Abstract
Bloating and distension are among the most common gastrointestinal complaints reported by patients with functional gut disorders and by the general population. These 2 complaints are also among the most prevalent of the severe symptoms reported by patients with irritable bowel syndrome. Nonetheless, only a limited number of published studies have specifically addressed bloating; it is infrequently studied as a primary endpoint, and what little systematic information exists has often been garnered from the assessment of secondary endpoints or the dissection of composite endpoints. This lack of data, and our consequent limited understanding of the pathophysiology of bloating, had hampered the quest for effective and targeted therapies until recently. Advances in the knowledge of underlying mechanisms, particularly with regard to the roles of diet, poorly absorbed fermentable carbohydrates, dysbiosis of the gut bacteria, alterations in visceral hypersensitivity, and abnormal viscerosomatic reflexes, have enabled the development of improved treatment options. The most significant recent advance has been a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, which significantly reduces patients’ symptoms and improves quality of life. Given the prevalence of bloating and its perceived severity, it is clear that further studies regarding the pathogenesis and treatment of this problem are needed.
Keywords: Bloating, distension, irritable bowel syndrome, FODMAP, fermentable carbohydrates, constipation, biofeedback, dietary therapy
The Clinical Problem
Bloating and distension, which are possibly separate but interrelated conditions, together comprise one of the most commonly described gastrointestinal symptoms. Bloating and distension have been reported in up to 96% of patients with irritable bowel syndrome (IBS)1 and in 20% to 30% of the general population,2,3 with the majority of those affected describing their symptoms as either moderate or severe. More than 50% of persons with bloating and distension report a significant impact on quality of life.4 Bloating is usually described as the most severe symptom in patients with functional gastrointestinal disorders, such as IBS, trumping the symptoms otherwise required for patients to meet the diagnostic criteria, such as abdominal pain and altered bowel frequency.5 Bloating is a pervasive symptom that frequently responds poorly to therapeutic interventions.
In general, the term bloating refers to the subjective sensation of increased abdominal pressure without an increase in abdominal size, whereas distension describes the same subjective sensation but with a corresponding objective increase in abdominal girth.6 Approximately 50% of patients with a sensation of bloating also describe abdominal distension.7 Bloating is more commonly seen in patients with IBS, whereas distension is more readily seen in patients with constipation and pelvic floor dysfunction,4,6 in whom plethysmographic studies confirm an increase in abdominal girth of as much as 12 cm.7
Bloating is a difficult endpoint because it is largely a subjective symptom. Patients’ descriptions of bloating vary significantly, but it is often vaguely described as a sensation of fullness, heaviness, tightness, or discomfort. Bloating ranges from mild to severe but is difficult to quantify and almost impossible to standardize. Although bloating commonly occurs in conjunction with functional gastrointestinal disorders, such as IBS, up to 50% of patients who present with bloating do not fulfill the Rome diagnostic criteria for IBS.8 The Rome III diagnostic criteria for functional gastrointestinal disorders fail to include bloating as a primary criterion for IBS or functional dyspepsia because of its nondiscriminatory nature. It is a supportive symptom for a diagnosis of IBS. Functional bloating (described as a recurrent feeling of bloating or visible distension with insufficient evidence for a diagnosis of IBS, functional dyspepsia, or a functional gastrointestinal disorder1) in isolation is relatively uncommon. As a result, few studies have included bloating as a primary endpoint, despite its clinical relevance. Fortunately, bloating is often included as a secondary endpoint or as part of a composite endpoint in studies targeted at IBS symptoms, and some data are therefore available for review.
Unfortunately, the overall pathogenic mechanisms behind such symptoms remain incompletely understood, although recent research indicates that they are likely to be multifactorial. As a result, treatment paradigms are often unsatisfactory because their empiric nature fails to adequately address the underlying pathophysiology. The effective management of bloating and distension requires therapies targeted at clearing the pathophysiologic mechanisms. To this end, a brief overview of the current knowledge regarding the pathophysiology of bloating is included here before a more comprehensive review of current treatment strategies is presented.
Pathophysiology
The Role of Luminal Contents
Intestinal Gas Endogenous gas production via the fermentation of luminal contents is a significant contributor to raised intra-abdominal volume. Lactulose, which is poorly absorbed in the small bowel and therefore fermented in the colon, has been shown to be associated with bloating and distension in healthy volunteers1 and in patients with chronic constipation,9 suggesting luminal gas production as a contributing mechanism. Fermentation and thus gas production are influenced by the substrate (ingested indigestible carbohydrates) and intestinal bacterial species, and studies show that alterations in the dietary intake of indigestible carbohydrates influence symptoms of bloating. In addition, reported alterations in the fecal microbiota of patients with IBS in comparison with those of healthy controls have been postulated to result in alterations of luminal fermentation,10 supported by the observation that patients with IBS have a lower cecal pH, presumably as a consequence of the increased production of short-chain fatty acids.11 Furthermore, the role of bacteria in the pathogenesis of bloating is supported by studies in which the modulation of bacteria with antibiotics resulted in symptomatic decreases in bloating.8
Despite this tidy hypothesis, the results of studies assessing the volume of intestinal gas have been unsatisfying. Argon gas washout techniques have shown no difference between gas production in patients with chronic bloating and that in healthy subjects,12 and overall studies to date have suggested that the total volume of intestinal gas is not significantly elevated in those who experience bloating. The volumes of intestinal gas on computed tomography (CT) scans are similar in patients with a functional gastrointestinal disorder, those with organic disorders, and, indeed, in healthy subjects,13-15 suggesting that other contributing mechanisms are likely. Furthermore, when similar dietary loads of indigestible carbohydrates are ingested, studies of breath hydrogen production have consistently shown similar volumes of gas production in patients with IBS and in healthy controls.16,17
Small-Intestinal Water Content Small-intestinal distension is also a potential cause of bloating. Slowly absorbed short-chain carbohydrates, such as fructose and mannitol, considerably increase the volume of water in the small-intestinal lumen by virtue of their osmotic effects, as shown by magnetic resonance (MR) imaging.18,19 The ingestion of these substances can induce gut symptoms that include bloating in patients with IBS. Malabsorption of some of the fructose or mannitol can lead to the relatively rapid production of gases such as hydrogen, but the symptomatic response is unrelated to whether malabsorption occurs.16,19
Colonic Contents Patients who are constipated commonly report bloating, with more than 80% describing severe symptoms.20 The pathogenesis of bloating in patients with constipation is likely multifactorial. First, the direct mechanical effects of fecal impaction and colonic loading can mimic bloating via the distensile effects of the fecal bolus. Second, colonic stasis may result in increased fermentation of the colonic contents by intestinal bacteria and, therefore, increased gas production. Finally, alterations in colonic motility may directly affect gas handling, leading to symptoms of bloating. The pathogenic association between constipation and bloating is further supported by the fact that therapies targeted at alleviating constipation (eg, prokinetics) also relieve bloating (see below).
Nongaseous Fermentation Products An alternative hypothesis for the benefits of dietary modification and probiotics is that products of fermentation other than gas influence symptoms of bloating by modulating visceral sensitivity. Short-chain fatty acids interact with specific free fatty acid receptors (FFA1 and FFA2) in the intestine, and these, in turn, interact with the enteric nervous system. Indeed, in animal studies, butyrate has been shown to influence visceral sensitivity in addition to affecting motility.21 However, the relevance of this potential mechanism to the human sensation of bloating requires further targeted investigation.
The Role of Gas Handling and Clearance
Rather than excessive gas production, it seems more likely that altered or dysfunctional gas handling and clearance are to blame. Intestinal transit is altered in patients who experience bloating; their prokinetics and colonic transit times are slower than those of healthy individuals,22 and distension directly correlates with transit times. The positive effect of prokinetics on bloating is testament to this. Furthermore, the proximal clearance of gas appears to be impaired in these patients, resulting in increased residual gas following attempted evacuation. This is most likely the consequence of altered reflex modulation of gas clearance.23
The Role of Visceral Sensitivity
Changes in visceral sensitivity have been proposed as the hallmark of IBS.24 Patients frequently describe marked symptoms in the absence of significant physiologic changes, so altered visceral sensitivity provides a ready hypothesis to explain the frequency and severity of the bloating that they experience. Most commonly, rectal hypersensitivity (lowered threshold to rectal distension) is observed in patients with IBS. Rectal hyposensitivity (elevated threshold to rectal distension) is also common, particularly in patients with constipation,25 and is associated predominantly with increased abdominal distension. Almost 90% of patients with “no-urge” constipation, which is associated with elevated rectal sensory thresholds, describe distension and bloating.26 In contrast, hypersensitive patients, in whom diarrhea-predominant IBS is more common, have increased symptoms of bloating in the absence of distension.27
Alterations in visceral sensitivity may explain why symptoms of bloating and distension may be worse in women during the perimenstrual phase.28 Visceral sensitivity varies throughout the menstrual cycle and is generally most acute in the perimenstrual phase,29 the period when symptoms are usually worse. Visceral pain thresholds are also lower in the setting of psychological stress,30 perhaps explaining why patients commonly note exacerbations of symptoms during periods of stress.31
Visceral sensation alone, however, is not sufficient to fully explain the symptom of bloating because more than one-third of patients with bloating and distension have normal visceral sensation on barostat testing.32 In addition, therapies targeted at reducing visceral sensitivity (eg, pregabalin and amitriptyline) have been disappointing in the treatment of bloating,33,34 although hypnotherapy, which has been postulated to modulate visceral afferent function via normalization of visceral sensation,35 appears to be of benefit.36,37
The Role of Altered Abdominal and Diaphragmatic Muscle Function
CT and abdominal muscle electromyographic testing indicate that the reflex control of abdominal and diaphragmatic muscles is altered in patients who experience bloating38,39 In response to an infusion of gas, healthy subjects exhibit diaphragmatic relaxation, costal expansion, and compensatory contraction of the upper rectus and external oblique abdominal muscles without changes in the lower abdominal muscle tone. In contrast, patients with bloating show a paradoxical contraction of the diaphragm and relaxation of the upper abdominal wall.38,40,41 The mechanism behind such disordered reflex function has not been fully elucidated. However, it has been postulated that abnormal behavioral responses occur as the result of subjective visceral hypersensitivity to gas distension.
The Role of Altered Pelvic Floor Function
Abnormal pelvic floor reflex function has been associated with bloating. In patients who have constipation and bloating, abnormal balloon expulsion is correlated with abdominal distension.42 Patients who have abdominal distension have been shown to have an altered rectoanal inhibitory response, a locally mediated rectal reflex, further supporting the hypothesis that altered evacuation may be contributory.43 A cause-and-effect relationship is supported by the fact that therapeutic interventions targeted at correcting anorectal dyssynergia, such as biofeedback, result in a decrease in the frequency and severity of bloating.20,44
When to Investigate
Several organic conditions may be associated with or result in bloating (Table). Although rare, these conditions should be considered. Bloating is a troublesome but benign condition, and the pursuit of numerous expensive tests is not recommended unless so-called alarm features are present (loss of weight, rectal bleeding, and nocturnal symptoms). A change in bowel habit in a patient older than 50 years of age is an appropriate trigger for colonoscopy; however, endoscopy in a young person without the aforementioned alarm features yields little. A patient who has a family history of celiac disease or symptoms suggestive of celiac disease should be screened with serology.45 If the patient has a family history of ovarian cancer or breast cancer or has experienced a recent change in menstruation, a pelvic ultrasound should be considered. All patients should be screened for psychological factors that may be contributing to their symptoms.
Table.
Causes of Bloating to Be Considered When Deciding on the Investigative Strategy
| Malabsorption |
|
| Dysmotility |
|
| Surgical intervention |
|
| Infections |
|
| Malignancy |
|
| Physiologic conditions |
|
SIBO, small intestinal bacterial overgrowth.
A plain radiograph to assess for fecal loading is often an invaluable tool for evaluating patients with refractory symptoms. Even though dietary fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) and small intestinal bacterial overgrowth (SIBO) may contribute to refractory symptoms, there is little convincing evidence that hydrogen breath tests reliably aid diagnosis or contribute significantly to management. These conditions should be evaluated clinically and treated empirically.
Treatment
The treatment of bloating has been notoriously difficult. However, now that the pathophysiologic mechanisms are better understood, several therapies directed at contributing factors—such as constipation, luminal distension, visceral hypersensitivity, dysfunctional motility responses, and abnormal viscerosomatic reflexes—have improved the ability to achieve symptom reduction, if not resolution.
Diet
The most significant advance in the treatment of bloating has been the identification of a group of poorly absorbed short-chain carbohydrates (FODMAPs). This term brings together carbohydrate subtypes with similar properties— fructo-oligosaccharides (fructans), galacto-oligosaccharides, lactose, fructose, sorbitol, and mannitol. All of these are of small molecular size and, thus, are osmotically active, and they always are or have the potential to be poorly absorbed or slowly absorbed in the human small intestine.
Ileostomy research has demonstrated that a high-FODMAP diet increases the amount of fluid exiting the small bowel.46 This may induce motility changes, distension of the bowel, and diarrhea. In addition, because of the poor absorption of FODMAP compounds, they are delivered to the microbiota of the distal small bowel and large intestine, where they are rapidly fermented to produce hydrogen, methane, and carbon dioxide gases.17 More recently, MR imaging techniques have been used to examine fluid and gas changes in the bowel following the ingestion of fructose and fructans, and it has been demonstrated that fructose, being a smaller molecule, increases the fluid content of the small bowel to a greater extent than fructans, which induce a more gaseous response19 because they are almost entirely delivered to the bacteria of the large bowel. Fructose, such as polyols (eg, sorbitol and mannitol), is slowly absorbed along the small intestine, and only a small proportion at worst reaches the large bowel, with a less gaseous response than that for fructans. Furthermore, fructose and mannitol induce symptoms in patients with IBS independently of whether they are poorly absorbed (ie, delivered to the large bowel).17,19 The different molecular sizes of the individual FODMAP compounds and their different absorption patterns are therefore likely to influence their effect on the gas and/or fluid content of the bowel, both of which will contribute to a sensation of bloating in sensitive individuals (likely to be those with visceral hypersensitivity).
Individually, these carbohydrates have been considered to be potential triggers of IBS symptoms for decades. Lactose intolerance has been the best-documented type of food intolerance related to a gastrointestinal symptomatic response. Studies in children and adults have highlighted the induction of symptoms, including bloating, in those who poorly absorb lactose.47,48 Poor absorption of fructose has also received significant attention, with dietary restriction of fructose providing overall symptomatic benefit as well as decreases in bloating specifically.49 Additional work that restricted dietary fructose and sorbitol in patients with IBS showed significant improvements in stool consistency, but bloating was not specifically assessed.50 Furthermore, the symptom response to fructans has been shown to be comparable with that to fructose and sorbitol.51 Additive effects on bloating have been noted for fructose plus fructans.52
With the grouping of these carbohydrates, the development of a comprehensive FODMAP food composition database, and well-controlled dietary trials, a low-FOD-MAP diet has now been confirmed as an effective therapy for the management of IBS symptoms, decreasing symptoms in at least 75% of patients.53-55 The documented 50% to 82% decrease in bloating following dietary FODMAP restriction confirms that a low-FODMAP diet is the most effective treatment option for managing bloating to date.
Other dietary modifications may be important. Given their abnormal handling of exogenous gas, it is advisable for these patients to avoid carbonated drinks. Similarly, dietary fiber has been shown to exacerbate bloating. Fiber intake is important for laxation and other putative general health benefits and may relieve some IBS symptoms, but it may worsen bloating. The choice of fiber needs to be carefully considered.56 Supplements rich in whole wheat fiber, such as wheat bran, should be avoided because they are usually rich in FODMAP compounds and are ineffective in patients with IBS.57 Fibers that are slowly fermented, such as psyllium/ispaghula husk, seem to be better tolerated in IBS, and there is some evidence, albeit weak, for their efficacy in IBS.58 A trial of a nonfermentable fiber, such as sterculia or methylcellulose, may be appropriate, and nonfermentable fiber may be far better tolerated than other fibers, although evidence for or against this approach is lacking.
Laxatives
The high prevalence of bloating in patients with chronic constipation and constipation-predominant IBS (IBS-C) suggests that initial treatment should be aimed at ensuring that constipation is adequately and aggressively managed. Indeed, many of the therapies advocated for the treatment of bloating are primarily aimed at improving the transit of stool through the colon. It must be noted, however, that bulking agents and some osmotic agents distend the bowel lumen by virtue of their water-holding effects and gas production from fermentation and the proliferation of bacteria. Prokinetic agents, which have evidence of good quality to support their use (see below), are generally preferable in patients who describe bloating in the setting of constipation.
Prosecretory and Promotility Agents
Prosecretory and promotility agents, such as linaclotide (Linzess, Forest Laboratories/Ironwood Pharmaceuticals), prucalopride, and lubiprostone (Amitiza, Sucampo/Takeda), are emerging as safe and efficacious treatments for chronic constipation and IBS-C. Data suggest that their use is associated with a concurrent decrease in bloating, but it remains uncertain whether this is a secondary response to reduced colonic loading or a direct effect on visceral sensation. The drugs have not been assessed in patients who have bloating without constipation.
Linaclotide Linaclotide, a minimally absorbed peptide that is an agonist of guanylate cyclase-C, has shown promising efficacy in IBS-C and chronic constipation. It increases fluid secretion and accelerates intestinal transit59 by activating the cystic fibrosis transmembrane conduction regulator. In addition, at higher doses in animal models, it has been shown to result in a reduction in visceral hypersensitivity via the direct inhibition of colonic nociceptors.60,61 In patients with IBS, linaclotide significantly reduces abdominal pain, and this reduction has been postulated to occur via the same mechanisms.60 A recent meta-analysis has provided good support that linaclotide improves bowel function, relieves abdominal pain, and decreases the overall severity of IBS-C and chronic constipation.62 In individual clinical trials, linaclotide significantly decreased all abdominal symptoms in patients with IBS-C, of which bloating was the most prevalent,63 and a recent preliminary report indicates that more than 1 in 3 patients with bloating and chronic constipation experienced relief.64 Indeed, several studies have reported decreased bloating associated with decreased constipation in patients who have IBS, as recently reviewed.65 Its low level of oral bioavailability and small number of systemic side effects, in addition to its positive effect on bloating, make linaclotide an attractive option.
Lubiprostone Lubiprostone, a member of the prostone class of compounds, also acts on chloride channels to relieve constipation and improve colonic transit.66 Most studies use the primary endpoint of increased frequency of spontaneous bowel motions, with decreases in abdominal pain and bloating as secondary endpoints. Once again, there appears to be a significant decrease in abdominal bloating.67-69 The effect on bloating would appear likely due to reduced colonic volume and improved transit because lubiprostone appears not to have an effect on visceral sensory thresholds.70 Further studies are required.
5-Hydroxytryptamine Agonists Prucalopride and tegaserod (Zelnorm, Novartis), which are 5-hydroxytryptamine receptor 4 (5-HT4) agonists, stimulate gastrointestinal motility and intestinal secretion. Tegaserod has been withdrawn from the market because of the risk for serious cardiac side effects. Prucalopride is a highly selective 5-HT4 agonist with few serious adverse events.71-73 Within the cohort of patients who have chronic constipation, prucalopride significantly reduces bloating, in addition to other symptoms associated with constipation.74,75 Emmanuel and colleagues recently reported on the efficacy of prucalopride in reducing bloating in patients with chronic pseudo-obstruction, but the numbers in the study were too small for any definitive conclusions to be drawn.76 There is evidence to suggest that prucalopride may enhance visceral sensation,75 an effect that requires further investigation, but the observation bodes well for its role in the treatment of bloating in the setting of constipation, in which symptoms commonly occur in the setting of rectal hyposensitivity.
Antidepressants
Current guidelines77 support the use of antidepressants for IBS, but the data on the reduction of bloating are less clear. Selective serotonin receptor inhibitors (SSRIs) are gaining favor over tricyclic antidepressants (TCAs) because of their better side effect profile. Data suggest that SSRIs do improve quality of life, but they do not appear to have a consistently positive effect on bloating.78,79 Some small studies have had positive results; for example, Vahedi and colleagues reported a smaller proportion of patients experiencing bloating in the fluoxetine group than in the placebo group,80 and a small but well-designed crossover study comparing citalopram and placebo revealed fewer days with bloating and less severe symptoms in the treatment phase.81 However, equally well-designed trials have shown no difference between fluoxetine82 and paroxetine78 in reducing bloating. Indeed, a meta-analysis concluded that there was no reduction in bloating in the SSRI group.83 Some evidence indicates that SSRIs may decrease orocecal transit time,84 which may make them an attractive treatment option for bloating in patients with IBS-C.
Data concerning the effects of TCAs on bloating are rare. Although several meta-analyses have attempted to qualify the studies of TCAs in functional gut disorders, with little concordance,85-88 none included bloating as an endpoint. A small, placebo-controlled trial of amitriptyline in adolescents did not show any decrease in bloating.89
Antibiotics
The gut microbiota is increasingly implicated in the pathophysiology of IBS and bloating.90 A major source of gas formation in the intestine is the bacterial fermentation of carbohydrates. Excessive growth of bacteria in the small intestine (SIBO) has been postulated as a cause of symptoms in a proportion of patients, and it has been suggested that dysbiosis of the gut microbiota contributes to gut dysfunction by virtue of interaction of the microbiota with the epithelium, immune system, enteric nervous system, and central nervous system.91 With this background, it is not surprising that manipulation of the gut microbiota with antibiotics, probiotics, and prebiotics is the focus of many studies in patients who have bloating in the context of IBS.
Rifaximin The only antibiotic for which high-quality evidence is available is rifaximin (Xifaxan, Salix). Rifaximin is a poorly absorbed antibiotic with a favorable side effect profile and presumably lower rates of clinically relevant bacterial resistance. In well-conducted trials, rifaximin had a modest effect in relieving IBS-related bloating in patients without constipation (combined odds ratio, 1.53 [95% CI][1.24-1.89]; P<.001).92 One investigating group indicated a greater response in patients with a lactulose breath hydrogen test suggesting SIBO,93 thus permitting more targeted therapy. However, the interpretation of lactulose hydrogen breath test results and the ability of the test to detect SIBO are controversial at best and probably invalid.94 Furthermore, in a study of patients with a negative lactulose hydrogen breath test, rifaximin led to a significant decrease in bloating.8 Therefore, the evidence that lactulose hydrogen breath testing should be used to guide the use of antibiotics is poor, and testing is not advised.
Neomycin The results of initial studies with neomycin for the treatment of SIBO and IBS were promising. Overall, IBS symptoms decreased,95 and the results of lactulose hydrogen breath tests were normalized in some studies.96 However, the risk for ototoxicity and significant, durable resistance has limited the use of neomycin clinically, and it has been largely superseded by rifaximin.
Probiotics
The information on probiotics and their benefit in bloating is largely inconclusive; there is considerable heterogeneity in the literature, with a preponderance of small studies having positive results. A systematic review concluded that bloating is significantly reduced by the probiotic Bifidobacterium infantis 35624. 97 B infantis has consistently shown benefit in reducing bloating and has shown superiority to Lactobacillus and placebo.98,99 One of the few studies to include abdominal inductance plethysmography as an endpoint reported a decrease in distension and an improvement in gut transit with the administration of Bifidobacterium lactis.100 VSL#3 (a composite probiotic containing predominantly Lactobacillus species, Bifidobacterium, and Streptococcus salivarius) had small but positive benefits for patients with bloating,101,102 but this effect was not replicated when a bloating severity score was adopted as the primary endpoint.103 Overall, the benefits of probiotics for bloating are restricted to those containing bifidobacteria, which do not generate gas during fermentation, and the effects have been modest in magnitude.
Behavioral and Psychological Therapies
It is recognized that the interaction between the enteric nervous system and the psychological state is implicated in the pathogenesis of IBS. As such, behavioral and psychological therapies are viewed with optimism, but the literature is insufficient, particularly with regard to bloating. Two Cochrane reviews, one on hypnotherapy104 and the other on psychological therapies in IBS,105 both concluded that their findings were hampered by small sample size, validity, heterogeneity, and outcome definition.
Hypnotherapy Hypnotherapy has been reported to reduce symptomatic bloating in patients with IBS, and the benefit is sustained over time.36,37,106 The decrease in bloating symptoms appears to be independent of the subtype of IBS. There is also evidence that hypnotherapy normalizes rectal sensory thresholds in patients with IBS,35,107 and such normalization might be expected to confer an alteration in the perception of bloating. A Cochrane review concluded that hypnotherapy may be useful in patients with IBS who have failed conventional therapy in comparison with either waiting list controls or IBS patients receiving standard care, but the review advised caution in interpreting the results because of poor methodology.104
Cognitive Behavioral Therapy The evidence for cognitive behavioral therapy (CBT) in IBS remains controversial, even though it has been more extensively investigated than any other psychological therapy. A recent systematic review and meta-analysis108 indicated an overall reduction in IBS symptoms; however, a Cochrane review concluded that, although CBT may be better than standard care, it is not superior to placebo, and its clinical relevance is uncertain.105 None of these studies identified bloating as an independent outcome measure, and only a minority used it as part of a composite endpoint. The benefit of CBT in bloating can only be inferred from IBS studies; whether CBT has a true effect on visceral anxiety, as hypothesized by Craske and colleagues,109 or merely relieves symptoms by enabling patients to cope with them more effectively is still debated.
Biofeedback and Neuromodulatory Techniques
Biofeedback Biofeedback and bowel retraining techniques have an established role in the management of functional hindgut disorders.110 Biofeedback involves a training program, usually led by a nurse or physiotherapist, in which instrument-based techniques are used to reinforce normal patterns of neuromuscular function.
Although no studies have assessed biofeedback for bloating in isolation, several small studies have shown that anorectal biofeedback relaxation techniques reduce symptoms of bloating in up to 70% of patients and concurrently reduce constipation, with the effect maintained for up to 12 months.20,111 This effect does not appear to be confined to those with slow transit or manometric abnormalities and is independent of the presence or absence of IBS.
Diaphragmatic breathing techniques are useful in aerophagia and rumination,112 and given the potential role of visceromotor reflexes in abdominal distension, they may confer benefit in the management of bloating. Unfortunately, no reliable studies have adequately explored this possible effect.
Neuromodulation Sacral nerve stimulation has been associated with a reduction in the frequency and severity of abdominal bloating and other symptoms of constipa-113,114 A study in patients with IBS also showed a significant reduction in bloating.115 However, sacral nerve stimulation is considered experimental, and as such, it cannot currently be recommended for the management of isolated bloating.
Interferential transabdominal electrical stimulation has had some success in patients with constipation and/or IBS, with improved bloating scores. However, this appears not to have significant benefit overall when compared with placebo.116,117
Complementary and Alternative Therapies
Simethicone and Charcoal The use of gas-reducing agents, such as simethicone and charcoal, to relieve bloating is widespread, but the evidence to support their efficacy is limited. When used alone, simethicone has been inefficacious, and the effects of activated charcoal have been inconsistent.118,119 There is a suggestion that gas-related discomfort is reduced with a combination of simethicone and loperamide.120,121 Likewise, the combination of simethicone, activated charcoal, and magnesium oxide significantly reduced bloating in patients with functional dyspepsia.122 The evidence to support the use of any of these agents alone in reducing bloating is insufficient, but there may be some benefit in combination.
Kiwifruit Extract There is emerging evidence that kiwifruit is effective in the treatment of constipation and bloating. Although the mechanisms remain unknown, kiwifruit appears to promote both laxation and gastric motility.123 Kiwifruit extract has had promising results for the treatment of constipation in randomized, placebo-controlled trials. A statistically significant reduction in bloating was noted in a study of patients with occasional constipation.124
STW5 STW5 (Iberogast, Flordis) is a preparation combining 20 different herbs with putative multiple-target action. Meta-analysis has confirmed STW5 to be more effective than placebo and cisapride (Propulsid, Janssen Pharmaceuticals) in alleviating the most troublesome symptoms of functional dyspepsia.125 The agent’s efficacy in reducing IBS symptoms has been embraced largely based on a single large, well-conducted trial.126 Although bloating was not studied, STW5 relieved the sensations of fullness and tension, which could be considered a surrogate for bloating. The tolerability and favorable side effect profile of STW5, in addition to its positive effects in functional dyspepsia and IBS, suggest that a trial in patients with bloating would be worthwhile.
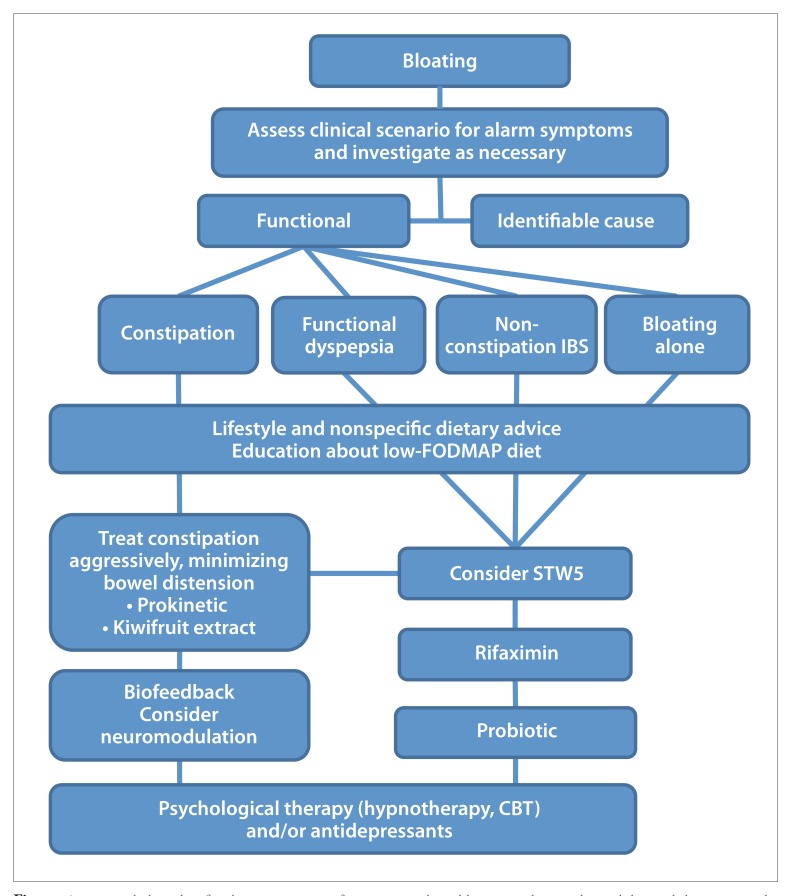
Treatment Algorithm for Patients With Functional Bloating
Although there is still a significant lack of data in regard to the management of bloating, as highlighted in this article, evidence is emerging that helps to guide therapy. A suggested treatment algorithm for the management of patients presenting with bloating is shown in the Figure.
Figure.
A suggested algorithm for the management of patients in whom bloating with or without abdominal distension is the predominant or most worrisome symptom.
CBT, cognitive behavioral therapy; FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome.
Conclusion
Bloating and distension are highly prevalent symptoms with a marked effect on health status and quality of life. Considerable progress has been made in understanding the pathogenesis of these symptoms over recent years, and multiple pathologic conditions are likely to contribute to their development. Emerging evidence indicates that targeting colonic motility, gut flora, visceral sensitivity, and dietary intake is helpful in controlling such symptoms, although few studies exist in which bloating is a primary endpoint. Fortunately, new therapies involving dietary manipulation (low-FODMAP diet) have proved highly successful in relieving symptoms of bloating and abdominal distension, with efficacy rates well exceeding those of drug therapies, such as antibiotics and prokinetic agents. However, additional studies are needed to further elucidate the pathologic mechanisms underlying bloating and distension, and targeted therapeutic studies are warranted. Because bloating is the most common and often the most troublesome functional gut symptom, it is only sensible that studies be performed with bloating as a primary endpoint so that data of better quality can be made available.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut. 1998;42(5):690–695. doi: 10.1136/gut.42.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008;103(5):1241–1248. doi: 10.1111/j.1572-0241.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000;45(6):1166–1171. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 5.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94(5):1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(12):3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 7.Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131(4):1003–1010. doi: 10.1053/j.gastro.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101(2):326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Attar A, Lémann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44(2):226–230. doi: 10.1136/gut.44.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):521–530. doi: 10.1111/j.1365-2982.2012.01891.x. e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer AD, Mohammed SD, Dukes GE, Scott SM, Hobson AR. Caecal pH is a biomarker of excessive colonic fermentation. World J Gastroenterol. 2014;20(17):5000–5007. doi: 10.3748/wjg.v20.i17.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasser RB, Bond JH, Levitt MD. The role of intestinal gas in functional abdominal pain. N Engl J Med. 1975;293(11):524–526. doi: 10.1056/NEJM197509112931103. [DOI] [PubMed] [Google Scholar]
- 13.McWilliams SR, McLaughlin PD, O’Connor OJ, et al. Computed tomography assessment of intestinal gas volumes in functional gastrointestinal disorders. J Neurogastroenterol Motil. 2012;18(4):419–425. doi: 10.5056/jnm.2012.18.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada JR. Intestinal gas and bloating: effect of prokinetic stimulation. Am J Gastroenterol. 2008;103(8):2036–2042. doi: 10.1111/j.1572-0241.2008.01866.x. [DOI] [PubMed] [Google Scholar]
- 15.Hernando-Harder AC, Serra J, Azpiroz F, et al. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol. 2010;105(4):876–882. doi: 10.1038/ajg.2010.75. [DOI] [PubMed] [Google Scholar]
- 16.Yao CK, Tan H-L, van Langenberg DR, et al. Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27(suppl 2):263–275. doi: 10.1111/jhn.12144. [DOI] [PubMed] [Google Scholar]
- 17.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 18.Marciani L, Cox EF, Hoad CL, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138(2):469–477. doi: 10.1053/j.gastro.2009.10.055. 477.e1. [DOI] [PubMed] [Google Scholar]
- 19.Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109(1):110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiotakakou-Faliakou E, Kamm MA, Roy AJ, Storrie JB, Turner IC. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42(4):517–521. doi: 10.1136/gut.42.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhoutvin SA, Troost FJ, Kilkens TO, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21(9):952–e76. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009;104(8):1998–2004. doi: 10.1038/ajg.2009.251. [DOI] [PubMed] [Google Scholar]
- 23.Salvioli B, Serra J, Azpiroz F, Malagelada JR. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101(8):1853–1857. doi: 10.1111/j.1572-0241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 24.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 25.Houghton LA, Whorwell PJ. Towards a better understanding of abdominal bloating and distension in functional gastrointestinal disorders. Neurogastroenterol Motil. 2005;17(4):500–511. doi: 10.1111/j.1365-2982.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 26.Harraf F, Schmulson M, Saba L, et al. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. 1998;43(3):388–394. doi: 10.1136/gut.43.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal A, Houghton LA, Lea R, Morris J, Reilly B, Whorwell PJ. Bloating and distention in irritable bowel syndrome: the role of visceral sensation. Gastroenterology. 2008;134(7):1882–1889. doi: 10.1053/j.gastro.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 28.Altman G, Cain KC, Motzer S, Jarrett M, Burr R, Heitkemper M. Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterol Nurs. 2006;29(1):4–11. doi: 10.1097/00001610-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50(4):471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilleri M, Ford MJ. Functional gastrointestinal disease and the autonomic nervous system: a way ahead? Gastroenterology. 1994;106(4):1114–1118. doi: 10.1016/0016-5085(94)90775-7. [DOI] [PubMed] [Google Scholar]
- 31.Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127(6):1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Houghton LA, Calvert EL, Jackson NA, Cooper P, Whorwell PJ. Visceral sensation and emotion: a study using hypnosis. Gut. 2002;51(5):701–704. doi: 10.1136/gut.51.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56(9):1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig Dis Sci. 2008;53(1):108–115. doi: 10.1007/s10620-007-9830-4. [DOI] [PubMed] [Google Scholar]
- 35.Lea R, Houghton LA, Calvert EL, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(5):635–642. doi: 10.1046/j.1365-2036.2003.01486.x. [DOI] [PubMed] [Google Scholar]
- 36.Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable bowel syndrome. Lancet. 1984;2(8414):1232–1234. doi: 10.1016/s0140-6736(84)92793-4. [DOI] [PubMed] [Google Scholar]
- 37.Gonsalkorale WM, Miller V, Afzal A, Whorwell PJ. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52(11):1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremolaterra F, Villoria A, Azpiroz F, Serra J, Aguadé S, Malagelada JR. Impaired viscerosomatic reflexes and abdominal-wall dystony associated with bloating. Gastroenterology. 2006;130(4):1062–1068. doi: 10.1053/j.gastro.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 39.Burri E, Barba E, Huaman JW, et al. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut. 2014;63(3):395–400. doi: 10.1136/gutjnl-2013-304574. [DOI] [PubMed] [Google Scholar]
- 40.Villoria A, Azpiroz F, Soldevilla A, Perez F, Malagelada JR. Abdominal accommodation: a coordinated adaptation of the abdominal wall to its content. Am J Gastroenterol. 2008;103(11):2807–2815. doi: 10.1111/j.1572-0241.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 41.Burri E, Cisternas D, Villoria A, et al. Accommodation of the abdomen to its content: integrated abdomino-thoracic response. Neurogastroenterol Motil. 2012;24(4):312–e162. doi: 10.1111/j.1365-2982.2011.01846.x. [DOI] [PubMed] [Google Scholar]
- 42.Shim L, Prott G, Hansen RD, Simmons LE, Kellow JE, Malcolm A. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010;105(4):883–887. doi: 10.1038/ajg.2010.54. [DOI] [PubMed] [Google Scholar]
- 43.Shim L, Hansen RD, Prott GM, Morris LA, Malcolm A, Kellow JE. Altered temporal characteristics of the rectoanal inhibitory reflex in patients with abdominal distension. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1343–G1346. doi: 10.1152/ajpgi.00400.2011. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull GK, Ritvo PG. Anal sphincter biofeedback relaxation treatment for women with intractable constipation symptoms. Dis Colon Rectum. 1992;35(6):530–536. doi: 10.1007/BF02050531. [DOI] [PubMed] [Google Scholar]
- 45.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656–676; quiz 677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31(8):874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 47.Leichter J. Lactose tolerance in a Slavic population. Am J Dig Dis. 1972;17(1):73–76. doi: 10.1007/BF02239264. [DOI] [PubMed] [Google Scholar]
- 48.Barr RG, Levine MD, Watkins JB. Recurrent abdominal pain of childhood due to lactose intolerance. N Engl J Med. 1979;300(26):1449–1452. doi: 10.1056/NEJM197906283002602. [DOI] [PubMed] [Google Scholar]
- 49.Johlin FC, Panther M, Kraft N. Dietary fructose intolerance: diet modification can impact self-rated health and symptom control. Nutr Clin Care. 2004;7(3):92–97. [PubMed] [Google Scholar]
- 50.Ledochowski M, Widner B, Bair H, Probst T, Fuchs D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand J Gastroenterol. 2000;35(10):1048–1052. doi: 10.1080/003655200451162. [DOI] [PubMed] [Google Scholar]
- 51.Rumessen JJ, Gudmand-Høyer E. Fructans of chicory: intestinal transport and fermentation of different chain lengths and relation to fructose and sorbitol malabsorption. Am J Clin Nutr. 1998;68(2):357–364. doi: 10.1093/ajcn/68.2.357. [DOI] [PubMed] [Google Scholar]
- 52.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 53.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 54.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24(5):487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 55.de Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67(9):895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 56.Muir JG, Gibson PR. Manipulating dietary carbohydrates to treat irritable bowel syndrome. Irritable Bowel Syndrome: Diagnosis and Management. In: Simrén M, Törnblom H, editors. London, United Kingdom: Future Medicine Ltd; 2013. [Google Scholar]
- 57.Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prior A, Whorwell PJ. Double blind study of ispaghula in irritable bowel syndrome. Gut. 1987;28(11):1510–1513. doi: 10.1136/gut.28.11.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Busby RW, Bryant AP, Bartolini WP, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649(1-3):328–335. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 60.Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology. 2013;145(6):1334–1346.e1-11. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 61.Eutamene H, Bradesi S, Larauche M, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil. 2010;22(3):312–e84. doi: 10.1111/j.1365-2982.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 62.Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(9):1084–1092.e3; quiz e68. doi: 10.1016/j.cgh.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12(4):616–623. doi: 10.1016/j.cgh.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 64.Lacy BE, Lembo A, Schey R, et al. Sa2008 efficacy and safety of linaclotide in chronic idiopathic constipation patients with abdominal bloating: phase 3b trial results. Gastroenterology. 2014;146(5):S–353. [Google Scholar]
- 65.Layer P, Stanghellini V. Review article: linaclotide for the management of irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2014;39(4):371–384. doi: 10.1111/apt.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 67.Schmulson M, Chang L. Review article: the treatment of functional abdominal bloating and distension. Aliment Pharmacol Ther. 2011;33(10):1071–1086. doi: 10.1111/j.1365-2036.2011.04637.x. [DOI] [PubMed] [Google Scholar]
- 68.Chey WD, Drossman DA, Johanson JF, Scott C, Panas RM, Ueno R. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2012;35(5):587–599. doi: 10.1111/j.1365-2036.2011.04983.x. [DOI] [PubMed] [Google Scholar]
- 69.Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27(8):685–696. doi: 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 70.Whitehead WE, Palsson OS, Gangarosa L, Turner M, Tucker J. Lubiprostone does not influence visceral pain thresholds in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23(10):944–e400. doi: 10.1111/j.1365-2982.2011.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58(3):357–365. doi: 10.1136/gut.2008.162404. [DOI] [PubMed] [Google Scholar]
- 72.Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation—a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29(3):315–328. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 73.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358(22):2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 74.Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014;26(1):21–27. doi: 10.1111/nmo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emmanuel AV, Roy AJ, Nicholls TJ, Kamm MA. Prucalopride, a systemic enterokinetic, for the treatment of constipation. Aliment Pharmacol Ther. 2002;16(7):1347–1356. doi: 10.1046/j.1365-2036.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 76.Emmanuel AV, Kamm MA, Roy AJ, Kerstens R, Vandeplassche L. Randomised clinical trial: the efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction—a double-blind, placebo-controlled, cross-over, multiple n=1 study. Aliment Pharmacol Ther. 2012;35(1):48–55. doi: 10.1111/j.1365-2036.2011.04907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 78.Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol. 2004;99(5):914–920. doi: 10.1111/j.1572-0241.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- 79.Creed F, Fernandes L, Guthrie E, et al. North of England IBS Research Group. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology. 2003;124(2):303–317. doi: 10.1053/gast.2003.50055. [DOI] [PubMed] [Google Scholar]
- 80.Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther. 2005;22(5):381–385. doi: 10.1111/j.1365-2036.2005.02566.x. [DOI] [PubMed] [Google Scholar]
- 81.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55(8):1095–1103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol. 2003;1(3):219–228. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 83.Rahimi R, Nikfar S, Abdollahi M. Selective serotonin reuptake inhibitors for the management of irritable bowel syndrome: a meta-analysis of randomized controlled trials. Arch Med Sci. 2008;4(1):71–76. [Google Scholar]
- 84.Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8(2):159–166. doi: 10.1111/j.1365-2036.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 85.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15(13):1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460. doi: 10.1002/14651858.CD003460.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 88.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 89.Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152(5):685–689. doi: 10.1016/j.jpeds.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 90.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM; Walkerton Health Study Investigators. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131(2):445–450; quiz 660. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 91.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292(7):852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 92.Pimentel M, Lembo A, Chey WD, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 93.Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36(11-12):1084–1093. doi: 10.1111/apt.12087. [DOI] [PubMed] [Google Scholar]
- 94.Gibson PR, Barrett JS. The concept of small intestinal bacterial overgrowth in relation to functional gastrointestinal disorders. Nutrition. 2010;26(11-12):1038–1043. doi: 10.1016/j.nut.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51(8):1297–1301. doi: 10.1007/s10620-006-9104-6. [DOI] [PubMed] [Google Scholar]
- 96.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98(2):412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 97.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104(4):1033–1049; quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 98.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 99.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 100.Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29(1):104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 101.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 102.Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51(1):24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 103.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 104.Webb AN, Kukuruzovic RH, Catto-Smith AG, Sawyer SM. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2007;(4):CD005110. doi: 10.1002/14651858.CD005110.pub2. [DOI] [PubMed] [Google Scholar]
- 105.Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev. 2009;(1):CD006442. doi: 10.1002/14651858.CD006442.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Palsson OS, Turner MJ, Johnson DA, Burnett CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig Dis Sci. 2002;47(11):2605–2614. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- 107.Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut. 1990;31(8):896–898. doi: 10.1136/gut.31.8.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 109.Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49(6-7):413–421. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rao SS. Biofeedback therapy for constipation in adults. Best Pract Res Clin Gastroenterol. 2011;25(1):159–166. doi: 10.1016/j.bpg.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Luo M-H, Qi Q-H, Dong ZL. Prospective study of biofeedback retraining in patients with chronic idiopathic functional constipation. World J Gastroenterol. 2003;9(9):2109–2113. doi: 10.3748/wjg.v9.i9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chitkara DK, Bredenoord AJ, Talley NJ, Whitehead WE. Aerophagia and rumination: recognition and therapy. Curr Treat Options Gastroenterol. 2006;9(4):305–313. doi: 10.1007/s11938-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 113.Kamm MA, Dudding TC, Melenhorst J, et al. Sacral nerve stimulation for intractable constipation. Gut. 2010;59(3):333–340. doi: 10.1136/gut.2009.187989. [DOI] [PubMed] [Google Scholar]
- 114.Kenefick NJ, Vaizey CJ, Cohen CR, Nicholls RJ, Kamm MA. Double-blind placebo-controlled crossover study of sacral nerve stimulation for idiopathic constipation. Br J Surg. 2002;89(12):1570–1571. doi: 10.1046/j.1365-2168.2002.02278.x. [DOI] [PubMed] [Google Scholar]
- 115.Fassov JL, Lundby L, Laurberg S, Buntzen S, Krogh K. A randomized, controlled, crossover study of sacral nerve stimulation for irritable bowel syndrome. Ann Surg. 2014;260(1):31–36. doi: 10.1097/SLA.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 116.Coban Ş, Akbal E, Köklü S, et al. Clinical trial: transcutaneous interferential electrical stimulation in individuals with irritable bowel syndrome—a prospective double-blind randomized study. Digestion. 2012;86(2):86–93. doi: 10.1159/000338301. [DOI] [PubMed] [Google Scholar]
- 117.Tremolaterra F, Pascariello A, Gallotta S, Ciacci C, Iovino P. Colonic gas transit in patients with bloating: the effect of an electromechanical stimulator of the abdominal wall. Tech Coloproctol. 2013;17(4):405–410. doi: 10.1007/s10151-012-0951-1. [DOI] [PubMed] [Google Scholar]
- 118.Hall RG, Thompson H, Strother A. Effects of orally administered activated charcoal on intestinal gas. Am J Gastroenterol. 1981;75(3):192–196. [PubMed] [Google Scholar]
- 119.Potter T, Ellis C, Levitt M. Activated charcoal: in vivo and in vitro studies of effect on gas formation. Gastroenterology. 1985;88(3):620–624. doi: 10.1016/0016-5085(85)90129-5. [DOI] [PubMed] [Google Scholar]
- 120.Hanauer SB, DuPont HL, Cooper KM, Laudadio C. Randomized, double-blind, placebo-controlled clinical trial of loperamide plus simethicone versus loperamide alone and simethicone alone in the treatment of acute diarrhea with gas-related abdominal discomfort. Curr Med Res Opin. 2007;23(5):1033–1043. doi: 10.1185/030079907x182176. [DOI] [PubMed] [Google Scholar]
- 121.Kaplan MA, Prior MJ, Ash RR, McKonly KI, Helzner EC, Nelson EB. Loperamide-simethicone vs loperamide alone, simethicone alone, and placebo in the treatment of acute diarrhea with gas-related abdominal discomfort. A randomized controlled trial. Arch Fam Med. 1999;8(3):243–248. doi: 10.1001/archfami.8.3.243. [DOI] [PubMed] [Google Scholar]
- 122.Coffin B, Bortolloti C, Bourgeois O, Denicourt L. Efficacy of a simethicone, activated charcoal and magnesium oxide combination (Carbosymag®) in functional dyspepsia: results of a general practice-based randomized trial. Clin Res Hepatol Gastroenterol. 2011;35(6-7):494–499. doi: 10.1016/j.clinre.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 123.Drummond L, Gearry RB. Kiwifruit modulation of gastrointestinal motility. Adv Food Nutr Res. 2013;68:219–232. doi: 10.1016/B978-0-12-394294-4.00012-2. [DOI] [PubMed] [Google Scholar]
- 124.Udani JK, Bloom DW. Effects of Kivia powder on gut health in patients with occasional constipation: a randomized, double-blind, placebo-controlled study. Nutr J. 2013;12(1):78. doi: 10.1186/1475-2891-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melzer J, Rösch W, Reichling J, Brignoli R, Saller R. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast) Aliment Pharmacol Ther. 2004;20(11-12):1279–1287. doi: 10.1111/j.1365-2036.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- 126.Madisch A, Holtmann G, Plein K, Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther. 2004;19(3):271–279. doi: 10.1111/j.1365-2036.2004.01859.x. [DOI] [PubMed] [Google Scholar]