbB2, whereas neither PKI nor CGP prevented phosphorylation of ErbB4 by NRG (Fig 1A). These data were confirmed by Western-blot, using antibodies specific for the phosphorylated form of either ErbB2 or ErbB4 (Fig 1B). Incubation for 48 hours with either tyrosine kinase inhibitor did not change the total protein level of both ErbB2 or ErbB4 (Fig 1C). We observed a trend for an increase in EGFR protein level after tyrosine kinase inhibitor treatment (PKI:1.6 fold vs. DMSO; CGP:1.5 fold vs. DMSO).
FIG. 1.
ErbB receptor phosphorylation and protein amount. A: ARVM cultured for 10 days were treated for 3h with tyrosine kinase inhibitors (PKI 1µM and CGP 1µM) followed by NRG 10 ng/ml for 10 minutes and immunoprecipitation/western blot was performed as indicated. NRG activated both ErbB2 and ErbB4 receptors; PKI blocked NRG-induced activation of ErbB2 whereas CGP did not. Neither PKI nor CGP inhibited NRG action on the ErbB4 receptor. B: ARVM were treated as in A and western blot was performed as indicated including phospho-specific antibodies for ErbB2 and ErbB4. C: ARVM cultured for 10 days were treated for 48h with DMSO 1µl/ml, PKI 1µM, CGP 1µM and western blot was performed for ErbB1/2/4 and actin as loading control. Neither PKI nor CGP reduced the receptor proteins.
ErbB2 inhibition leads to myofibrillar structural damage
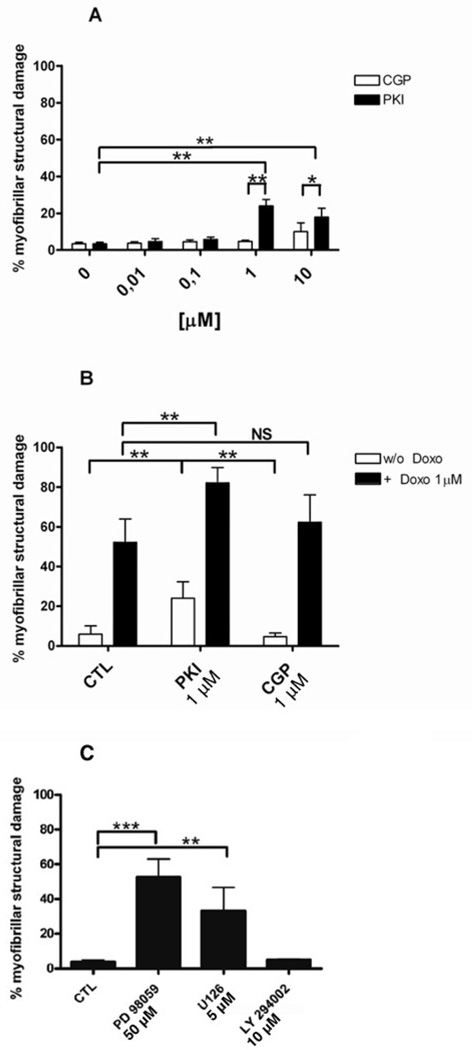
The effect of the dual tyrosine kinase inhibitor PKI and the EGFR inhibitor CGP on sarcomere structure was investigated in ARVM at 12 days in culture. Myocytes were exposed to 0.01, 0.1, 1 and 10µM of PKI or CGP for 48 hours, and myofibril structure was assessed by immunolabeling of the M-band protein myomesin and of filamentous actin in fixed cells (Fig. 2). The concentration range used in this experiment is in accordance with measurements of cancer patients serum in a clinical trial (from 0.18µM to 7.1µM) [13]. Fluorescence microscopy of the PKI treated ARVM revealed a rarification of the actin filaments and paucity of myomesin compared to the control condition (Fig 2). These structural changes were similar to that seen in ARVM treated with monoclonal antibodies against ErbB2 and anthracyclines[10]. In contrast to PKI, CGP had no effect on the myofibrillar structure. Quantification of the myofibrillar structural damage showed that PKI had no effect at concentrations ≤ 0.1 µM (Fig 3A). At higher doses there was a significant increase of myofibrillar damage compared to untreated and to CGP treated myocytes. In contrast to PKI, CGP had no significant effect on myofibrillar structure at all concentrations tested (Fig 3A). Since anthracyclines showed a synergistic effect when combined with anti-ErbB2 antibodies in a previous study [10], we tested if Doxo (1 µM) would worsen PKI-induced myofibrillar damage. Doxo alone induced significant myofibrillar structural damage that was additive to the PKI effect but not to CGP (Fig 3B).
FIG. 3.
Quantification of myofibrillar structural damage. A: Comparison of different doses of PKI and CGP; B: PKI 1 µM and CGP 1 µM were combined with Doxo 1 µM (filled bars); C: Cardiomyocytes were treated with PD98059 50 µM, U126 5 µM or LY294002 10 µM. A–C: *p<0.01; **p<0.001; One-way ANOVA p<0.00001. N=6 independent experiments, 700 cells counted per condition.
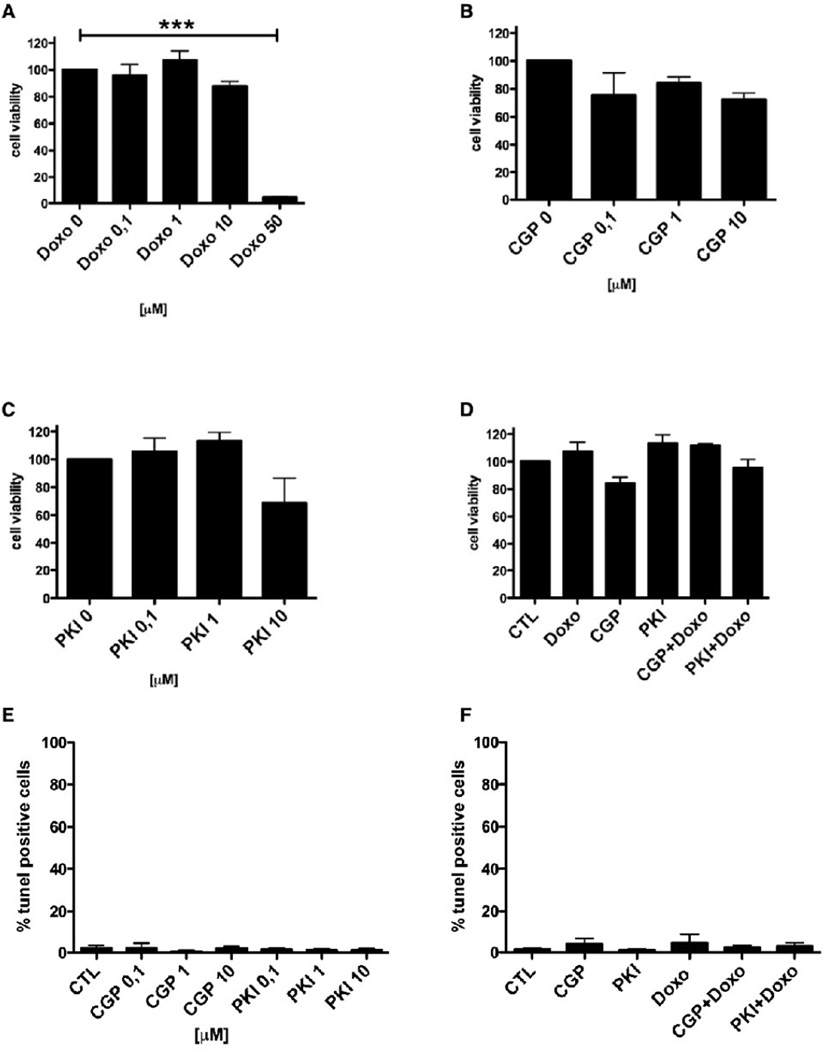
EGFR and ErbB2 inhibition does not induce cell death in cardiac myocytes
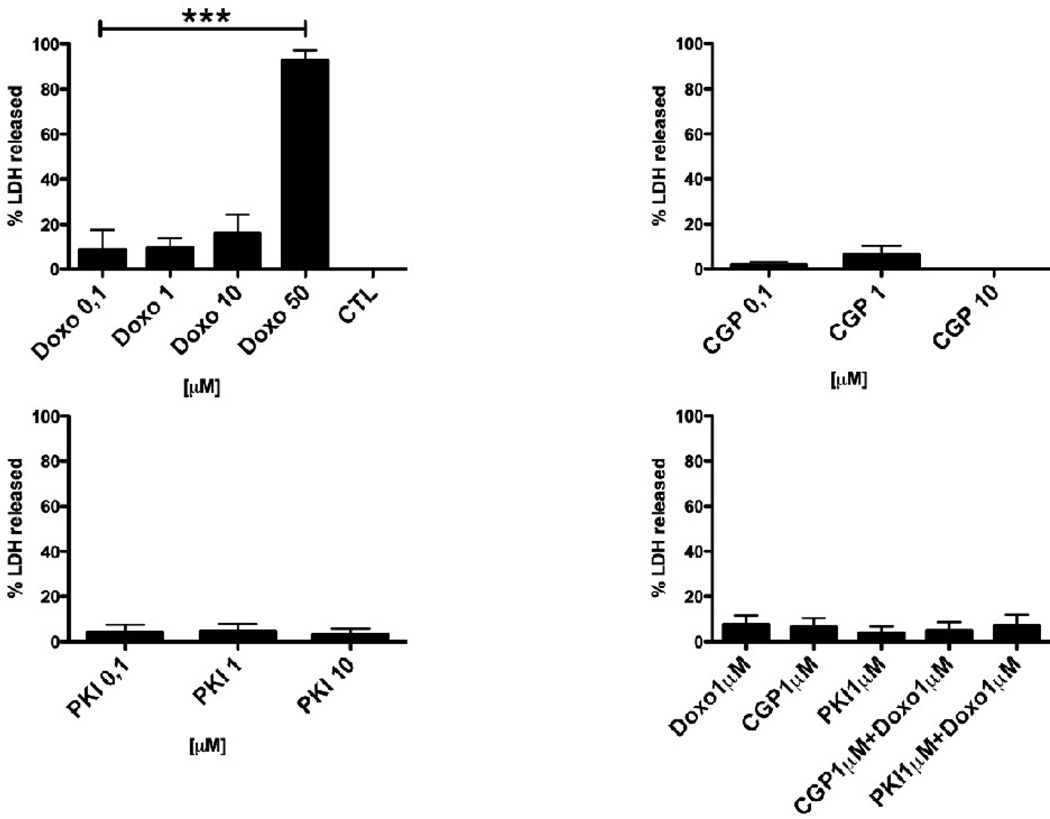
Single or dual inhibition of EGFR and ErbB2 by tyrosine kinase inhibitors induces cell death in several cancer cell lines [14]. Therefore, we tested the effect of PKI or CGP on necrosis and apoptosis. ARVM were treated with PKI or CGP for 48 hours and the release of lactate dehydrogenase (LDH) in the medium was measured as a marker of plasma membrane damage and cell necrosis (Fig 4). Although PKI at a concentration of ³1 µM induced myofibrillar damage, LDH release could not be detected at any concentration of inhibitors used. Similarly, CGP did not induce LDH release, indicating that neither dual EGFR/ErbB2 nor EGFR inhibition leads to myocyte necrosis. In contrast, exposure to Doxo (1, 10 and 50 µM) for 48 hours led to a dose-dependent increase of LDH release (1 µM 9.5±4.4%, n.s. vs. CTL, 10 µM 16.2±8.2%, n.s. vs. CTL and 50 µM 92.5±4.6%, p<0.001 vs. CTL; ANOVA p<0.0001). LDH release after treatment with Doxo 1 µM in combination with either PKI 1 µM or CGP 1 µM was not significantly different from CTL.
FIG. 4.
LDH release assay. Myocytes cultured for 10 days were treated for 48h with increasing doses of Doxo, PKI, CGP, or combinations of Doxo (1µM) with either PKI (1µM) or CGP (1µM). A significant increase in LDH release was observed only with Doxo 50 µM. ***p<0.0001
An anti-ErbB2 antibody has been reported to alter myocyte mitochondrial function in cultured neonatal cardiomyocytes [15]. We examined whether inhibition of EGFR alone or EGFR/ErbB2 inhibition would impair mitochondrial respiration and cell viability. ARVM were treated with PKI or CGP for 48 hours, followed by measurement of MTT conversion as an indicator of mitochondrial respiration and cell viability (Fig 5 A–D). Cells treated with PKI or CGP developed MTT levels comparable to CTL (set as 100%), indicating that inhibition of EGFR or EGFR/ErbB2 inhibition did not affect mitochondrial respiration. In contrast, a high concentration of Doxo (50 µM) induced a significant reduction in the MTT levels (4.3±0.35% of CTL p<0.0001 vs. CTL; n=5 experiments).
FIG. 5.
A-D: Mitochondrial activity (MTT assay). Myocytes cultured for 10 days were treated for 48h with increasing doses of Doxo, PKI, CGP, or combinations of Doxo (1µM) with either PKI (1µM) or CGP (1µM). Only with Doxo at 50µM a significant decrease in MTT conversion was observed. ***p<0.0001. E–F: DNA-degradation (TUNEL assay). Myocytes cultured for 10 days were treated for 48h with increasing doses of PKI or CGP (left), or combinations of Doxo (1µM) with either PKI (1µM) or CGP (1µM)(right). A significant increase in TUNEL-positive nuclei was not observed.
We then investigated, if EGFR/ErbB2 inhibition causes myocyte apoptosis. ARVM were treated with 0.01–10 µM PKI or CGP for 48 hours and tested for DNA degradation by TUNEL assay. Neither PKI- nor CGP-treated ARVM displayed signs of DNA fragmentation (Fig 5 E–F). Doxo 1 µM alone or in combination with PKI 1 µM or CGP 1 µM also did not induce apoptosis.
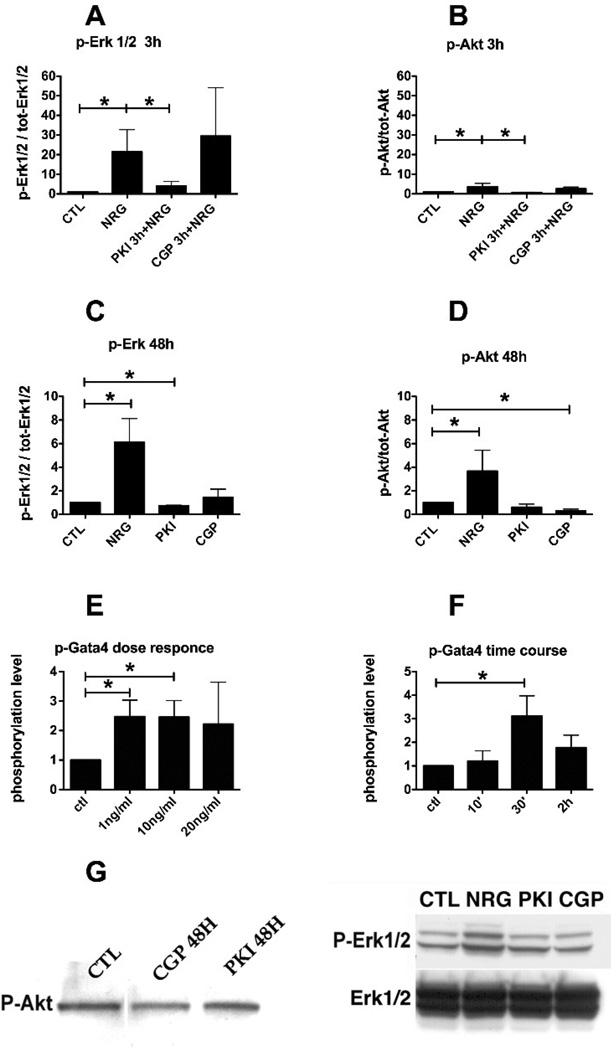
ErbB2 inhibition reduces the basal phosphorylation of Erk1/2
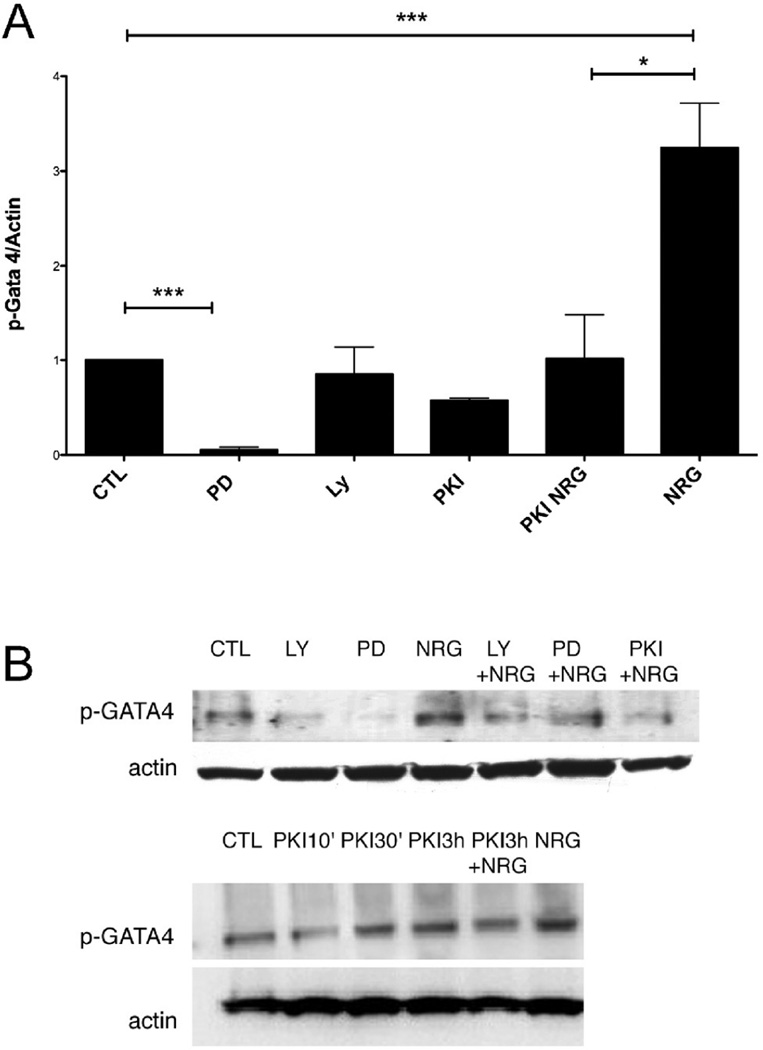
Known downstream signaling kinases of the ErbB system in cardiac myocytes are the MAP-kinase and PI3-kinase pathways [16]. We examined the effect of a 3 hours treatment in ARVM cultured for 10 days with 1 µM PKI or 1 µM CGP on baseline or NRG-induced phosphorylation of Erk1/2 and Akt in ARVM (Fig. 6A and 6B). PKI alone was able to block phosphorylation of Erk1/2 or Akt induced by NRG (10 minutes), whereas CGP had no effect on NRG stimulation. A 48h treatment with PKI 1 µM reduced the basal phosphorylation of Erk1/2 (0.7 fold of CTL) (Fig. 6C) whereas both PKI and CGP reduced the basal phosphorylation of Akt (0.6 and 0.3 fold of CTL respectively) (Fig. 6D). GATA4 is a transcription factor that modulates several genes encoding sarcomeric and other cardiac proteins, and is required during heart development and hypertrophy [17]. ARVM were treated with increasing doses of NRG for 30’ and lysates were probed for p-GATA4 (Fig 6E). Already the lowest concentration tested (1 ng/ml) induced a significant phosphorylation of GATA4 (Fig 6E). Cardiomyocytes were also treated for increasing durations with NRG 10 ng/ml and lysates were probed for p-GATA4 (Fig 6F). Here we observed a significant phosphorylation of GATA4 at the time point of 30 minutes (Fig 6F). ARVMs were treated with PD98059, LY294002, PKI, NRG, and PKI followed by NRG. We observed that inhibition of Erk1/2 but not of AKT led to a significant decrease in GATA4 phosphorylation. PKI alone did not significantly reduce GATA4 basal phosphorylation, but significantly blocked the NRG effect (Fig 7). Representative images of Western blots used for densitometry are shown in Fig 6G for p-Akt (left) and p-ERK, total-ERK (right).
FIG. 6.
Signaling pathways downstream of ErbB2. A and B: Cardiomyocytes were treated for 3h with either PKI 1 µM or CGP 1 µM followed by stimulation with NRG 10ng/ml for 10 minutes. C and D: Cardiomyocytes were treated for 48h with either PKI 1 µM or CGP 1 µM. E: cardiomyocytes were treated with increasing doses of NRG for 30’ and probed for p-GATA4. F: cardiomyocytes were treated for increasing durations with NRG 10 ng/ml and probed for p-GATA4. G: Western blot for p-Akt (left) and p-Erk1/2 (right) with inhibitors PKI 1 µM, CGP 1 µM. A–E, and F: *p<0.01; **p<0.001; ***p<0.0001; One-way ANOVA p<0.01.
FIG. 7.
GATA4 and NRG-activated signaling pathways. A. Cardiomyocytes were treated as indicated or for 30’ with either PD98059 50 µM (PD), LY294002 10 µM, PKI 1 µM, NRG 10 ng/ml, or with PKI 1 µM followed by NRG 10 ng/ml. B. representative western blots. N=3. *p<0.01, ***p<0.0001, One-Way ANOVA p<0.001.
Inhibition of p-Erk1/2 but not p-AKT induces myofibrillar structural damage
The analysis of the downstream signaling pathways (Fig. 6) suggested that possibly the maintenance of myofibrils depends on the MAPK/Erk signaling rather than on the PI3 kinase pathway. To test this hypothesis, cardiomyocytes were treated for 48h with MEK1/2 inhibitors (PD98059 and U126) or a PI3 kinase inhibitor LY294002 (LY). Only inhibition of MEK/Erk induced myofibrillar structural damage similar to that seen with inhibition of EGFR/ErbB2 by PKI (Fig 3C) or by anti-ErbB2 antibodies as previously reported [7].
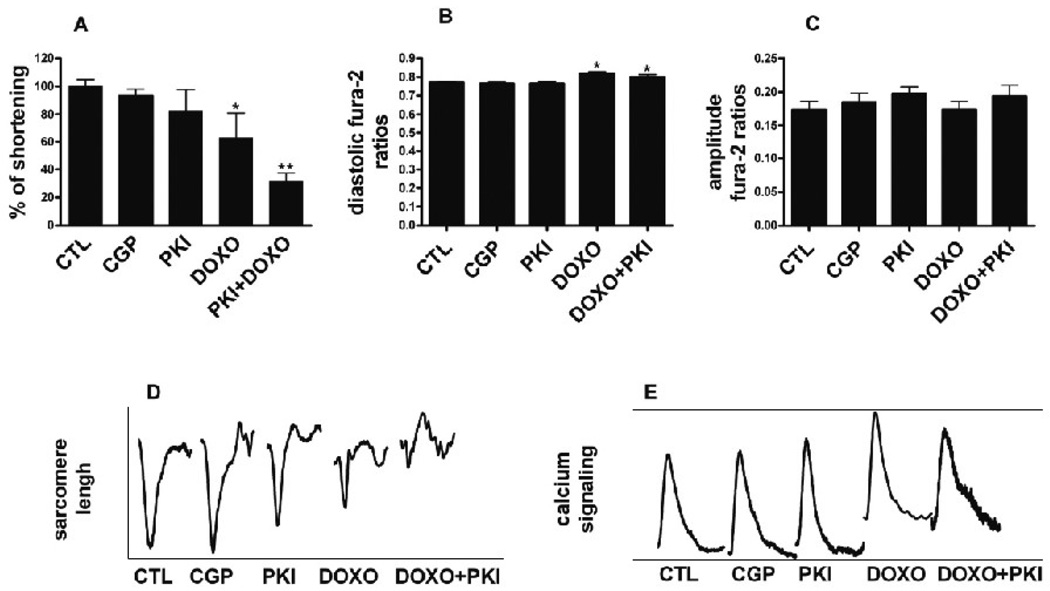
ErbB2 inhibition in combination with doxorubicin reduces myocytes contractility
Cultured cardiomyocytes were treated for 48h with PKI 1µM, CGP 1µM, or Doxo 1µM and electrically stimulated to study their contractile function (Fig. 8). The inhibition of EGFR by CGP had no effects on contractility, whereas there was a tendency towards inhibition by PKI (mean, 82.8% of CTL, n.s.) (Fig 8A and 8D). Doxo significantly reduced the fractional shortening of sarcomeres (63.2% of CTL p<0.01 vs. CTL) (Fig 8A and 8D), and the combination of PKI and Doxo almost completely abolished it (31.7% of CTL, p<0.001 vs. CTL) (Fig 8A and 8D). The amplitude of calcium transients (Fig 8C and 8E) was similar to untreated cells in all conditions, although in Doxo-treated cells diastolic calcium was significantly increased as previously reported (Fig 8B)[18]. Tracings of the contraction and calcium transients obtained by averaging the curves of several cells showed the loss of discernable myofibril contractions in the Doxo/PKI-treated ARVM (Fig 8D), while calcium transients were still present (Fig 8E) (n=3, 13 cells for each experimental condition measured).
FIG. 8.
Excitation-contraction coupling. Cardiomyocytes cultured for 10 days were treated for 48h with PKI 1 µM, CGP 1 µM, Doxo 1 µM, or PKI 1 µM in combination with Doxo 1 µM. Sarcomere shortening and calcium transients were determined in 2 Hz paced cells, as described in methods. Tracings (D and E) represent the average contraction and calcium transients of 39 cells (n=3, 13 cells for experiment). Results were normalized for the averaged values measured in untreated cells. A. Fractional shortening. *p<0.01 vs. CTL; **p<0.001 vs. CTL; One-way ANOVA p<0.00001. B. Diastolic fura-2 ratios. *p<0.01 vs. CTL; One-way ANOVA p<0.00001. C. Calcium amplitude D. Averaged contraction transients. E. Averaged calcium transients.
Discussion
Early in vivo experiments and recent clinical and biological data demonstrated a critical role for the ErbB family and its ligands in the heart. Mice lacking either ErbB2 or ErbB4 die in utero at day 10.5 due to cardiac malformation during development [1, 19]. At this stage ErbB2 and ErbB4 are expressed in the trabeculae, structures essential for blood circulation in the fetus. Mice lacking ErbB2 or ErbB4 did not develop such structures, which likely leads to death in utero. Systemic deletion of EGFR (ErbB1) leads to several phenotypes, mainly due to defects in epithelial tissue development [20, 21]. The expression of a mutated form of the EGF receptor in the heart caused valve defects [22, 23]. Concerning the ErbB3 isoform and ligands of the ErbB receptors, it was found that post-natal cardiac myocytes express the receptors ErbB2 and ErbB4, but not ErbB3, while cardiac microvascular endothelial cells express the NRG-1 ligand [11]. More recently cardiac restricted knock-out mice were obtained for ErbB2 or ErbB4. In both cases, mice developed dilated cardiomyopathy 8 weeks after birth with enlarged cardiac chambers and expression of fetal genes, such as ANP (atrial natriuretic peptide) and skeletal a-actin[3, 24, 25]. Isolated myocytes were more susceptible to Doxo treatment, as demonstrated by the higher incidence of cell death. Additionally, a role of the neuregulin/ErbB2 signaling system in the heart is illustrated by the fact, that serum ErbB2 levels are increased in patients with chronic heart failure [26].
In accordance with these in vivo models, inhibition of the ErbB2 receptor with the tyrosine kinase inhibitor PKI in cultured cardiomyocytes led to myofibrillar structural damage when given as single agent, whereas inhibition of the EGFR signaling by CGP did not lead to structural changes in the myocytes. Doxo is one of the most effective cancer therapeutic agents, with well-known cardiotoxic effects. The mechanisms of Doxo-induced cardiotoxicity comprises myofibrillar structural damage which can be seen both in endomyocardial biopsies of Doxo-treated cancer victims, as well as in myocyte cell culture as we have observed here [10, 27, 28]. The concomitant treatment of cardiomyocytes with Doxo and PKI significantly increased the induction of myofibrillar structural damage, suggesting that ErbB2 modulates either Doxo-induced activation of this degradative pathway, or the capacity of the myocyte to respond to this injury. Alternatively, ErbB2-dependent modulation of sarcomere stabilization via a focal adhesion kinase pathway may account for this synergistic injury [29]. This myofibrillar structural damage could be a fundamental mechanism for early cardiac dysfunction in the presence of ErbB2 inhibition [30].
Similar to studies in neonatal cardiomyocytes [31], we did not find DNA-degradation in cells treated with PKI or an antibody to ErbB2 in combination with clinically relevant doses of anthracyclines. The lack of apoptosis after inhibition of EGFR and ErbB2 would in theory be a form of reversible injury. It is remarkable that many of the patients with trastuzumab-related cardiac dysfunction can fully recover upon the initiation of cardiovascular treatment or ending trastuzumab therapy[32], consistent with the reversibility of this form of injury.
Analysis of the PI3-kinase and MAPK signalling pathways showed, that both PKI and CGP were able to reduce basal phosphorylation of Akt in culture, whereas only PKI decreased phosphorylation of Erk1/2, as did the ErbB2 monoclonal antibody clone 7.16.4 as observed in a previous study in the same culture model [7]. Specific inhibition of Erk1/2 by PD98059 or U126 (but not of Akt by LY294002) led to myofibrillar structural damage thereby confirming the essential role of Erk1/2 in the maintenance of myofibrils. The MAPK-Erk1/2 pathway is present in all cell types where it promotes cell proliferation and growth; in cardiomyocytes the role of Erk1/2 has been extensively investigated for years, but its role in the organization and maintenance of myofibrils is not yet fully understood. In cultured neonatal cardiomyocytes both PD98059 and U126, specific inhibitors of MEK1/2, were able to block myofibril reorganization and Erk1/2 activation upon stimulation with NRG [16, 33]. GATA4 is a transcription factor that is activated by the MAPK pathway in the heart and modulates several genes encoding sarcomeric proteins in development and hypertrophy [17]. In our experiments we observed that NRG induces phosphorylation of the transcription factor GATA4. These data taken together suggest the possibility that changes in the activity of ErbB2, Erk1/2, and GATA4 are linked to the induction of myofibrillar structural damage. Still, we cannot rule out the involvement of other mechanisms such as unspecific effects of the tyrosine kinase inhibitors on other signaling pathways.
The other signaling pathway induced by the ErbB receptors, the PI3-kinase pathway, has been found mainly involved in the protection of cardiomyocytes against cell death. Activation of Akt protects cultured cardiomyocytes against anthracycline-induced apoptosis [31] and promotes survival during ischemia-reperfusion injury [34], whereas inhibition of the Akt pathway increases cell death induced by Doxo treatment. Here, we observed that Akt phosphorylation was reduced after treatment with receptor tyrosine kinase inhibitors, but no increase in cell death was observed. It is possible, that cell death appears at higher levels of cellular stress with concomitant inhibition of the ErbB2-pathway. Consistent with the disruption of myofibrils, we observed a reduction in contractility when cells were treated with PKI and Doxo. It is interesting to note, that the magnitude of calcium transients was not affected by PKI, leading to the conclusion that the reduction in contractility is due to alterations of the contractile structure. Thus neither EGFR nor ErbB2 at baseline appear to regulate Ca++ handling as it pertains to excitation-contraction coupling.
Independent of cancer therapy, it is interesting to consider whether ErbB2-dependent sarcomere stabilization plays a role in the structural changes of myofibrils observed in other forms of cardiac injury and heart failure. Hibernation, stunning (both called “ischemic syndromes”), and heart failure are characterized by metabolic dysfunctions leading to ATP depletion, which may result in ErbB2 degradation and ‘resistance’ of myocytes to stimulation by endogenous NRG [35]. There is disruption of ErbB2 expression in patients with heart failure [36], which supports the overall notion that ErbB2 signaling plays an important role in maintenance of cardiac sarcomeres in general, and not only in the setting of exposure to chemotherapeutic agents.
Acknowledgments
This work was supported by research grants by Hoffmann La-Roche to D.B. Sawyer and T.M. Suter, Swiss National Science Foundation grants to T.M. Suter (SCORE-A 32-54985.98, 32-55136.98) and to C. Zuppinger (3100A0-120664), and by grant HL068144 from the National Institutes of Health to D.B. Sawyer. L. Pentassuglia was supported by a grant of the Swiss University Conference in the framework of the Swiss Cardiovascular Research and Training Network and by a fellowship from the Swiss National Science Foundation. F. Timolati was supported by a grant of the Gebert-Ruef foundation (GRS-038/01) to J.C. Perriard (Inst. of Cell Biology, ETHZ, Zurich). We thank Peter Traxler Ph.D. (Novartis Pharma) for materials and helpful discussions. We thank Franziska Seifriz for excellent technical assistance. We thank Prof. Robert Friis and Yitzhak Zimmer Ph.D. for carefully reading the manuscript. Laura Pentassuglia would like to thank in particular all the volunteers of the A.N.D.O.S. Varese (National Association of Women Operated for Breast Cancer) for sustaining her work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 2.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 3.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Pentassuglia L, Timolati F, Seifriz F, Abudukadier K, Suter TM, Zuppinger C. Inhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Exp Cell Res. 2007;313:1588–1601. doi: 10.1016/j.yexcr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Kondo RP, Apstein CS, Eberli FR, Tillotson DL, Suter TM. Increased calcium loading and inotropy without greater cell death in hypoxic rat cardiomyocytes. Am J Physiol. 1998;275:H2272–H2282. doi: 10.1152/ajpheart.1998.275.6.H2272. [DOI] [PubMed] [Google Scholar]
- 9.Eppenberger HM, Zuppinger C. In vitro reestablishment of cell- cell contacts in adult rat cardiomyocytes. Functional role of transmembrane components in the formation of new intercalated disk-like cell contacts. FASEB J. 1999;13:S84–S89. doi: 10.1096/fasebj.13.9001.s83. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 12.Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, O’Reilly T, Wood J, Zimmermann J. Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev. 2001;21:499–512. doi: 10.1002/med.1022. [DOI] [PubMed] [Google Scholar]
- 13.Hoekstra R, Dumez H, Eskens FA, van der Gaast A, Planting AS, de Heus G, Sizer KC, Ravera C, Vaidyanathan S, Bucana C, Fidler IJ, van Oosterom AT, Verweij J. Phase I and pharmacologic study of PKI166, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2005;11:6908–6915. doi: 10.1158/1078-0432.CCR-05-0720. [DOI] [PubMed] [Google Scholar]
- 14.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 15.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231–2238. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 16.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 17.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 18.Wang YX, Korth M. Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circ Res. 1995;76:645–653. doi: 10.1161/01.res.76.4.645. [DOI] [PubMed] [Google Scholar]
- 19.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 21.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 22.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 23.Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130:4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- 24.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 26.Perik PJ, de Vries EG, Gietema JA, van der Graaf WT, Smilde TD, Sleijfer DT, van Veldhuisen DJ. Serum HER2 levels are increased in patients with chronic heart failure. Eur J Heart Fail. 2006 doi: 10.1016/j.ejheart.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62:865–872. [PubMed] [Google Scholar]
- 28.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pentassuglia L, Sawyer DB. The role of Neuregulin/ErbB signaling in the heart. Exp Cell Res in press. 2008 doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 33.Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S, Sawyer DB. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem. 2005;280:13148–13152. doi: 10.1074/jbc.M410838200. [DOI] [PubMed] [Google Scholar]
- 36.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium Depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]