Summary
The aim of this study is to investigate the effect of the native, citrullinated or carbamylated type II human collagen T cell‐ and B cell‐epitopes on the adaptive immune response in rheumatoid arthritis (RA). Peripheral blood T and B cells obtained from a human leucocyte D4‐related (antigen DR4− HLA‐DR4)+ woman with early RA, her healthy monozygotic twin and an unrelated HLA‐DR3+ woman with early RA were analysed for activation (CD154/CD69), apoptosis (annexin/7‐aminoactinomycin), cytokine production [interferon (IFN)γ/interleukin (IL)−17/IL‐4/IL‐10/IL‐6] and functional phenotype (CD45Ra/CCR7) after stimulation with the collagen native T cell epitope (T261‐273), the K264 carbamylated T cell epitope (carT261–273), the native B cell epitope (B359–369) or the R360 citrullinated B cell epitope (citB359–369), and the combinations of these. The T cell memory compartment was activated by T cell epitopes in both discordant DR4+ twins, but not in the DR3+ RA. The collagen‐specific activation of CD4+ T cells was induced with both the native and carbamylated T cell epitopes only in the RA twin. Both T cell epitopes also induced IL‐17 production in the RA twin, but a greater IL‐4 and IL‐10 response in the healthy twin. The citrullinated B cell epitope, particularly when combined with the carbamylated T cell epitope, induced B cell activation and an increased IL‐6/IL‐10 ratio in the RA twin compared to a greater IL‐10 production in the healthy twin. Our data suggest that circulating collagen‐specific T and B cells are found in HLA‐DR4+ subjects, but only RA activated cells express co‐stimulatory molecules and produce proinflammatory cytokines. Carbamylation and citrullination further modulate the activation and cytokine polarization of T and B cells.
Keywords: carbamylation, citrullination, rheumatoid arthritis, type II human collagen
Introduction
Rheumatoid arthritis (RA) is a multi‐factorial chronic inflammatory disease with an autoimmune pathogenesis leading to preponderant joint damage and severe disability 1. The importance of genetic and environmental factors in RA is well represented by the incomplete concordance in monozygotic (MZ) twins 2, 3 and by the disease familiar occurrence 4, 5. RA is associated with class II human leucocyte antigen (HLA) alleles DR4 and DR1 2, and the binding of self‐antigens to HLA molecules is pivotal to RA pathogenesis 6. Type II human collagen is an established self‐antigen involved in RA pathogenesis 7, 8, based on its representation in the joint cartilage, the murine model induced by collagen in the Aq or human DR1/DR4 transgenic mouse 9, 10 and the presence of serum and synovial autoantibodies to type II collagen in patients with RA 11. Furthermore, anti‐collagen antibodies can induce arthritis in mice lacking both B and T cells 12.
B and T cells recognize different epitopes on human type II collagen with B cells targeting the 359–369 peptide (B359–369, ARGLTGRPGDA) 13 and T cells the DR4/DR1‐restricted 261–273 peptide (T261–273, AGFKGEQGKGEP) 14. In the joint inflammatory milieu, collagen is cleaved with the release of peptides T261–273 and B359–369 15 which undergo post‐translational modifications in vivo, mainly glycosylation, but also citrullination and carbamylation 11. Post‐translational modifications have a complex impact on antigen presentation and immune cell activation, potentially explaining the breakdown of self‐tolerance. Both citrullination and carbamylation have an important role in the diagnosis of RA 16, but their complex role in RA pathogenesis remains to be elucidated 17, 18, 19, 20.
Changes of immunodominant epitope sequences may affect the interaction between antigen‐presenting cells and T cells inducing apoptosis, activation‐induced death or anergy 21, 22. Of note, single amino acid substitutions in the collagen T261–273 epitope induces different T cell activation states or anergy, as well as a change in cell cytokine polarization towards a protective T helper type 2 (Th2) phenotype 23. Furthermore, epitope affinity changes in T cell receptors with a different expression of co‐stimulatory molecules may influence B cell receptor engagement inducing B and T cell regulatory phenotypes, instead of B and T cell activation 24.
To understand more clearly the impact of naive and post‐translationally modified collagen in RA we investigated the effects of the B and T cell epitopes in their native (B359–369, T261–273), citrullinated (citB359–369) and carbamylated (carT261–273) forms on the adaptive immune response in the unique model of HLA‐DR4‐carrying MZ twin sisters discordant for RA.
Materials and methods
Peripheral blood mononuclear cells (PBMCs) were obtained from a treatment‐naive HLA‐DR4+ 27‐year‐old woman with early RA [with positive serum rheumatoid factor, negative serum anti‐cyclic citrullinated peptides (anti‐CCP) antibody, elevated C reactive protein (CRP)], bilateral wrist arthritis for 3 months, disease activity score (DAS)28‐CRP 3·1, her healthy MZ twin sister (negative for both rheumatoid factor and anti‐CCP, normal CRP, with no history or signs of arthritis) and a treatment‐naive unrelated HLA‐DR3+ woman with early active RA (DAS28–CRP 5·11) and positive rheumatoid factor and anti‐CCP. The study was approved by the local Institutional Review Board (IRB) and all patients and controls signed an informed consent to participate.
The choice to include one HLA‐DR3+, anti‐CCP+ woman with RA as control was based on the need to compare findings in the absence of HLA‐DR4, but with immune response against citrullinated self‐epitopes. Further, we speculate that the more active disease observed in the unrelated RA case may illustrate the possible role of active inflammation on T and B cell activation. Collagen peptides were synthesized in native, T261–273 and B359–369, and modified post‐translationally, carT261–273 (carbamylated in K264) and citB359–369 (citrullinated in R360) forms (Biosynthesis Inc., Lewisville, TX, USA). From each subject, 106 fresh PBMCs were cultured in duplicate in 24‐well plates with 1 ml RPMI [10% of fetal bovine serum, 1% penicillin and streptomycin, 1% glutamine and 1% of HEPES [4‐(2‐hydroxyethyl)−1‐piperazine ethanesulphonic acid] for 3 days with 20 μg/ml 7 of T261–273 or carT261–273, B359–369 or citB359–369, T261–273 + B359–369 or carT261–273 + citB359‐369 or without peptides (referred thereafter as unstimulated cells). As reported previously, this peptide concentration was chosen based on its capability to stimulate memory T cells in RA 7.
To discriminate the influence of collagen epitopes on CD4+ T cell cytokine phenotype, experiments were performed in the absence of phorbol myristate acetate (PMA)/ionomycin or anti‐CD3/anti‐CD28, thus observing a weak cytokine production in all conditions, as expected. After stimulation, cells were analysed by flow cytometry [CD3‐allophycocyanin (APC)‐H7, CD4‐phycoerythrin (PE)‐Cy7, CD8‐Alexa700, CD19‐hydrazone (HZN) V500] for functional phenotype [CD45RO‐PE‐cyanin 5 (Cy5), CCR7‐PE‐Cy7, CD45RA‐HZN V500, CD24‐ PE‐Cy7, CD1d‐PE and CD5‐Alexa700], activation [CD154‐APC, CD69‐peridinin chlorophyll (PerCP)‐Cy5·5], apoptosis [annexin‐fluorescein isothiocyanate (FITC), 7‐ 7‐amino‐actinomycin D (AAD)] and cytokine polarization [interferon (IFN)‐γ‐FITC, IL‐17‐HZN V450, IL‐4‐PE, IL‐10‐PE‐CF594, IL‐6‐APC] (all from BioLegend, San Diego, CA, USA) using LS Fortessa (BD Biosciences, San Jose, CA, USA). Briefly, cells were transferred from wells to fluorescence activated cell sorter (FACS) tubes and diluted with 1 ml of FACS buffer (BioLegend); FACS tubes were then centrifuged for 5 min at 400 g. Cells were first stained with extracellular markers at 4°C in dark conditions for 30 min with 5 μl of CD3, CD4, CD8, CD45RO, CD45RA, CCR7 (for effector memory and central memory phenotyphe reff) and CD154 (surrogate for antigen‐specific activation) 25 in T cell analysis or of CD19 and CD69 in B cell analysis. After adding 2 ml of FACS buffer, FACS tubes were centrifuged for 5 min at 400 g. Cells were resuspended in 1 ml of citofix fixation buffer (BioLegend) and then incubated for 20 min at room temperature in dark conditions.
To analyse intracellular cytokines, 10 μg of brefeldin was added in the last 12 h of culture. For intracellular staining, cells were resuspended in 1 ml of perm/wash buffer (BioLegend) and incubated at room temperature for 30 min in dark conditions with 5 μl of IFN‐γ, IL‐17, IL‐4 and IL‐10 for T cell analysis or of IL‐6 and IL‐10 for B cell analysis. Cells were resuspended in 2 ml of perm/wash buffer and centrifuged for 5 min at 400 g.
To analyse apoptosis, cells were washed twice with cold cell staining buffer and then resuspended in annexin V binding buffer (1 × 106 cells/ml) (BioLegend). Cells were then stained with 5 μl of annexin V and 10 μl of 7‐AAD and incubated for 15 min at room temperature in the dark. Cells were analysed by flow cytometry within 1 h.
Data were expressed as number, percentages or mean fluorescence intensity (MFI).
Results
T cell activation and cytokine polarization
Following a 3‐day culture without peptide stimulation, effector memory T cells (CD3+CD4+CD45RO+CCR7–) were represented more in the DR4+ RA twin compared to the DR4+ healthy twin (Table 1). CCR7 MFI increased significantly with both native and carbamylated T cell epitopes (T261–273 and carT261–273) in the DR4+ RA twin and in her healthy twin (Table 1 and Fig. 1a,b). The increased CCR7 MFI was observed both in the central memory pool and in the effector memory pools of both twins without changes in the relative number of the populations (Fig. 1c,d). Only a weak CCR7 increased expression was found in 2 DR4+ patients with long‐standing RA in remission with therapy (see Supporting information). Effector memory T cells were also more numerous in the DR3+ RA case, but did not increase after epitope stimulation (Table 1). This result has been replicated in a DR3+ healthy subject (see Supporting information). The expression of CCR7 did not increase after stimulation with B cell epitopes alone (data not shown). These results suggest that both T cell epitopes can be presented in the HLA‐DR4 complex to T cells, thus alerting the entire T helper compartment in DR4+ subjects regardless of the clinical phenotype.
Table 1.
Central memory (CM) and effector memory (EM) CD4+ T cells, CCR7 mean fluorescence intensity (MFI) on CD4+ T cells and activated (CD69+) B cells after collagen epitope stimulations in two human leucocyte antigen DR4‐related (HLA‐DR4)+ monozygotic twins discordant for rheumatoid arthritis (RA) and in an unrelated HLA‐DR3+ case of rheumatoid arthritis (RA)
| DR4+ RA twin | DR4+ healthy twin | DR3+ RA patient | |
|---|---|---|---|
| CM CD4 T cells (%): unstimulated | 82·9 | 87·8 | 74·2 |
| T261–273 | 81·9 | 84·2 | 81·2 |
| carT261–273 | 85·4 | 83·5 | 77·0 |
| T261–273+B359–369 | 85·0 | 81·3 | 63·1 |
| carT261–273+citB359–369 | 83·2 | 83·0 | 81·3 |
| EM CD4 T cells (%): unstimulated | 15·3 | 10·8 | 23·8 |
| T261–273 | 16·5 | 14·6 | 16·5 |
| carT261–273 | 13·4 | 14·4 | 18·3 |
| T261–273+B359–369 | 14·2 | 17·2 | 34·2 |
| carT261–273+citB359–369 | 15·7 | 15·0 | 14·3 |
| CCR7 CD4 T cells (MFI): unstimulated | 1099 | 1822 | 1835 |
| T261–273 | 6370 | 6781 | 1828 |
| carT261–273 | 6845 | 5688 | 1854 |
| T261–273+B359–369 | 6828 | 5796 | 1946 |
| carT261–273+citB359–369 | 6388 | 6582 | 1847 |
| CD69 B cells (%): unstimulated | 17·3 | 16·0 | 47·2 |
| B359–369 | 24·3 | 21·4 | 38·6 |
| citB359–369 | 24·9 | 36·3 | 28·6 |
| T261–273+B359–369 | 22·9 | 46·9 | 28·0 |
| carT261–273+citB359–369 | 29·9 | 46·1 | 34·0 |
| CD69 B cells (MFI): unstimulated | 583 | 570 | 812 |
| B359–369 | 653 | 354 | 786 |
| citB359–369 | 659 | 803 | 606 |
| T261–273+B359–369 | 639 | 809 | 600 |
| carT261–273+citB359–369 | 709 | 801 | 780 |
Figure 1.
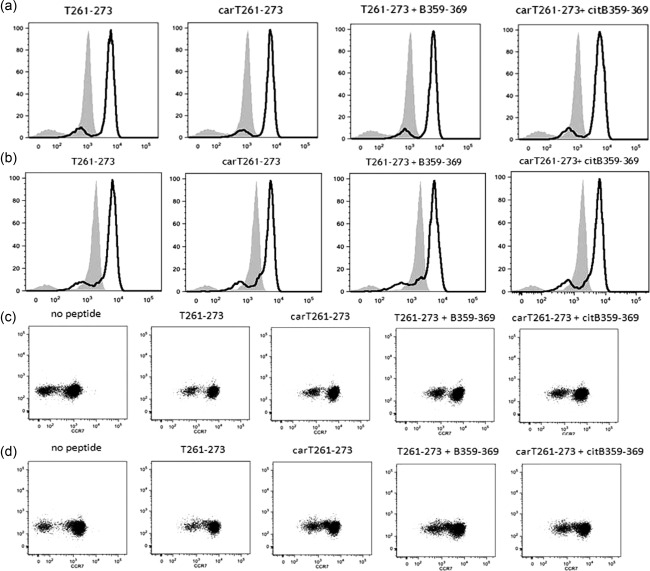
Increased CCR7 expression on CD4+ T cells of the DR4+ rheumatoid arthritis (RA) twin (a) and of the DR4+ healthy twin (b) after collagen T cell epitope stimulations compared to unstimulated cells (white peaks versus grey peak, respectively). Increased CCR7 expression (right shift) on the central memory (CM, right cell cloud) and on the effector memory (EM, left cell cloud) CD4+ T cells of the DR4+ RA twin (c) and of the DR4+ healthy twin (d) after collagen epitope stimulations. Stimulating peptides include native (T261‐273) and carbamylated (carT261‐273) T cell epitopes and the combinations with native (B359‐369) or citrullinated (citB359‐369) B cell epitopes, respectively.
Collagen‐specific T cells were identified by CD154 expression as CD154+CD4+ T cells upon epitope stimulation, i.e. activated and able to co‐stimulate B cells. A relevant expression of CD154 on CD4+ T cells was observed only in the DR4+ RA twin in response to the stimulation with both T cell epitopes (T261–273 and carT261–273) (Fig. 2). A higher expression of CD154 on unstimulated T cells was observed in the DR3+ RA patient compared to the DR4+ RA twin, due possibly to the more active disease, but no further increase in CD154 expression was induced by collagen epitope stimulations (Fig. 2). The stimulation with both native and citrullinated B cell epitopes (B359–369 or citB359–369) did not alter CCR7 or CD154 expression (Fig. 2).
Figure 2.
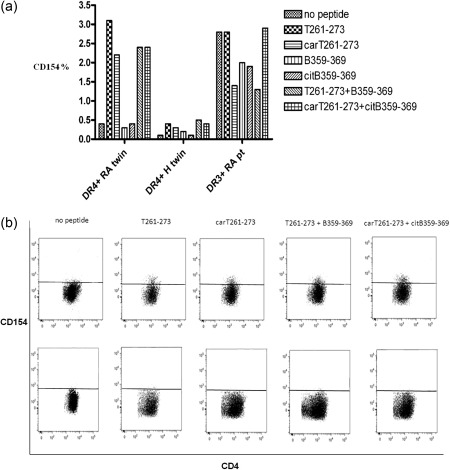
Increased CD154 (CD40L) expression on the CD4+ T cells of the DR4+ rheumatoid arthritis (RA) twin (a) after collagen epitope stimulations compared to the DR4+ healthy (H) twin (b) and the DR3+ RA patient (pt). Stimulating peptides include native (T261‐273) and carbamylated (carT261‐273) T cell epitopes and native (B359‐369) and the combinations with native (B359‐369) or citrullinated (citB359‐369) B cell epitopes, respectively.
We observed a predominant IL‐17 production in the DR4+ RA twin compared to IL‐10 and IL‐4 in all conditions; conversely, in the DR4+ healthy twin, a greater IL‐10 and IL‐4 production was found (Fig. 3a). In the DR3+ RA case, the more active disease was associated with a significant expression of IL‐17 in unstimulated CD4+ T cells and IL‐17 production increased further after the addition of modified T and B cell epitopes (carT261–273, citB359–369) along with IL‐4, possibly suggesting a regulatory Th2 expression. Of note, modified T and B cell epitopes also induced an increased IL‐17 production in the CD8+ T cells of the DR3+ RA patient, thus suggesting their recognition as non‐self‐peptides via type I HLA (Fig. 3c). The data suggest cumulatively that in the DR4+ RA twin collagen‐specific CD4+ T cells are committed to produce pathogenic cytokines and that carbamylation and citrullination of T and B cell epitopes, respectively, can induce a pathogenic response in a HLA‐DR3+ RA patient.
Figure 3.
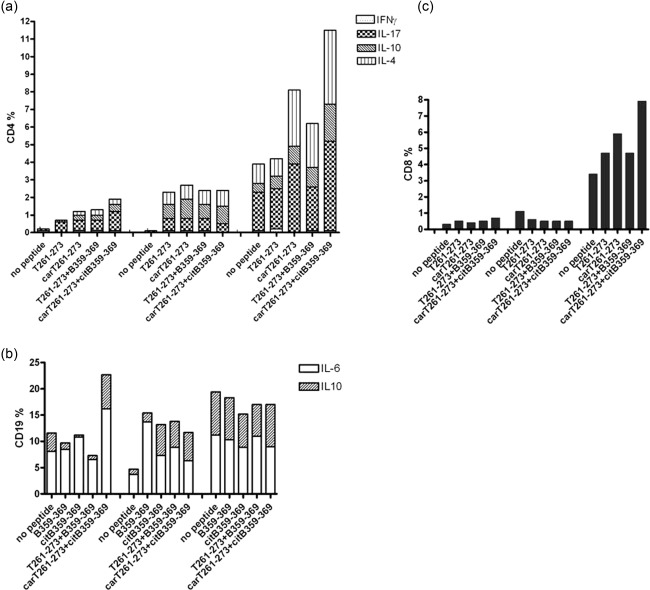
(a) Increased interleukin (IL)−17 production in the CD4+ T cells of the DR4+ rheumatoid arthritis (RA) twin after collagen epitope stimulations; greater IL‐10 and IL‐4 production in the DR4+ healthy (H) twin; significant expression of IL‐17 after post‐translationally modified collagen epitope stimulations in the DR3+ RA patient (pt). (b) Increased IL‐6 production after citB359‐369 stimulation and decreased IL‐10 production after B359‐369 or citB359‐369 stimulations in the B cells of the DR4+ RA twin. Increased B cell production of IL‐6 and, greatly, of IL‐10 compared to unstimulated B cells in the DR4+ H twin after collagen epitope stimulations. In the DR3+ RA pt the collagen epitope stimulations did not alter IL‐6 production, but slightly decreased IL‐10 expression. (c) Increased IL‐17 production in the CD8+ T cells of the DR3+ RA patient after collagen epitope stimulations compared to the DR4+ twins. Stimulating peptides include native (T261‐273) and carbamylated (carT261‐273) T cell epitopes and the combinations with native (B359‐369) or citrullinated (citB359‐369) B cell epitopes, respectively.
B cell activation and cytokine polarization
Unstimulated B cells from the DR4+ RA twin were not more activated (estimated by CD69 expression) compared to the DR4+ healthy twin (Table 1, Fig. 4). In the DR4+ RA twin, the citB359–369 epitope induced the activation of a small population of B cells, while the combined use of modified T and B cell epitopes was associated with a weakly enhanced B cell activation, particularly in the HLA‐DR4+ healthy twin (Fig. 4). CD69 expression did not change significantly after stimulation with T cell epitopes alone (data not shown). These results suggest that citrullination of the B cell epitope may be sufficient to activate B cells regardless of the disease state, albeit the use of PBMCs remains a heterogeneous cell system and activated B cells are almost dead after 3‐day culture. As such activation is amplified in presence of the T cell epitopes, we hypothesize a role for antigen‐activated T cells as B cell‐helper in DR4+ subjects. CD69 was over‐expressed in B cells of the DR3+ RA patient, but its expression decreased after B cell stimulation (Table 1).
Figure 4.
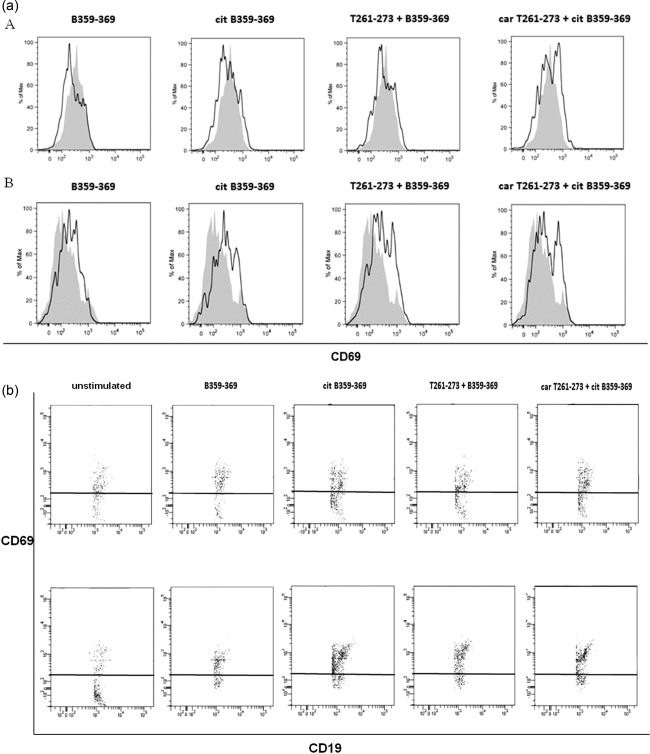
Activated B cells in the DR4+ rheumatoid arthritis twin (a) after the stimulation with the modified collagen epitopes (citB359‐369 and carT261‐273 + citB359‐369) and in the DR4+ healthy twin (panel b) (citB359‐369, T261‐273 + B359‐369 and carT261‐273 + citB359‐369). Stimulating peptides include native (B359‐369) and citrullinated (citB359‐369) B cell epitopes and the combinations with native (T261‐273) and carbamylated (carT261‐273) T cell epitopes, respectively.
As expected, and similar to CD69 expression, IL‐6 production by unstimulated B cells was higher in the DR4+ RA twin. A further IL‐6 production was induced by citB359–369, especially in combination with carT261–273, while a decreased IL‐10 production was observed with both B359–369 and citB359–369 stimulations in the DR4+ RA twin (Fig. 3b). Epitope challenge induced B cells from the DR4+ healthy twin to produce both IL‐6 and IL‐10 compared to unstimulated cells. In the DR3+ RA patient, B cell epitope stimulations did not alter IL‐6 production, already higher in basal condition due to the more active RA, and weakly reduces IL‐10 expression (Fig. 3b). These observations suggest cumulatively that B cell activation by collagen B cell epitopes is associated with an altered balance between inflammatory and regulatory cytokines, favouring inflammation in RA.
T and B cell survival
Unstimulated CD154+CD4+ T cells from the HLA‐DR4+ RA twin manifested an increased survival (annexin– 7‐AAD–) compared to the healthy twin, in which the small subpopulation of CD154+CD4+ T cells was invariably in late apoptosis (annexin+ 7‐AAD+) (Fig. 5).
Figure 5.

Increased survival of unstimulated CD154 + CD4+ T cells in the rheumatoid arthritis (RA) patient(pt)s compared to the healthy (H) twin. Increased survival of antigen‐activated T cells in the DR4+ RA twin after the stimulation with the T cell epitopes, especially with the native T cell epitope (T261‐273). Stimulating peptides include native (T261‐273) and carbamylated (carT261‐273) T cell epitopes and the combinations with native (B359‐369) or citrullinated (citB359‐369) B cell epitopes, respectively.
CD154+CD4+ T cells from the DR4+ RA twin showed increased survival after stimulation with carT261–273 (up to 60%) or T261–273 (up to 100%) (Fig. 5). Despite the low number of events, we observed an increased survival of CD154+CD4+ T cells in the DR4+ healthy twin.
Unstimulated CD154+CD4+ T cells from DR3+ RA case manifested a survival similar to the DR4+ RA twin, and this did not increase after epitope stimulation (Fig. 5). These data suggest that antigen‐activated CD4+ T cells may have a survival advantage in RA, possibly favouring chronic inflammation, even if we cannot exclude proliferation of this selected subpopulation.
Independent of the stimulation, the majority of activated B cells were in late apoptosis in all subjects (data not shown).
Discussion
The aetiology and pathogenesis of RA remain largely enigmatic, despite numerous lines of evidence in support of the role of autoimmune mechanisms and environmental factors 26, possibly through epigenetic changes of a susceptible genetic background 27. A recent large twin study report a concordance for anti‐CCP‐positive RA of 8·3% for MZ twins, with an overall heritable component of 39%, and of 44% in the case of anti‐CCP‐positive RA, thus suggesting a strong influence of environmental or stochastic variables 28. No data are available on twin concordance for seronegative RA, while we submit that data from other rheumatic diseases fall short of 50% concordance rates overall. A common complexity across seronegative or anti‐CCP‐positive cases of RA is also based on similar co‐segregation in families 29. The data presented herein suggest the hypothesis that an altered recognition of type II collagen epitopes by T and B cells could be involved in the pathogenesis of RA and that epitope post‐translational modifications, more specifically carbamylation and citrullination of T and B cell epitopes, respectively, may also contribute to tolerance breakdown and perpetuate inflammation. The inclusion of a MZ twin set carrying the HLA‐DR4 allele represents a unique case and control series which allows that the T‐dependent collagen epitope is presented to T cells in both subjects while minimizing the bias due to genetic diversity. Also using an HLA‐DR3‐positive unrelated RA case allows exploration of collagen immunity beyond the DR4‐CD4‐restricted pathway.
Human type II collagen contains a large number of lysine residues that are often hydroxylated post‐translationally and, subsequently, glycosylated with a β‐D‐galactopyranosyl‐ or an α‐D‐glucopyranosyl‐β‐D‐galactopyranosyl unit 30. The T cell epitope T263‐271 contains two glycosylation sites, K264 and K270. T cells recognize the peptide glycosylated in K264 or in K270 bound to HLA‐DR4, but the β‐D‐galactopyranose unit located in the hydroxylysine 264 seems to be immunodominant 31. This provided the main rationale for testing the K264 carbamylated T261–273. Both K264 and K270 are flanked by non‐polar amino acids and so they are equally prone to carbamylation 32; in addition, in our previous work we generated a computational model showing that F263 of the T cell epitope is bound in the large non‐polar P1 pocket of HLA‐DR4, and this link may be favoured by the neutral homocitrulline in the close position 264 compared to the basic lysine, while K270 extend prominently into solvent where they can be contacted by T cell receptor 8.
The effects of collagen glycosylation on immune recognition remain to be determined, but this modification is believed to enhance the peptide immunogenic potential by slowing down its processing rate 33, thus suggesting that other post‐translational modifications of these sites could alter the epitope immune recognition. Carbamylation involves the non‐enzymatic reaction of urea‐derived cyanate with free NH2 groups on lysine residues to yield homocitrulline leading to an impaired protein function due to the altered charge distribution 22. This process can be mediated in vivo by myeloperoxidase (MPO), the enzyme responsible for the inflammation‐driven carbamylation of proteins 34. Notably, it was shown that collagen is easily carbamylated in vivo and that such modification may be linked directly to granulocyte activation and protease release 31, as observed in the inflamed RA joint, but no reports have investigated the effect of carbamylation of the immunodominant collagen T cell epitope.
Our data demonstrate that both native and carbamylated collagen T cell epitopes induces the enhanced expression of CCR7, a chemokine receptor involved in lymphocyte migration 35, in the T cell memory pool of the DR4+ twins, but not of the DR3+ RA patient. This transient phenomenon was described after T cell receptor engagement by Lanzavecchia and colleagues in both central memory and effector memory T cells 36, and suggests that both epitopes could be presented in the DR4 complex to T cells, thus alerting T helper compartment to migrate into lymph nodes or inflamed tissue. In physiological conditions, joint cartilage lacks vascularization and collagen is thus hidden from the immune system, but during the course of RA not only is T261–273 exposed to the immune system, but it is also presented in a class II HLA complex manner as a non‐self‐peptide. These conditions seem to be sufficient to activate T cells in HLA‐DR4+ subjects. However, only the RA twin T cells had an increased expression of CD154 or CD40L, the co‐stimulatory molecule necessary for an effective T–B cell co‐operation in the adaptive immune response 37. Moreover, while we observed an increased IL‐17 production in T cells of the DR4+ RA twin, a greater Th2 and Treg response was demonstrated in her healthy twin sister after T cell epitope stimulation. In the healthy twin, the native T cell epitope exposure to the immune system or its carbamylation with the creation of a neoepitope may not be sufficient to activate self‐reactive T clones co‐stimulating B cells and producing pathogenic cytokines. Of note, in the DR4+ healthy twin the collagen‐ mediated T cell alerting led to regulatory cytokine production, more suggestive of peripheral self‐tolerant antigen‐experienced T cells, rather than of anergic cells, as in the case of antigen engagement without adequate co‐stimulation 38.
Carbamylation of the collagen T cell epitope did not alter the T cell response in DR4+ subjects compared to the native epitope, suggesting that homocitrulline in K264 does not influence the DR4‐T cell receptor complex. Further, the carbamylated T cell epitope induced IL‐17 expression in the DR3+ RA patient, thus suggesting that this post‐translational modification of the immunodominant type II collagen T cell epitope can induce a pathogenic response independently of the class II HLA haplotype, possibly through the activation of CD8+ T cells, similarly to what happens in CD8 cross‐presentation 39. We are aware that these results could be influenced by the high disease activity of the HLA‐DR3+ woman with RA and by the presence of anti‐CCP, a mark of lost tolerance against citrullinated self‐epitopes without supporting cross‐reactivity.
Citrullination is a post‐translational modification occurring predominantly during apoptosis and opening intracellular protein conformation to favour protein cleavage. Citrullination changes the protein charge distribution and the ability to form H‐bonds, and in‐vitro evidence demonstrated that the modification of more than 10% of arginine residues leads to the denaturation of a protein 35. Of note, peptidyl arginine deiminase (PAD) can be released in the inflamed joint and citrullinate extracellular proteins, including collagen, contributing to neoantigen formation 16, 17. Albeit similar, carbamylation and citrullination target different amino acid residues, lysine and arginine, respectively. Therefore, homocitrulline and citrulline residues map at different positions along the protein sequence, as in the case of collagen, with the T cell epitope T261–273 possibly carbamylated and B cell epitope B359–369 citrullinated. The B359–369 epitope contains two citrullination sites, i.e. R360 and R365, with the former being more probably modified, as R365 is adjacent to a proline 39; this led to the choice of studying R360 citrullination. Our data suggest that the citrullinated B cell epitope can activate B cells, while the native form can induce a pathogenic response only in combination with the T cell epitope, as in the case of a linked recognition of T and B cell epitopes. Despite the anti‐CCP high specificity for RA, the humoral response does not appear to be sufficient to cause the disease; in fact, RA murine model requires the entire or the majority of the collagen molecule to induce arthritis 40, thus suggesting that the presence of both T and B cell‐specific epitopes are important in disease pathogenesis. In fact, anti‐CCP in RA are linked to the shared epitope QΚRAA or QRRAA in the majority of patients 41, a conserved sequence of the P4 pocket, the main determinant of the antigen‐binding site of the β chain of some alleles of HLA‐DR4 e DR1 associated specifically to RA 16, of which T261–273 is the immunodominant T cell epitope 7, 8. A potential pathogenic mechanism, explaining both humoral and cellular response in RA, is the linked recognition of B359–369 and T261–273. T cells can recognize peptides derived from the core regions of proteins presented in class II HLA, while B cells can recognize surface epitopes on the same proteins. According to this, B cells are expected to recognize a surface epitope as the B359–369 epitope through the B cell receptor, internalize the whole protein or a large part of it, cleave it and present an internal epitope as the T261–273 epitope on class II HLA, recognized ultimately by pathogenic T cells. T cells, besides producing proinflammatory cytokines could, in turn, help B cells in autoantibody production. The clinical efficacy of abatacept targeting B and T cell co‐stimulation via cytotoxic T lymphocyte (CTLA)−4 in RA appears to support this fascinating hypothesis 42, 43. Besides a mechanistic view of the benefits of abatacept, additional therapeutic potential implications stem from this study and may include the development of oral vaccines 44 or chimeric antigen receptor T cells expressing collagen‐specific T cell receptors, but also redirected to a regulatory cytokine profile 45, expanding the horizon of RA treatments from monoclonal antibodies towards cell‐based therapy.
It should also be determined whether post‐translational modifications of extracellular peptides in RA could take place on neutrophil extracellular traps (NETs), considering that both PAD and MPO, the enzymes catalyzing citrullination and carbamylation, respectively, are found on NETs 46 and the major role of neutrophils in producing IL‐17 47. Our data need to be confirmed in a larger cohort of patients carrying HLA‐DR4 or the shared epitope and compared either to a large group of non‐DR4 RA patients with similar disease activity or healthy subjects carrying or not DR4. Indeed, the RA twin had an early disease with mild activity and was seronegative for anti‐CCP, while the DR3 RA case had an active long‐standing disease and was seropositive for anti‐CCP, and we cannot exclude the influence of the disease status on the results. We are convinced that results should be confirmed using other DR4‐restricted peptides as well as irrelevant sequences.
In conclusion, data obtained from the natural model of MZ twins may be summarized in two major issues. First, T cells recognizing the type II collagen 261‐273 epitope are present in the memory pool of DR4+ subjects, but are self‐reactive and committed to produce IL‐17 only in RA, while being tolerant and committed to produce IL‐4 and IL‐10 in physiological conditions. Of note, the carbamylation of the collagen T cell epitope may induce a pathogenic response even in non‐DR4+ RA. Secondly, the linked recognition of native T and B cell epitopes seems necessary, while the citrullination of the B cell epitope is sufficient to activate B cells in DR4+ subjects. A higher pathogenic versus protective cytokine ratio was observed in B cells of patients with RA after collagen B cell epitope stimulations. Taken altogether, our data suggest the post‐translational modifications of collagen epitopes may be pivotal to RA pathogenesis, while providing new targets for new treatments acting upstream of proinflammatory cytokines and signalling pathways.
Author contributions
This study was designed and supervised by M. D. S.; A. C., F. C. and N. I. performed the experiments. E. G., M. M. and C. C. collected samples from patients and clinical data. Data were analysed by M. D. S., H. U. S., C. M. and C. S. All authors participated in the reporting of this study results and were involved in writing the paper.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. CCR7 expression in the DR3+ rheumatoid arthritis (RA) patient (a), in two DR4+ long‐standing RA patients in remission with therapy (b,c) and in a DR3+ healthy subject (d).
References
- 1. Atzeni F, Benucci M, Salli S, Bongiovanni S, Boccassini L, Sarzi‐Puttini P. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev 2013; 12:575–9. [DOI] [PubMed] [Google Scholar]
- 2. Svendsen AJ, Hjelmborg JV, Kyvik KO et al The impact of genes on the occurrence of autoantibodies in rheumatoid arthritis. A study on disease discordant twin pairs. J Autoimmun 2013; 41:120–5. [DOI] [PubMed] [Google Scholar]
- 3. Bogdanos DP, Smyk DS, Rigopoulou EI et al Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun 2012; 38:J156–69. [DOI] [PubMed] [Google Scholar]
- 4. Selmi C, Lu Q, Humble MC. Heritability versus the role of the environment in autoimmunity. J Autoimmun 2012; 39:249–52. [DOI] [PubMed] [Google Scholar]
- 5. Miller FW, Alfredsson L, Costenbader KH et al Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun 2012; 39:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakkas LI, Bogdanos DP, Katsiari C, Platsoucas CD. Anti‐citrullinated peptides as autoantigens in rheumatoid arthritis‐relevance to treatment. Autoimmun Rev 2014; 13:1114–20. [DOI] [PubMed] [Google Scholar]
- 7. Ria F, Penitente R, De Santis M et al Collagen‐specific T‐cell repertoire in blood and synovial fluid varies with disease activity in early rheumatoid arthritis. Arthritis Res Ther 2008; 10:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Rosa MC, Giardina B, Bianchi C et al Modeling the ternary complex TCR‐Vbeta/CollagenII(261‐273)/HLA‐DR4 associated with rheumatoid arthritis. PLoS One 2010; 5:e11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosloniec EF, Brand DD, Myers LK et al An HLA‐DR1 transgene confers susceptibility to collagen‐induced arthritis elicited with human type II collagen. J Exp Med 1997; 185:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosloniec EF, Brand DD, Myers LK et al Induction of autoimmune arthritis in HLA‐DR4 (DRB1*0401) transgenic mice by immunization with human and bovine type II collagen. J Immunol 1998; 160:2573–8. [PubMed] [Google Scholar]
- 11. Uysal H, Bockermann R, Nandakumar KS et al Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med 2009; 206:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nandakumar KS, Backlund J, Vestberg M et al Of T or B cells but the arthritis progression is enhanced by CII‐reactive T cells . Arthritis Res Ther 2004; 6:R544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burkhardt H, Sehnert B, Bockermann R, Engstrom A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol 2005; 35:1643–52. [DOI] [PubMed] [Google Scholar]
- 14. Rosloniec EF, Whittington KB, Brand DD, Myers LK, Stuart JM. Identification of MHC class II and TCR binding residues in the type II collagen immunodominant determinant mediating collagen‐induced arthritis. Cell Immunol 1996; 172:21–8. [DOI] [PubMed] [Google Scholar]
- 15. Van den Steen PE, Proost P, Brand DD, Kang AH, Van Damme J, Opdenakker G. Generation of glycosylated remnant epitopes from human collagen type II by gelatinase B. Biochemistry 2004; 43:10809–16. [DOI] [PubMed] [Google Scholar]
- 16. Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol 2008; 26:651–75. [DOI] [PubMed] [Google Scholar]
- 17. Shi J, Knevel R, Suwannalai P et al Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA 2011; 108:17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turunen S, Koivula MK, Risteli L, Risteli J. Anticitrulline antibodies can be caused by homocitrulline‐containing proteins in rabbits. Arthritis Rheum 2010; 62:3345–52. [DOI] [PubMed] [Google Scholar]
- 19. Mydel P, Wang Z, Brisslert M et al Carbamylation‐dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol 2010; 184:6882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scinocca M, Bell DA, Racape M et al Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol 2014; 41:270–9. [DOI] [PubMed] [Google Scholar]
- 21. Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum 1998; 41:1152–60. [DOI] [PubMed] [Google Scholar]
- 22. Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res 2000; 2:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myers LK, Tang B, Rosioniec EF, Stuart JM, Kang AH. An altered peptide ligand of type II collagen suppresses autoimmune arthritis. Crit Rev Immunol 2007; 27:345–56. [DOI] [PubMed] [Google Scholar]
- 24. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012; 30:221–41. [DOI] [PubMed] [Google Scholar]
- 25. Mackey MF, Barth RJ Jr, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol 1998; 63:418–28. [DOI] [PubMed] [Google Scholar]
- 26. Miller FW, Pollard KM, Parks CG et al Criteria for environmentally associated autoimmune diseases. J Autoimmun 2012; 39:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meda F, Folci M, Baccarelli A, Selmi C. The epigenetics of autoimmunity. Cell Mol Immunol 2011; 8:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hensvold AH, Magnusson PK, Joshua V et al Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA‐positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 2015; 74:375–80. [DOI] [PubMed] [Google Scholar]
- 29. Frisell T, Hellgren K, Alfredsson L, Raychaudhuri S, Klareskog L, Askling J. Familial aggregation of arthritis‐related diseases in seropositive and seronegative rheumatoid arthritis: a register‐based case–control study in Sweden. Ann Rheum Dis 2016; 75:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 1995; 64:403–34. [DOI] [PubMed] [Google Scholar]
- 31. Backlund J, Carlsen S, Hoger T et al Predominant selection of T cells specific for the glycosylated collagen type II epitope (263‐270) in humanized transgenic mice and in rheumatoid arthritis. Proc Natl Acad Sci USA 2002; 99:9960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang G, Zhou Y, Zhang Y, Li BQ, Zhang N, Cai YD. Prediction of carbamylated lysine sites based on the one‐class k‐nearest neighbor method. Mol Biosyst 2013; 9:2729–40. [DOI] [PubMed] [Google Scholar]
- 33. Dzhambazov B, Nandakumar KS, Kihlberg J, Fugger L, Holmdahl R, Vestberg M. Therapeutic vaccination of active arthritis with a glycosylated collagen type II peptide in complex with MHC class II molecules. J Immunol 2006; 176:1525–33. [DOI] [PubMed] [Google Scholar]
- 34. Sirpal S. Myeloperoxidase‐mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci 2009; 116:681–95. [DOI] [PubMed] [Google Scholar]
- 35. Forster R, Davalos‐Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8:362–71. [DOI] [PubMed] [Google Scholar]
- 36. Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen‐primed nonpolarized T lymphocytes in vivo . J Exp Med 2001; 193:987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 2001; 58:4–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006; 38:1662–77. [DOI] [PubMed] [Google Scholar]
- 40. Kidd BA, Ho PP, Sharpe O et al Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther 2008; 10:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987; 30:1205–13. [DOI] [PubMed] [Google Scholar]
- 42. Cutolo M, Nadler SG. Advances in CTLA‐4‐Ig‐mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun Rev 2013; 12:758–67. [DOI] [PubMed] [Google Scholar]
- 43. Romo‐Tena J, Gomez‐Martin D, Alcocer‐Varela J. CTLA‐4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun Rev 2013; 12:1171–6. [DOI] [PubMed] [Google Scholar]
- 44. Huang X, Wu H, Lu Q. The mechanisms and applications of T cell vaccination for autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol 2014; 47:219–33. [DOI] [PubMed] [Google Scholar]
- 45. Jethwa H, Adami AA, Maher J. Use of gene‐modified regulatory T‐cells to control autoimmune and alloimmune pathology: is now the right time? Clin Immunol 2014; 150:51–63. [DOI] [PubMed] [Google Scholar]
- 46. Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther 2014; 16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin‐17 and innate immunity in infections and chronic inflammation. J Autoimmun 2015; 60:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. CCR7 expression in the DR3+ rheumatoid arthritis (RA) patient (a), in two DR4+ long‐standing RA patients in remission with therapy (b,c) and in a DR3+ healthy subject (d).


