Figure 1.
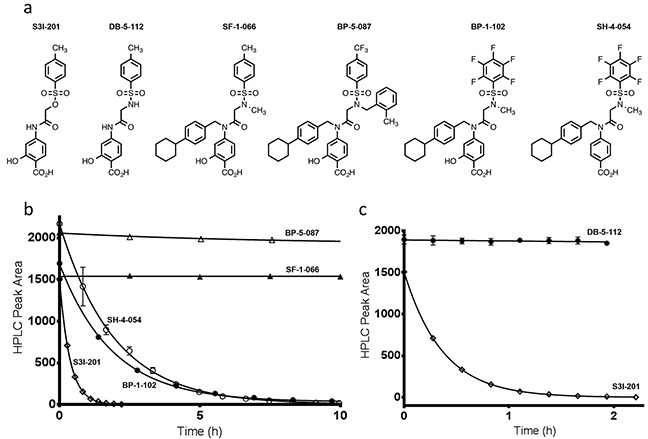
a. Chemical structures of S3I-201, DB-5-112, SF-1-066, BP-5-087, BP-1-102, and SH-4-054. b. Reaction time course for the decay in HPLC peak area for BP-5-087, SF-1-066, SH-4-054, BP-1-102, and S3I-201 in the presence of 10 mM GSH, 10 mM TCEP-HCl and 50 mM HEPES pH 7.4 (fitted parameters available in Supplementary Table S2). c. Shows the reactivity differences between the O-tosyl group of S3I-201 and the sulfonamide analogue DB-5-112 under identical reaction conditions, over the same reaction time course (fitted parameters available in Supplementary Table S3).
