Summary
Gut microflora and dysbiosis as an environmental factor has been linked to the pathogenesis of enthesitis‐related arthritis (JIA‐ERA); thus, we performed a proof‐of‐concept study of probiotics to modulate the gut‐flora and study the effects on immune and clinical parameters of children having JIA‐ERA. Forty‐six children with active JIA‐ERA were randomized to placebo or probiotic therapy along with non‐steroidal anti‐inflammatory drugs (NSAIDs) for 12 weeks. Patients were assessed using a six‐point composite disease activity index (mJSpADA) based on morning stiffness, joint count, enthesitis count, sacroiliitis/inflammatory back pain, uveitis and erythrocyte sedimentation rate/C‐reactive protein (ESR/CRP). Frequencies of T helper type 1 (Th1), Th2, Th17 and regulatory T cells in blood were measured using flow cytometry. Serum cytokines interferon (IFN)‐γ, interleukin (IL)−4, IL‐17, IL‐10, tumour necrosis factor (TNF)‐α and IL‐6 were measured by cytokine bead array using flow cytometer. The average age of 46 children (44 boys) was 15 ± 2.5 years and duration of disease was 3.5 ± 3 years. There was no significant difference in improvement in mJSpADA between the two groups (P = 0·16). Serum IL‐6 levels showed a decrease (P < 0·05) in the probiotic‐group. Th2 cell frequency (P < 0·05) and serum IL‐10 levels (P < 0·01) showed an increase in the placebo group, but again the probiotic use did not show a significant change in immune parameters when compared to the placebo. Adverse effects among the probiotic and placebo groups were diarrhea (36 versus 45%), abdominal pain (9 versus 20%), minor infections (4·5 versus 20%) and flatulence (23 versus 15%), respectively. Thus, we can conclude that probiotic therapy in JIA‐ERA children is well tolerated, but failed to show any significant immune or clinical effects over NSAID therapy.
Keywords: clinical trial, cytokine, juvenile arthritis, outcome measures, probiotics
Introduction
Enthesitis‐related arthritis (ERA) is a category of juvenile idiopathic arthritis (JIA), defined under the International League of Associations for Rheumatology (ILAR) classification 1. It is characterized by enthesitis and lower limb arthritis. JIA‐ERA has clinical similarities to adult spondyloarthritis (SpA), although development of radiographic sacroiliitis and inflammatory back pain occurs late in the course of disease. The classification of spondyloarthritis in adults and children is approached differently. Using the ILAR system of classification, most juvenile SpA is classified as ERA 2.
The pathogenesis of ERA is unknown, and current evidence indicates that genetic factors such as human leucocyte antigen (HLA)‐B27 renders a person susceptible to the influence of environmental factors which trigger the disease 3. Several clinical and laboratory observations have suggested a role of the gut in the pathogenesis of SpA and JIA‐ERA. Associations of intestinal inflammation with enteropathic forms of SpA such as reactive arthritis and inflammatory bowel disease (IBD) are well established. Subclinical gut inflammation with increased gut permeability has also been linked to SpA 4, 5.
JIA‐ERA shows a wide geographical difference with increased prevalence in Asia, which may be related to higher gastrointestinal infections in this region leading to recurrent gut inflammation and microbiome modulation 6. 16S ribosomal‐DNA sequencing of stool samples of patients with ankylosing spondylitis (AS) and JIA‐ERA revealed the differences in gut flora compared to healthy controls 7, 8. In AS, HLA‐B27 has been hypothesized to shape the gut flora leading to dysbiosis, thus affecting the immune system 9, 10. Frequent and early antibiotic exposure before diagnosis has been associated with an increased rate of developing JIA, perhaps mediated through gut flora modulation 11. Thus, commensal gut flora and its dysbiosis influenced by genetic and environmental factors can contribute to the pathogenesis of JIA‐ERA.
Probiotics can lead to immunomodulation via gut microflora alterations, as shown in the IL‐10 knock‐out mouse model (mice model for IBD) and IBD patients with pouchitis. The administration of probiotics has resulted in changes of serum cytokine levels favouring an anti‐inflammatory response, an increase in IL‐10 and transforming growth factor (TGF)‐β and a decrease in tumour necrosis factor (TNF)‐α 12, 13. This translates into a clinical benefit by prevention of pouchitis and maintenance of remission in ulcerative colitis patients 14, 15 and improvement of abnormal intestinal permeability in IBD patients 16. Although there is preclinical evidence of effect on arthritis by modulation of gut microbiome, a single randomized controlled trial in SpA patients did not show improvement with probiotic therapy. However, probiotic therapy did not cause any adverse effect 14, 15, 16, 17.
VSL#3 contains eight different strains, namely Streptococcus thermophilus, Bifidobacterium breve, B. longum, B. infantis, Lactobacillus acidophilus, L. plantarum, L. paracasei and L. delbrueckii. B. longus has been shown to improve immune function in elderly people as well as cause generation of healthy gut flora in young infants. Both Bifidobacterium and Lactobacilli have been shown to inhibit growth of pathogenic bacteria 18, 19. Probiotic VSL#3, along with standard of care, led to a higher rate of remission and fewer flares in children with ulcerative colitis 20 and also reduced endoscopic recurrence after surgery in Crohn's disease 21. Based on this evidence, we conducted a proof‐of‐concept study of probiotics (VSL#3) to determine their effect on clinical and immune inflammatory parameters in JIA‐ERA.
Methods
Patients
The study was conducted at the Department of Clinical Immunology at Sanjay Gandhi Postgraduate Institute of Medical Sciences, India. Ethical approval was obtained from the institute's ethics committee. Written informed consent was taken from all children and guardians (for children aged < 18 years) in accordance with the Declaration of Helsinki. Forty‐six children meeting the ILAR classification criteria for JIA‐ERA and active disease were enrolled from May 2013 to January 2015. Active disease was defined as the presence of arthritis (at least a single swollen joint) or enthesitis [Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) ≥ 2] with elevated erythrocyte sedimentation rate (ESR) > 30 mm at 1 h. The age range for inclusion into the study was 6–20 years. Children with diagnosed IBD, isolated axial disease without peripheral arthritis defined as juvenile AS and reactive arthritis were excluded. Thus, the study cohort included children with undifferentiated juvenile SpA that also satisfied the ILAR criteria for ERA. Children exposed to antibiotics, intra‐articular or systemic steroids, any other immunosuppressive drugs or symptomatic gastrointestinal infection in the last 6 weeks were also excluded from the study. They were advised to avoid curd or commercial probiotic preparations during the study period.
The trial was registered prospectively (CTRI/2012/08/002871) at the Clinical Trials Registry of India, an online public record system for clinical trials registration hosted by the Indian Council of Medical Research. Children were randomized to receive either probiotics or placebo for 12 weeks. Both groups were prescribed non‐steroidal anti‐inflammatory drugs (NSAIDs), had counselling and were advised physiotherapy according to need. The NSAIDs and maximum doses used were naproxen 15 mg/kg/day, indomethacin 4 mg/kg/day and etoricoxib 120 mg daily (only for those aged > 18 years). The dose and duration of the drug was recorded. An independent clinician using the variable permuted block randomization method performed randomization. The treatment allocation was performed using sequentially numbered sealed opaque containers that ensured blinding of both patient and assessor during the study period. The allocation code was revealed only after the completion of data analysis using the A and B groups to ensure unbiased statistical analysis.
Study treatment
VSL3 capsules manufactured by Sun Pharmaceuticals (Mumbai, India) containing eight different strains of 112·5 billion bacterial cells per capsule were given orally twice daily for 12 weeks as probiotics. Capsules filled with corn powder were used as placebo, prepared in the department; care was taken to ensure the same colour, appearance and texture of both drugs. Children were followed‐up at the end of weeks 6 and 12. The study drug was dispensed as two sets, one at the time of enrolment and the second at week 6, in a numbered sealed opaque container having 84 capsules. They were advised to keep the drug in a cool, moisture‐free, dark place, preferably a refrigerator at 2–8°C. The remaining drugs and containers were collected to ensure proper compliance. Toxicity of intervention for example diarrhoea, constipation, flatulence, bloating, vomiting or any other unexpected symptoms or infections was documented at each visit, along with the clinical assessment.
Clinical assessment
Efficacy was assessed using clinical parameters such as patient global improvement (PGI, %), duration of early morning stiffness (EMS, min), number of swollen joints (SJC, 66 joint count excluding hip joints), number of tender joints (TJC, 68 joint count), acute anterior uveitis, enthesitis count (MASES plus documentation of any other sites involved), presence of clinical sacroiliitis [tenderness on palpation with positive Patrick's Flexion, ABduction, and External Rotation (FABER) test] or inflammatory back pain (IBP) as per the assessment of the Spondyloarthritis International Society (ASAS) criteria 22. In addition, NSAIDs use was monitored to determine any reduction with the use of probiotics.
Laboratory tests
Acute‐phase proteins, ESR (Westergren method) and serum C‐reactive protein (CRP; nephelometry) were estimated as laboratory markers of disease activity. DNA was extracted from ethylene diamine tetraacetic acid (EDTA) blood samples using the salting‐out method. The amplification refractory mutation system polymerase chain reaction (PCR) was used for human leucocyte antigen (HLA)‐B27 typing applying three B27‐specific primers: 1, forward B1 (5′‐GCT ACG TGG ACG ACA CGC T‐3′) and 2, reverse B2 (5′‐CTC GGT CAG TCT GTG CCT T‐3′) and B3 (5′‐TCT CGG TAA GTC TGT GCC TT‐3′). A conserved intronic region of HLA‐DR was also amplified as an internal control using two primer sets: C1 (5′‐TGC CAA GTG GAG CAC CCA A‐3′) and C2 (5′‐GCA TCT TGC TCT GTG CAG AT‐3′) 23.
Staining of cells for flow cytometry
Venous blood was collected at baseline in tubes containing lithium heparin (before starting probiotic administration) and at the end of probiotic treatment (week 12); 500 µl whole blood was cultured in 500 µl RPMI media supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic. Cells were stimulated with 50 ng phorbol myristate acetate (PMA) (Sigma, St Louis, MO, USA) and 1 µg/ml ionomycin (Sigma). Ten µg/ml brefeldin A (Sigma) was added as secretion inhibitor. Cells were cultured in an incubator for 6 h at 37°C with 5% carbon dioxide followed by intracellular staining. Samples were acquired using a Beckman Coulter (Pasadena, CA, USA) flow machine and analysed with Navios software.
Cells were surface‐stained with anti‐CD4 fluorescein isothiocyanate (FITC) and anti‐CD3 allophycocyanin (APC). Anti‐interferon (IFN)‐γ phycoerythrin (PE), anti‐IL‐17A peridinin chlorophyll (Per‐CP) and anti‐interleukin (IL‐4) PE (BD Bioscience, San Jose, CA, USA) were used as intracellular antibodies. CD4+IFN‐γ+ cells, CD4+IL‐4+ cells and CD4+IL‐17A+ cells were measured in CD3 gate and considered as T helper type 1 (Th1), Th2 and Th17, respectively.
For regulatory T cell (Treg) staining the forkhead box protein 3 (FoxP3) staining kit (BD Pharmingen, Franklin Lakes, NJ, USA) was used. Cells were surface‐stained with anti‐CD4 FITC and anti‐CD25 APC and anti‐FoxP3 PE was used as intracellular antibody. Staining was performed as per the manufacturer's instructions. CD25+Foxp3+ cells in CD4 gate were considered as Treg cells.
Measurement of cytokine levels
Serum (2·5ml) was separated from the venous blood samples collected at baseline and week 12 and stored at −80°C. IFN‐γ, IL‐4, IL‐17, IL‐10, IL‐6 and tumour necrosis factor (TNF)‐α levels were measured using the BD cytometric bead array (CBA) human Th1/Th2/Th17 cytokine kit (BD Bioscience) following the instructions given in the manual using a flow cytometer (BD Canto II). The limits of detection for IFN‐γ, IL‐4, IL‐17, IL‐10, IL‐6 and TNF‐α were 3·7 pg/ml, 4·9 pg/ml, 18·9 pg/ml, 4·5 pg/ml, 2·4 pg/ml and 3·8 pg/ml, respectively. Data were analysed using FCAP Array software.
Outcome measures
The primary outcome measure was a statistically higher improvement in modified juvenile spondyloarthropathy disease activity (mJSpADA) score in the probiotic group compared to the placebo group. mJSpADA is a six‐point composite disease activity index based on morning stiffness, joint count, enthesitis count, sacroiliitis/inflammatory back pain, uveitis and ESR/CRP. This score is derived from a recently validated index (JSpADA) 24. Each item is scored as 1, thus the score varied from 0 to 6. Composite measures avoid problems of multiple comparisons.
The secondary outcome was skewing of immune parameters, i.e. T cell subset frequencies and serum cytokine towards an anti‐inflammatory response (decrease in Th1/Th17 and increase in Th2/Treg, decrease in serum IL‐6, TNF‐α, IFN‐γ, IL‐17 and increase in IL‐10/IL‐4) with probiotic therapy. Additional outcome measures included improvement from baseline in individual parameters of disease activity and safety of probiotic use in children.
Statistical analysis
The trial was a proof‐of‐concept study, so the sample size was kept small. The sample size of 40 was calculated based on the following assumptions: 95% confidence interval, 80% power, expected difference in mJSpADA index change between two groups of 2 and variance of 5, as the score has a value from 0 to 6. Adding 15% dropout to this calculation, the final sample size was 46. The data were analysed and projected graphically using IBM SPSS Statistics Software, version 16 and GraphPad Prism, version 6·00 for Mac (trial version; GraphPad Software, San Diego, CA, USA). The Mann–Whitney U‐test was used for intergroup comparison while Wilcoxon's signed‐rank test was used for intragroup comparison. P < 0·05 was considered significant.
Results
Patients
The average age of 46 children (44 boys) at enrolment was 15 ± 2·5 years and disease duration was 3·5 ± 3 years. Table 1 shows the baseline characteristics of all the children and two groups separately. There was no difference between the groups at baseline; 76% (35) of children had a disease duration ≤ 5 years and 94% (43) were first visits to the clinic; 48% (22) had episodic history of arthritis while others had a chronic progressive course; and 28% (13) had at least one damaged joint. All children at the time of enrolment were using suboptimal doses of NSAID therapy. None had used steroids or other immunosuppressive drugs during the last 6 weeks. Twenty‐two per cent (10) of children had a past history of steroid use, 13% (six) had used methotrexate and 17% (eight) had used sulphasalazine. They were never treated with biological drugs, for example anti‐TNF therapy.
Table 1.
Baseline characteristics, disease activity and immune parameters of the participants.
| Placebo group (n = 23) | Probiotic group (n = 23) | |
|---|---|---|
| Baseline characteristics | ||
| Enrolment age | 15 (14–16) | 16 (13–19) |
| Disease duration | 3 (1–6) | 3 (1·5–5) |
| Age at onset | 11 (10–14) | 13 (10–14) |
| Gender | All boys | 2 girls |
| Enthesitis | 11 | 18 |
| HLA‐B27‐positive | 22 | 21 |
| Past uveitis | 3 | 1 |
| IBP | 7 | 5 |
| Sacroilitis | 11 | 13 |
| Family history | 5 | 5 |
| Disease activity parameters | ||
| EMS min | 45 (23–60) | 30 (0–60) |
| TJC (68) | 3 (2–4·5) | 3 (2–5) |
| SJC (66) | 2 (2–3) | 2 (2–3) |
| ESR mm at 1 h | 80 (47–95) | 80 (40–115) |
| CRP mg/dl | 2·8 (1·4–6) | 8 (3–9·5) |
| mJSpADA‐ESR (6) | 3·5 (2·5–4·3) | 3 (2–4·5) |
| mJSpADA‐CRP (6) | 3·5 (2·5–4·8) | 3 (2·5–4·5) |
| Immunological parameters | ||
| Th1 (%) | 6·5 (3·6–9·2) | 6·6 (5–8·8) |
| Th2 (%) | 0·3 (0·1–0·5) | 0·6 (0·2–1·1) |
| Th17 (%) | 1·2 (0·8–1·7) | 1·5 (0·7–1·7) |
| Treg (%) | 2·7 (1·6–3·7) | 2·6 (1·8–3·3) |
| IL‐6 pg/ml | 33 (24–122) | 53 (17–135) |
| TNF‐α pg/ml | 1 (0·4–3·8) | 0·9 (0·1–2·3) |
| IFN‐γ pg/ml | 0 (0–2·2) | 0 (0–1·8) |
| IL‐4 pg/ml | 0 (0–1·7) | 0 (0–0) |
| IL‐17 pg/ml | 44 (24–60) | 36 (4–57) |
| IL‐10 pg/ml | 1·1 (0·7–1·4) | 1 (0·6–2·1) |
IBP = inflammatory back pain; EMS = early morning stiffness; mJSpADA‐ESR and mJSpADA‐CRP = six‐point composite disease activity index using erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), respectively; Treg = regulatory T cells; Th = T helper; TJC = total joint count; SJC = swollen joint count; HLA = human leucocyte antigen.
Compliance
Forty children completed the trial, with the exclusion of six (13%). One child in the placebo group was excluded due to a drug‐dispensing error (unlabelled box being delivered). The outline of the trial is shown in Fig. 1. All the containers were collected at weeks 6 and 12 follow‐up visits, respectively. Most of the drug dispensed (84 capsules) was consumed with a pill‐count of 83 [interquartile range (IQR) = 78–84] and 83 (IQR = 80–84) at weeks 6 and 12 of follow‐up, respectively.
Figure 1.
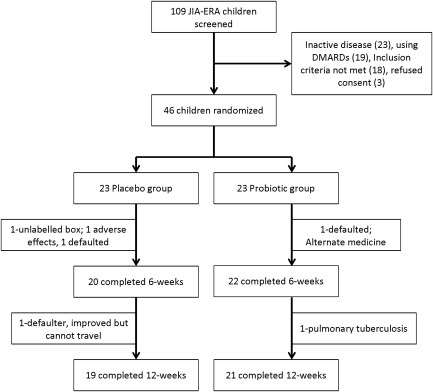
Flow diagram representing the trial layout.
Clinical response and NSAID requirement
All the children were treated with optimal doses of regular NSAIDs with the probiotic or placebo in the respective groups. The NSAIDs used were indomethacin (30, 17 in the placebo group and 13 in the probiotic group), naproxen (6, two in the placebo‐group and four in the probiotic‐group) and etoricoxib (four, all in the probiotic group). The difference in NSAID use was not different between two groups [χ2 = 2·72; P = not significant (n.s.)]. Both groups had a significant clinical response at the end of the trial (Supporting information, Table S1), but the primary outcome variable (difference in change in mJSpADA) between the two groups was not significantly different (Table 2). At week 6, a change in the NSAID was required for three children, two in the placebo group and one in the probiotic group, while a dose increase was required for one child in each group. There was no reduction in the NSAID dose requirement after 12 weeks of therapy, the median percentage dose reduction being 0% (IQR = 0–20), and there was also no difference between the groups, placebo 0% (IQR = 0–12) and probiotic 0% (IQR = 0–21).
Table 2.
Comparison of median changes after 12 weeks in clinical and immune parameters between the probiotic and placebo group.
| Probiotic group (n = 21) | Placebo group (n = 19) | P‐value | |
|---|---|---|---|
| Disease activity parameters | |||
| mJSpADA‐ESR | −0·5 (−2 to 0) | −2 (−2·5 to −1) | 0·06 |
| mJSpADA‐CRP | −1 (−2·8 to 0) | −2 (−2·5 to −1·5) | 0·16 |
| PGI % | 70 (35 to 80) | 70 (25 to 90) | 0·8 |
| EMS (min) | −10 (−60 to 5) | −30 (−60 to 0) | 0·6 |
| TJC (0–68) | −1 (−3·5 to 1·5) | −2 (−4 to −2) | 0·06 |
| SJC (0–66) | −1 (−2·5 to −1) | −2 (−3 to −1) | 0·15 |
| Enthesitis count | 0 (−2 to 1·5) | 0 (0 to 2) | 0·5 |
| ESR mm | −15 (−61 to 10) | −40 (−66 to 2) | 0·35 |
| CRP mg/dl | −3·3 (−8·3 to 0) | −1·5 (−4 to −0·2) | 0·36 |
| Immune parameters | |||
| Th1% | 0·1 (−1·9 to 2·5) | 0·8 (−3·3 to 3·5) | 0·7 |
| Th2% | 0·2 (−0·2 to 0·6) | 0·3 (−0·1 to 1) | 0·5 |
| Th17% | −0·07 (−0·8 to 0·45) | 0·4 (−0·4 to 0·8) | 0·3 |
| Treg% | 0·4 (−1·6 to 1·3) | 1·1 (−0·4 to 1·9) | 0·2 |
| IL‐6 pg/ml | −37 (−102 to −1·7) | −9·2 (−40 to 16·8) | 0·13 |
| TNF‐α pg/ml | −0·75 (−2·3 to 0·5) | 0·14 (−2·2 to 0·9) | 0·5 |
| IFN‐γ pg/ml | 0 (−1·2 to 0) | 0 (−2·4 to 0) | 0·5 |
| IL‐4 pg/ml | 0 (0 to 1) | 0 (−8·6 to 0·5) | 0·3 |
| IL‐17 pg/ml | 2·8 (−26 to 29) | −20 (−42 to 6) | 0·26 |
| IL‐10 pg/ml | −0·75 (−2 to 0·6) | 1 (−0·6 to 1·9) | 0·013* |
Values expressed as median with interquartile range, Mann–Whitney U‐test used to compare the medians. *P < 0·05. mJSpADA‐ESR and mJSpADA‐CRP, six‐point composite disease activity index using erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), respectively; PGI = patient‐reported global improvement; EMS = early morning stiffness; TJC = tender joint count; SJC = swollen joint count; Treg = regulatory T cells; IL = interleukin; TNF = tumour necrosis factor; Th = T helper.
Immune response
Whole blood T cell subsets, for example Th1, Th2, Th17 and Treg frequency, did not show significant changes with 12 weeks of probiotic therapy. There was an increase in median Th2 cell frequencies from 0·3 to 0·6% in the placebo‐group, which was statistically significant (P < 0·05). Serum cytokine levels, for example IFN‐γ, IL‐4, IL‐17, IL‐10 and TNF‐α, also did not show significant changes with the probiotic therapy. Serum IL‐6 levels showed a decline from 53 pg/ml (IQR = 12–15) to 11·4 pg/ml (IQR = 6·6–21) with the use of probiotics (P = 0·007). The median serum IL‐10 level increased in the placebo group 1 pg/ml (IQR = −0·6 to 1·9, P = 0·01) (Supporting information, Table S1). The difference between increase in serum IL‐10 after treatment was higher in the placebo group (P = 0·013) (Table 2).
Adverse effects
Adverse effects in the probiotic and placebo groups were diarrhoea, abdomen pain, flatulence, constipation, appetite loss and minor infections; for example, upper respiratory tract and skin infections. The adverse effects were not higher in the probiotic group (Table 3). Two patients were withdrawn due to side effects, one in the placebo group due to severe diarrhoea and the other in the probiotic group due to pulmonary tuberculosis.
Table 3.
Adverse events in children exposed to therapy.
| Adverse effects | Placebo n = 20 (%) | Probiotic n = 22 (%) |
|---|---|---|
| Any adverse event | 11 (55) | 9 (41) |
| Serious adverse event* | 1 (5) | 1 (4·5) |
| Diarrhea | 9 (45) | 8 (36) |
| Flatulence | 3 (15) | 5 (23) |
| Abdomen pain | 4 (20) | 2 (9) |
| Minor infections | 4 (20) | 1 (4·5) |
| Arthritis flare | 4 (20) | 1 (4·5) |
| Constipation | 2 (10) | 0 |
| Appetite loss | 2 (10) | 1 (4·5) |
*Requiring withdrawal of the treatment.
Discussion
Gut microflora regulates the immune response and is implicated in various rheumatic diseases, including JIA‐ERA; thus, the use of either prebiotics or probiotics are attractive therapeutic candidates for methods to modulate it. This proof‐of‐concept study failed to show any benefit, clinical or immunological, of probiotic over the regular use of NSAIDs in patients with JIA‐ERA. The groups had significant clinical improvement as well as serum cytokine level changes in favour of anti‐inflammatory milieu; for example, a fall in IL‐6 and a rise in IL‐10 and Th2 cells, but the changes were not different between the groups. Probiotic use was safe in the children.
Data on probiotic usage for treating chronic inflammatory disease is limited. Studies in ulcerative colitis showed modest benefits with reduction in flare rates and better quality of life 25. The present work is a proof‐of‐concept study, so the number of patients enrolled was small. Similarly, studies in arthritis are limited and also have small sample sizes 26, 27, 28, 29. In patients with rheumatoid arthritis (RA), studies with small number of patients showed a reduction in disease activity with probiotic usage 26, 27 while others failed to show a response 28. The randomized controlled trial in adult SpA also did not show a clinical response with probiotic therapy 17. Similar observations were reported in an internet‐based randomized controlled trial among SpA patients 29. Together, these data suggest that the clinical efficacy of probiotics in autoimmune diseases still needs to be established.
At the outset of the trial, one of our prime concerns was safety and dose of probiotic use in children. The adverse effects with the dose of probiotics used in the study were similar to the placebo group. Most of the studies using probiotics in adults have reported minimal adverse effects, such as gastrointestinal disturbances 17, 25, 26, 27, 28, 30, 31.
The clinical efficacy of probiotics may be due to multiple mechanisms; for example, increase in epithelial integrity, competition for nutrients with pathobionts and effects on systemic immune responses 32. In addition to clinical efficacy, we studied the effects of probiotic on systemic immune parameters, i.e. serum cytokines and relative T cell subset frequencies in blood. A 12‐week probiotic therapy had no effect on these parameters. Certain anti‐inflammatory changes seen in these parameters were not specific to the probiotic group, and can be attributed to the anti‐inflammatory effects of NSAID therapy. Most of the data on T cells have been generated in animals using gut‐associated lymphoid tissue and mesenteric lymph nodes 33, 34, 35. In animals fed with probiotics, local T cells have been shown to skew towards Tregs in contrast to Th17 cells in response to antigens or subdue arthritis by inhibiting the Th1 response 33, 34, 35.
Human data for the effect of probiotics on systemic immune parameters are limited. In RA patients, daily probiotic supplementation containing L. casei resulted in decreased serum levels of IL‐6, IL‐12 and TNF‐α and an increase in IL‐10 levels 26, while another study in RA patients using probiotics containing L. rhamnosus and L. reuteri showed insignificant changes in serum cytokine levels 28. Thus, the immune modulation of probiotic might be limited to the local gut mucosal immunity, with minor effects reflected on the systemic immune parameters. These effects may also vary with the strains of bacteria present in the probiotic used. In humans, and particularly children, it is ethically challenging to study the effects using local intestinal biopsies and thus the effects on gut immune cells and cytokines.
One prime reason for the lack of probiotic effect in this study could be its contents. A recent study addressing dysbiosis in JIA‐ERA reported less abundance of the Faecalibacterium prausnitzii and Lachnospiraceae families and higher abundance of Bifidobacterium and Bacteroides compared to healthy controls 7. Our own data have shown an increase in Bacteroides and decrease in Prevotella in patients with ERA (unpublished work). In contrast, a gut microbiome study in RA has shown an increase in Prevotella and a decrease in Bacteroides species 36. The study in adult patients with AS has shown an increase in Lachnospiraceae, Ruminococcaceae, Rickenellaceae and Bacteroidaceae and a decrease in Veillonellaceae and Prevotellaceae 37. The different studies show different dysbiosis, and thus it is highly possible that use of the same probiotic content for all autoimmune diseases may not work. For example, in Crohn's disease, probiotics containing Lactobacillus alone failed to show any clinical benefit while those containing both Bifidobacterium and Lactobacillus improved the symptoms 25, 30, 31. It is probable that the use of specific probiotic to correct the dysbiosis in a particular autoimmune disease may result in clinical efficacy.
There were certain limitations to our study. The children had an established disease with a median duration of 3·5 years. The duration of probiotic therapy was 12 weeks. A study by Yatsuneko et al. has shown that gut microflora takes almost 3 years after birth to evolve towards a stable configuration 38. Therefore, it might be difficult to modulate gut microflora in older children and with short duration of the therapy. Further use of NSAIDs as a background drug may have masked the efficacy of probiotics. These drugs are known to have high clinical efficacy in SpA 39. NSAID use can also modify gut microflora 40.
Our knowledge regarding dysbiosis and ways to modulate dysbiosis and its effect on the immune system in joint diseases is still limited and evolving. Thus, the present study in the light of its limitations cannot completely refute the clinical efficacy of probiotics.
Disclosure
The authors have declared no disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Changes after 12 weeks from baseline in disease activity and immune parameters within the groups.
Acknowledgements
We thank all the patients who participated in this study.
References
- 1. Petty RE, Southwood TR, Manners P et al International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton 2001. J Rheumatol 2004; 31:390–2. [PubMed] [Google Scholar]
- 2. Colbert RA. Classification of juvenile spondyloarthritis: enthesitis‐related arthritis and beyond. Nat Rev Rheumatol 2010; 6:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007; 369:767–78. [DOI] [PubMed] [Google Scholar]
- 4. Picco P, Gattorno M, Marchese N et al Increased gut permeability in juvenile chronic arthritides. A multivariate analysis of the diagnostic parameters. Clin Exp Rheumatol 2000; 18:773–8. [PubMed] [Google Scholar]
- 5. Orlando A, Renna S, Perricone G, Cottone M. Gastrointestinal lesions associated with spondyloarthropathies. World J Gastroenterol 2009; 15:2443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saurenmann RK, Rose JB, Tyrrell P et al Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum 2007; 56:1974–84. [DOI] [PubMed] [Google Scholar]
- 7. Stoll ML, Kumar R, Morrow CD et al Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis‐related arthritis. Arthritis Res Ther 2014; 16:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill T, Asquith M, Rosenbaum JT, Colbert RA. The intestinal microbiome in spondyloarthritis. Curr Opin Rheumatol 2015; 27:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin P, Bach M, Asquith M et al HLA‐B27 and human β2‐microglobulin affect the gut microbiota of transgenic rats. PLOS ONE 2014; 9:e105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenbaum JT, Davey MP. Hypothesis: time for a gut check: HLA B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum 2011; 63:3195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arvonen M, Virta LJ, Pokka T, Kröger L, Vähäsalo P. Repeated exposure to antibiotics in infancy: a predisposing factor for juvenile idiopathic arthritis or a sign of this group's greater susceptibility to infections? J Rheumatol 2015; 42:521–6. [DOI] [PubMed] [Google Scholar]
- 12. Schultz M, Veltkamp C, Dieleman LA et al. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin‐10‐deficient mice. Inflamm Bowel Dis 2002; 8:71–80. [DOI] [PubMed] [Google Scholar]
- 13. Borruel N, Carol M, Casellas F et al Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut 2002; 51:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gionchetti P, Rizzello F, Helwig U et al Prophylaxis of pouchitis onset with probiotic therapy: a double‐blind, placebo‐controlled trial. Gastroenterology 2003; 124:1202–9. [DOI] [PubMed] [Google Scholar]
- 15. Kruis W, Fric P, Pokrotnieks J et al Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004; 53:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H et al Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol 2008; 43:842–8. [DOI] [PubMed] [Google Scholar]
- 17. Jenks K, Stebbings S, Burton J, Schultz M, Herbison P, Highton J. Probiotic therapy for the treatment of spondyloarthritis: a randomized controlled trial. J Rheumatol 2010; 37:2118–25. [DOI] [PubMed] [Google Scholar]
- 18. Kalina WV, Mohamadzadeh M. Lactobacilli as natural enhancer of cellular immune response. Discov Med 2005; 5:199–203. [PubMed] [Google Scholar]
- 19. Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 2005; 22:495–512. [DOI] [PubMed] [Google Scholar]
- 20. Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 2009; 104:437–43. [DOI] [PubMed] [Google Scholar]
- 21. Fedorak RN, Feagan BG, Hotte N et al The probiotic VSL#3 has anti‐inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol 2015; 13:928–35. [DOI] [PubMed] [Google Scholar]
- 22. Sieper J, van der Heijde D, Landewé R et al New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009; 68:784–8. [DOI] [PubMed] [Google Scholar]
- 23. Tonks S, Marsh SG, Bunce M, Bodmer JG. Molecular typing for HLA class I using ARMS‐PCR: further developments following the 12th International Histocompatibility Workshop. Tissue Antigens 1999; 53:175–83. [DOI] [PubMed] [Google Scholar]
- 24. Weiss PF, Colbert RA, Xiao R et al Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res (Hoboken) 2014; 66:1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saez‐Lara MJ, Gomez‐Llorente C, Plaza‐Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int 2015; 2015:505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaghef‐Mehrabany E, Alipour B, Homayouni‐Rad A, Sharif SK, Asghari‐Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014; 30:430–5. [DOI] [PubMed] [Google Scholar]
- 27. Alipour B, Homayouni‐Rad A, Vaghef‐Mehrabany E et al Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double‐blind clinical trial. Int J Rheum Dis 2014; 17:519–27. [DOI] [PubMed] [Google Scholar]
- 28. Pineda ML, Thompson SF, Summers K, de Leon F, Pope J, Reid G. A randomized, double‐blinded, placebo controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit 2011; 17:CR347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brophy S, Burrows CL, Brooks C, Gravenor MB, Siebert S, Allen SJ. Internet‐based randomized controlled trials for the evaluation of complementary and alternative medicines: probiotics in spondyloarthropathy. BMC Musculoskelet Disord 2008; 9:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujimori S, Tatsuguchi A, Gudis K et al High dose probiotic and prebiotic cotherapy for remission induction of active Crohn's disease. J Gastroenterol Hepatol 2007; 22:1199–204. [DOI] [PubMed] [Google Scholar]
- 31. Steed H, Macfarlane GT, Blackett KL et al Clinical trial: the microbiological and immunological effects of synbotic consumption – a randomized double‐blind placebo‐controlled study in active Crohn's disease. Aliment Pharmacol Ther 2010; 32:872–83. [DOI] [PubMed] [Google Scholar]
- 32. Howarth GS, Wang H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 2013; 5:58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsumoto S, Hara T, Hori T et al Probiotic Lactobacillus‐induced improvement in murine chronic inflammatory bowel disease is associated with the down‐regulation of pro‐inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol 2005; 140:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng B, van Bergenhenegouwen J, Overbeek S et al Bifidobacterium breve attenuates murine dextran sodium sulfate‐induced colitis and increases regulatory T cell responses. PLOS ONE 2014; 9:e95441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1‐mediated murine colitis by inducing IL‐10 and IL‐10‐dependent TGF‐beta‐bearing regulatory cells. J Immunol 2005; 174:3237–46. [DOI] [PubMed] [Google Scholar]
- 36. Scher JU, Sczesnak A, Longman RS et al Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013; 2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Costello ME, Ciccia F, Willner D et al Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol 2015; 67:686–91. [DOI] [PubMed] [Google Scholar]
- 38. Yatsunenko T, Rey FE, Manary MJ et al Human gut microbiome viewed across age and geography. Nature 2012; 486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kroon FP, van der Burg LR, Ramiro S et al Non‐steroidal anti‐inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non‐radiographic axial spondyloarthritis). Cochrane Database Syst Rev 2015; 7:CD010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rogers MA, Aronoff DM. The influence of non‐steroidal anti‐inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016; 22:178.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Changes after 12 weeks from baseline in disease activity and immune parameters within the groups.


