Abstract
Importance
Obstetricians and gynecologists frequently deal with hemorrhage so they should be familiar with management of patients who refuse blood transfusion. Although there are some reports in the literature about management of Jehovah’s Witness patients in obstetrics and gynecology, most of them are case reports and a comprehensive review about these patients including ethico-legal perspective is lacking.
Objective
This review outlines the medical, ethical and legal implications of management of Jehovah’s Witness patients in obstetrical and gynecological settings.
Evidence Acquisition
A search of published literature using Pubmed, Ovid Medline, EMBASE and Cochrane databases was conducted about physiology of oxygen delivery and response to tissue hypoxia, mortality rates at certain hemoglobin levels, medical management options for anemic patients who refuse blood transfusion and ethical/legal considerations in Jehovah’s Witness patients.
Results
Early diagnosis of anemia and immediate initiation of therapy is essential in patients who refuse blood transfusion. Medical management options include iron supplementation and erythropoietin. There are also some promising therapies that are in development such as anti-hepcidin antibodies and hemoglobin based oxygen carriers. Options to decrease blood loss include anti-fibrinolytics, desmopressin, recombinant Factor VII and factor concentrates. When surgery is the only option, every effort should be made to pursue minimally invasive approaches.
Conclusion and Relevance
All obstetricians and gynecologists should be familiar with alternatives and ‘less invasive’ options for patients who refuse blood transfusions.
Target Audience
Obstetricians and gynecologists, family physicians
Background
This is the story of a Jehovah’s Witnesses patient in her own words:
‘Refusing blood transfusion has personally affected me four times. Once when my mother had a postpartum hemorrhage and her doctor refused to treat her. The second was when my daughter was born and thankfully my doctor figured out an alternative way to stop my hemorrhage and was supportive of my choice. The third was at the emergency room when I had bleeding and anemia. Three gynecologists said there was nothing they could do other than iron if I refuse a transfusion. One physician even told me I was choosing death! The fourth time was recently when I had heavy vaginal bleeding. Thank you for your kind, respectful bedside manner and working on an alternative solution for me. Speaking strictly for myself and my experiences, it seems as though once I mention I won’t accept a blood transfusion, some physicians think I am saying I won’t accept any treatment while all I’m really asking for is alternative solutions. If your article improves the approach of only one physician, it could be lifesaving.’
This Christian community was initially formed in 1872 in Pittsburg, PA and now includes around 1 million members in the USA and 6 million worldwide. Their belief of refusal of blood transfusion comes from about 36 bible verses and the most well-known are:
- Genesis 9:4 "But flesh (meat) with…blood…ye shall not eat"
- Leviticus 17:12-14 "…No soul of you shall eat blood…whosoever eateth it shall be cut off"
- Acts 15:29 "That ye abstain…from blood…"
- Acts 21:25 "…Gentiles…keep themselves from things offered to idols and from blood…"
As a result, their members refuse the four major components of blood (red blood cells, white blood cells, platelets and plasma) according to the Watchtower Society’s declaration in 1945 (1). The Watchtower Society is the governing council of Jehovah’s Witnesses, which has the ultimate authority on all issues related to their faith tradition. Autologous blood transfusions from stored blood are not acceptable either. However, the administration of fractionates such as albumin, recombinant human erythropoietin (rhEPO) and factor concentrates are now permissible as the declarations have changed in recent years. Also procedures in which, blood is taken out of the body and continuously reinfused (e.g. hemodialysis, intraoperative cell saver) is up to the individual’s preference (2).
Obstetricians and gynecologists frequently deal with hemorrhage, therefore, they should be familiar with management of patients who refuse blood transfusion. The aim of this paper is to review the medical, ethical and legal implications of management of Jehovah’s Witness patients in obstetrical and gynecological settings.
Physiological Considerations
Oxygen delivery and the response to tissue hypoxia
The physiology of oxygen delivery to tissues is the standpoint for understanding the response to anemia and, therefore, treatment options of patients who refuse blood transfusion. The amount of blood and oxygen delivered to the whole body (DO2) is determined by the equation below and is related to two major components:
DO2= Arterial Oxygen Content (AOC) × Cardiac Output (CO). (3)
The oxygen content in arterial system can be in one of two forms, either bound to hemoglobin (Hb) or dissolved in plasma water. The Hb-bounded oxygen is the major component of AOC and the molecular structure of hemoglobin determines the oxygen’s binding affinity. Hemoglobin consists of four globin chains, each of which contains a heme ring that causes flexibility for the bound, leading to a high affinity at high PO2 levels in the lungs and low affinity at low PO2 levels in the tissues.
AOC= % saturation × 1.39* × Hb (g/L) (4,5)
* 1 g fully saturated Hb binds 1.39 ml of oxygen
If these two equations are combined, then the equation for the delivery of the oxygen content to the body is
DO2= % saturation × 1.39 × Hb × Cardiac Output (CO)
This equation shows the major determinants of hypoxia at the cellular level: % saturation, Hb level and CO. CO is the amount of blood pumped into the general circulation and is calculated by multiplying the stroke volume and heart rate. Stroke volume is affected by preload, afterload and contractility.
There are several variables affecting the blood supply to different organ systems in acute situations and the response mechanisms. A range of 4 to 10 ml/min/kg, translates to a DO2 of 280 to 700 ml/min in an average 70 kg patient. In a patient with an Hb level of 10 g/dl, 99% saturation and 5 L/min CO, DO2 will be 688 ml/min, suggesting maximal global oxygen delivery occurs at Hb levels of ≥ 10 g/dl. Below this level, although oxygen carrying capacity is decreased, tissue oxygenation is still preserved due to the response mechanisms. These mechanisms include an increase in 2,3 diphosphoglycerate (DPG) concentrations in red blood cells which causes a right shift in the oxygen dissociation curve, increase in erythropoietin levels to enhance erythropoiesis, and increase in stroke volume and heart rate to increase cardiac output (5).
The critical Hb content below which adequate tissue oxygenation is impaired seems to be 4 g/dl (DO2 of 275 ml/min) in a healthy individual (5) however it is expected to be higher in patients with comorbid conditions and those on certain medications. For example, beta-blockers impair the compensatory CO increase. Similarly, patients with coronary heart disease require a higher baseline critical DO2, as the perfusion of coronary arteries is impaired due to shortened diastolic phase and increased heart rate.
Mortality Rates at Certain Hemoglobin Levels
It is estimated that about 1000 Jehovah’s Witnesses die annually due to refusal of blood transfusion (6). In Jehovah’s Witness patients, the question of the critical Hb level for transfusion to decrease mortality can be transformed to the critical Hb level to start medical management or to administer blood substitutes. Weis and colleagues demonstrated that the response mechanisms can maintain satisfactory tissue oxygenation down to a Hb level of 5 g/dl (7). However, acute isovolemic anemia in healthy young individuals decreases neurocognitive function and central neural processing below Hb levels of less than 6 and 5 g/dl, respectively (8). The studies assessing the mortality risk of patients with very low Hb levels who refuse blood transfusion reveal that Hb levels less than 5-6 g/dl are strongly associated with mortality (5). Carson et al. (8) published the largest series of patients with postoperative Hb levels ≤ 8 g/dl, who declined blood transfusion for religious reasons. They reported that the odds of death in patients with a postoperative Hb level ≤ 8 g/dl increased 2.5 times for each gram decrease in Hb levels. In patients with Hb levels between 5-7 g/dl, the mortality was about 9%. In patients with Hb level between 7 -8, although there were no deaths, the serious morbidity (arrhythmia, congestive heart failure, myocardial infarction, bacteremia, pneumonia, deep wound infection) rate was 9.4%. A recent review (10) of 134 Jehovah’s Witness patients found no mortality at a Hb concentration of higher than 5 g/dl, however, below this level, the mortality rate was found to be 44%. All these data suggest that when RBC transfusion is not an option alternative treatments should be considered before the Hb level decreases to 5-6 g/dl.
In addition to Hb levels, there are other factors that increase the risk of mortality. A recent study developed mortality risk stratification in severely anemic Jehovah’s Witness patients (11). Eleven significant mortality risk factors were identified: age ≥ 45 years, non-European, non-Maori ethnicity, weight ≥ 90 kg, acute admission, hypertension, cardiac arrhythmia, angina, previous myocardial infarction, valvular heart disease, heart failure, hemodialysis and Hb ≤ 8 g/dL on admission. Among all eleven, age (RR: 8.85), acute admission (RR: 14.99) and Hb ≤ 8 g/dL (RR: 6.8) were found to be most strongly correlated mortality risk factors (Table 1). Also each patient was assigned mortality risk stratification according to the total number of these risk factors present on admission to the hospital. Patients were divided into four groups according to the developed mortality stratification 0–3, 4–5, 6–7 and ≥ 8 factors and mortality rates were 4.4%, 31.8%, 50% and 83.3% respectively.
Table1.
Mortality risk factors for Jehovah’s Witness patients (11)
| Risk Factors | RR |
|---|---|
| Acute admission | 14.9 |
| Age ≥ 45 | 8.8 |
| Hb ≤ 8 g/dL | 6.8 |
| Cardiac arrythmia | 5.2 |
| Hypertension | 4.9 |
| Non-European, Non-Maori ethnicity | 4.1 |
| Hemodialysis | 4.0 |
| Previous myocardial infarction | 3.3 |
| Weight ≥ 90 kg | 3.2 |
| Heart Failure | 3.1 |
| Angina | 2.6 |
| Valvular Heart Disease | 2.5 |
Medical Considerations
Iron Supplementation
Since JW patients will not accept blood transfusion, any degree of anemia should be immediately treated. Iron deficiency anemia is the most common type and its overall management is not different in JW patients. As oral iron therapy takes at least 6 weeks and up to 6 months to replete iron stores and the amount of bleeding generally exceeds the ability of the GI tract to absorb iron, this will not be effective in acute settings. The maximum amount of elemental iron that can be absorbed through gastrointestinal tract is 25 mg/day, whereas 1000 mg of elemental iron can be administered following a single infusion intravenous iron (12). Also oral iron does not synergize well with erythropoietin stimulating agents in patients with acute blood loss (13). For these stimulating agents to be optimally effective, the iron stores should be repleted beforehand or faster.
Available IV iron preparations include iron dextran, ferric gluconate, iron sucrose, ferumoxytol, ferric carboxymaltose and iron isomaltoside (Table 2).
Table 2.
FDA approved parenteral iron preparations in the US
| Iron concentration (mg/ml) |
Max approved dose (mg elemental) |
Vial volume (ml) |
Test dose | Premedication | Total dose infusion possible |
Route | |
|---|---|---|---|---|---|---|---|
| Iron dextran (high molecular weight) |
50 | 100* | 1-2 | Required γ | No | Yes | IM IV (preferred) |
| Iron dextran (low molecular weight) |
50 | 100* | 2 | Required γ | No | Yes | IM IV (preferred) |
| Ferric gluconate | 12.5 | 125• | 5 | Not required |
No | No | IV only |
| Iron sucrose | 20 | 200ε | 5 | Not required |
No | No | IV only |
| Iron Polyglucose sorbitol carboxymethylether |
30 | 510 κ | 17 | Not required |
No | No | IV only |
The formula used for calculation of parenteral iron dose: Required iron dose (mg) = (2.4 × (target Hb-actual Hb) × pre-pregnancy weight (kg)) + 1000 mg for replenishment of stores (81)
Doses ≤ 300 mg, slow iv push up to 50 mg/minute; or diluted in 100-250 ml normal saline. For total dose infusion, the dose should be diluted to normal saline (250-1000 ml) and after a test dose, the solution can be infused over 4-6 hours.
25 mg over 5 minutes. Observe patient for 1 hour.
IV infusion of 125 mg diluted in 100 ml normal saline over 60 minutes daily for 5 doses maximum per week, up to a cumulative dose of 1 gram. Transferrin saturation and serum iron levels should be checked 48 hours after the third dose to assist to detect iron accumulation. No need for daily infusions if the transferrin saturation is more than 40%.
100mg IVP over 2-5 minutes; 100 mg/ 100ml normal saline over 15 minutes; 200mg / 250ml normal saline over 2 - 4 hours for a total dose of 1,000mg over a 14-day period. If more than 600mg is needed for iron repletion, a transferrin saturation and serum iron levels should be checked 72 hours after the completion of weekly dose to assist in detection of iron accumulation. May stop infusions unless transferrin saturation is less than 40%
5-10 mg IV push
ref: Adamson JW. Iron deficiency and other hypoproliferative anemias. In: Braunwald E, Fauci AS, Kasper DL, editors. Harrison's textbook of internal medicine. 17th ed. New York: McGraw Hill; 2008. pp. 628–33.
The choice of iron preparation
There is clear evidence that iron stores should be repleted in patients with low Hb levels for erythropoiesis stimulating agents to be more effective (14), however there is no IV preparation which has been proved to be superior over another. At this point, the choice of the preparation is generally based on patient characteristics (e.g. drug allergy) or drug effectiveness and safety.
The most common concern about IV iron preparations is the anaphylactic reaction. Although high molecular weight IV iron preparations are associated with higher incidence of systemic reactions (4.7%), adverse events with low molecular weight reactions are rare (< 1/200000) (15). Low molecular weight iron dextran, iron sucrose, ferric gluconate complex and ferumoxytol were found to be safe in randomized controlled trials and meta-analyses (16). Although rare, anaphylactic type reactions are generally seen with high molecular weight IV iron dextran and due to this issue, FDA recommends that equipment and trained personnel for anaphylactic type reactions be available during IV iron dextran administration. If the patient has asthma or more than one drug allergy, IV premedication with steroids should be considered for all IV iron formulations (17). Premedication with antihistamines is discouraged.
Erythropoietin
Erythropoietin (EPO) use reduces the need for RBC transfusion (18) with an average Hb increase of 1.44 g/dl per week (19). However there is no consensus about the dose and schedule. Ball et al (20) reviewed fourteen cases of critically ill Jehovah’s Witnesses who needed erythropoietin therapy as a blood alternative. In each clinical situation, erythropoietin was found to be effective to enhance erythropoiesis, however time period to initiate therapy, dosages, route of administration and treatment duration varied widely. For elective non-cardiac non-vascular surgeries, there is now a FDA approved dosage regimen of epoetin alpha:
- If preoperative period is ≥ 3 weeks, 600 U/kg, subcutaneous, every week, total of four doses (last dose is at the day of surgery)
- If preoperative period is < 3 weeks, 300 U/kg once daily, a total of 15 doses.
It is not clear whether weight based regimen is superior to standard dose regimens (dose of a standard 70 kg patient). rHuEPO reveal low volume of distribution (0.042-0.07 L/kg) (20) and it can be assumed that the drug stays within intravascular space rather than widely distributed in tissues. Therefore standard dosing (i.e. 20000 units vs 40000 units for a 70 kg patient) is also reasonable to minimize drug waste.
Erythropoietin does not only stimulate the bone marrow, but it also has different properties. It acts as a cytokine with anti-apoptotic effects (21-23) with protective properties from hypoxemia and ischemia, which might provide additive benefit in severely anemic patients who refuse blood transfusion. The overexpression of the erythropoietin receptors in multiple tissues is also suggestive of this protective effect (21). Iron, folate and vitamin B12 should also be added to keep up with enhanced erythropoiesis.
Darbapoietin alfa is the modified version of the rHuEPO with a three times longer half life which gives a benefit of less frequent dose regimens however it has not been widely studied in this patient population. The most serious side effect with erythropoietin use is thrombotic events, which can be especially seen when Hb concentrations are ≥ 12 g/dl (21-23).
Agents to minimize bleeding
Antifibrinolytics
Antifibrinolytics, categorized as serine protease inhibitors or lysine analogues, are used in various clinical scenarios to minimize bleeding. Aprotinin is a serine protease inhibitor that inhibits various proteases including plasma and tissue kallikrein leading to weakening of intrinsic coagulation pathway and inhibition of fibrinolysis (24). Tranexamic acid and e-aminocaproic acid are synthetic lysine analogues which act by blocking the lysine binding sites on plasminogen molecules, leading to inhibition of the formation of plasmin and therefore inhibiting fibrinolysis (25).
Among these agents, tranexamic acid is the mostly studied agent in obstetrics and gynecology literature. Although aprotinin is effective in decreasing blood loss, it has become a matter of debate because of its side effects after a study in cardiac surgery (26). The study investigated the risk of excessive bleeding in cardiac surgery in which patients were randomized to receive either aprotinin, tranexamic acid or e-aminocaproic acid to investigate the bleeding minimizing effects during surgery, however it was interrupted due to increased 30 day mortality rate in the aprotinin arm of 6%, whereas mortality rate was 3.9% for tranexamic acid and 4% for e-aminocaproic acid arm. However a recent Cochrane analysis (27) investigating the effects of anti-fibrinolytic use for minimizing perioperative allogenic blood transfusion did not show an increased risk of myocardial infarction (RR 0.87, 95% CI 0.69 to 1.11), stroke (RR 0.82, 95% CI 0.44 to 1.52), renal dysfunction (RR 1.10, 95% CI 0.79 to 1.54) or overall mortality (RR 0.81, 95% CI 0.63 to 1.06) regarding aprotinin use. Aprotinin was found to reduce the RBC transfusion requirement 34% (relative risk [RR] 0.66, 95% confidence interval [CI] 0.60 to 0.72). The relative risk for RBC transfusion was found to be 0.61 (95% CI 0.53 to 0.70) 0.81 (95% CI 0.67 to 0.99) with tranexamic acid and e-aminocaproic acid use, respectively.
These drugs have been shown to be effective in the obstetrical and gynecological setting. A systematic review by Mattison et al (28) about management of heavy menstrual bleeding reported that tranexamic acid was related to 26-54% decrease in menstrual blood flow. A review, which investigated the effects of lysine analogues during pelvic surgery included both urologic and gynecologic procedures. Among 56 articles that are reviewed, 21 were related to gynecologic surgery and lysine analogues were found to decrease blood loss significantly in abdominal hysterectomies, c-sections, cervical conizations and hysteroscopic resectoscopes without increasing the thromboembolic events (29). Although not specifically related to gynecologic surgery, a recent Cochrane systematic review also investigated the effect of tranexamic acid on surgical bleeding and found that it reduced receiving blood transfusion by one third (RR 0.62, 95% CI 0.58 – 0.65; P < 0.001) (30). A very recent Cochrane review showed that tranexamic acid, in addition to uterotonic medications, decreases postpartum blood loss and prevents postpartum hemorrhage and blood transfusions following vaginal birth and CS in women at low risk of postpartum hemorrhage (31).
All these data suggest that antifibrinolytic agents are effective to decrease blood loss. Although there is a theoretical risk for thromboembolic events, high quality studies have not found increased risk that were hypothesized when these agents were introduced.
Desmopressin
In addition to its approval for bleeding disorders to increase the plasma levels of factor VIII and von Willebrand Factor (32), Desmopressin also gained attraction to minimize bleeding in surgical procedures. Currently, there is no publication in the literature specifically investigating the effects of Desmopressin on blood loss and transfusion needs in gynecologic surgery, however the largest review and meta-analysis in the literature investigating the effects of Desmopressin on transfusion needs concluded that Desmopressin was associated with reduced requirements of blood product transfusion (standardized mean difference: − 0.29 [−0.52 to −0.06] units per patient; P < 0.01), that were more pronounced in noncardiac surgical patients. Thromboembolic events were not increased in the Desmopressin group (57/1,002 = 5.7% in the Desmopressin group vs. 45/979 = 4.6% in the placebo group; P = 0.3) (33). In most of the included studies the dosage was 0.3 microgram/kg, which was administered prophylactically over a 15 to 30 minute period.
Factor VII
Factor VII (Novoseven® RT, Novo Nordisk, Bagsvaerd, Denmark) is produced by recombinant technology and is approved by the FDA for surgeries and procedures in adults and children with hemophilia A or B with inhibitors, congenital Factor VII deficiency, and people with Glanzmann's thrombasthenia with decreased or absent response to platelet transfusions. However it has been also used as ‘off-label’ in various settings including trauma, surgery and postpartum hemorrhage. Its administration leads to the activation of factor X and thrombin (34) causing an enhanced primary and secondary hemostasis (35) and it also activates thrombin activatable fibrinolytic inhibitor and FXIII (36,37). To have the optimum effect of rFVII, there should be an optimal hemostatic environment: hematocrit should be around 30%, platelets > 50000/ml, fibrinogen level more than 1 g, arterial pH > 7.2, normal blood calcium levels and temperature > 34.8 C and PT/PTT should be less than 1.5 × upper normal range (38).
Although there is no consensus on the optimal dose for off label use such as in postpartum hemorrhage, in most of the case series 90 mcg/kg was used. This is also the recommended dose for hemophilia patients. The dose can be repeated in 30-60 minutes if there is no improvement and it is assured that there is no arterial bleeding, as the drug has a short half life of about 2 hours (38). One of the most comprehensive reviews in the literature analyzed a total of 272 women with postpartum hemorrhage and at a median dose of 81.5 mcg/kg, rFVIIa was found to b5-10 mg IV e effective in stopping or reducing bleeding in 85% of the cases (39). The most serious complication with rFVII is thromboembolic events, however, it is reported in less than 1% of cases (40).
Factor concentrates
Prothrombin Complex Concentrate
Prothrombin complex concentrates (PCCs) are extracted from human plasma and mainly indicated for reversal of vitamin K antagonists. There are two types of PCCs in the market as ‘four factor’ concentrates (Kcentra, CSL, Behring and Octaplex, Octapharma, Switzerland), which include factors II, VII, IX, X and ‘three factor’ concentrates (Bebulin, Baxter, CA, USA and Prolfinine, Grifols, CA, USA), which include factors II, IX, X. These concentrates also contain various amounts of anti-thrombotic constituents such as protein C, protein S and protein Z (41,42) as well as some anti- thrombin III and small amounts of heparin (0.5 IU heparin per IU factor IX) to balance the potential pro-thrombotic effects (43). These concentrates include 25 times higher factors than that of plasma and are standardized according to their factor IX content (44). The efficacy of PCCs in patients who need urgent surgery or invasive intervention has been shown in various studies in the literature (45,46). In the study by Goldstein et al (47), 168 patients who needed reversal of vitamin K antagonist for an urgent surgery or invasive procedure were randomized to have either PCC or fresh frozen plasma (FFP) (4fPCC=87; plasma= 81). All patients received vitamin K concomitant with a single dose of either 4F-PCC (Beriplex/Kcentra/Confidex; CSL Behring, Marburg, Germany) or plasma, with dosing based on international normalized ratio (INR) and weight. Effective hemostasis was achieved in 90% of patients in the 4F-PCC group compared with 75% of patients in the plasma group and rapid INR reduction was achieved in 55% of patients in the 4F-PCC group compared with 10% of patients in the plasma group. The study demonstrated that PCCs are superior to plasma for effective hemostasis and rapid INR reversal without significant difference in adverse events. Rapid normalization of factors II, VII, IX and X was achieved within 30 minutes with PCCs while it takes minimum of 3 hours for FFP. The updated the American College of Chest Physicians guidelines recommend administration of 4-factor PCC, rather than FFP, and the additional use of 5 to 10 mg of iv vitamin K for rapid reversal of vitamin K antagonists (48).
Cryoprecipitate
Each unit of cryoprecipitate is commonly prepared from 1 unit of fresh frozen plasma (FFP) by centrifugation of thawed FFP at 1-6 ° C and includes fibrinogen (150-250 mg), FVIII (80-150 units), vWF (100-150 units) and FXIII (50-75 units) (49). Although the indications include hemophilia, von Willebrand disease, hypofibrinogenemia, afibrinogenemia, its use in obstetrics and gynecology is usually in the setting of massive postpartum hemorrhage and disseminated intravascular coagulation (50). One pool (5 units) of cryoprecipitate is expected to raise fibrinogen levels by 50 mg/dl in an average weight (70 kg) woman (49). However the dose should be individualized based on severity of consumption. It has been successfully used to increase fibrinogen levels during obstetric hemorrhage (50).
Fibrinogen Concentrate
Fibrinogen Concentrate (RiaSTAP™) is produced from pooled human plasma and is used in bleeding episodes with fibrinogen deficiency. One vial contains 900 mg to 1300 mg of fibrinogen concentrate (51). There is strong evidence that a low fibrinogen level is an accurate predictor for severe postpartum hemorrhage (52,53). In the case of a severe hemorrhage, monitoring of patient’s fibrinogen level is recommended during treatment and a target fibrinogen level of 100 mg/dl should be maintained until hemostasis is achieved. Point of care testing of coagulation using thromboelastography (TEG!; Haemonetics, Braintree MA, USA), or thromboelastometry (ROTEM; TEM GmBH, Munich, Germany) is uncommon in labor and delivery suites, however rapid bedside assessment of coagulation could be beneficial in a Jehovah’s Witness patient who is refusing blood transfusion. ROTEM FIBTEM was used successfully to predict fibrinogen levels during postpartum hemorrhage (53). As an average, a FIBTEM of 15 mm equated to a fibrinogen level of 300 mg/dl, 10 mm to 200 mg/dl, and 6 mm to 100 mg/dl.
The dosage and route of administration of medical therapies are summarized in Table 3.
Table 3.
Medications used to minimize bleeding
| Medication | Mechanism of Action |
Common Indications | Dose/Route | Comments/ Adverse effects |
|---|---|---|---|---|
| Tranexamic acid (82) |
*Antifibrinolytic *Block lysine binding sites on plasminogen |
*Abnormal uterine bleeding *Postpartum hemorrhage |
*1300 mg TID (Max 5 days) *1000 mg (single dose) |
Theoretical concern for thromboembolic events |
| e-aminocaproic acid (82) |
*Antifibrinolytic *Block lysine binding sites on plasminogen |
*Abnormal uterine bleeding, *Postpartum hemorrhage |
4-5 g IV X 1, then 1 g/h IV 5 g po X 1, then 1 g tabs q1h cont X 8 or hours or until the bleeding controlled Max: 30 g/day |
Theoretical concern for thromboembolic events |
| Desmopressin (82) |
*Increase plasma levels of FVIII and vWF |
*Abnormal uterine bleeding *Postpartum hemorrhage, *Surgical bleeding |
0.3 mcg/kg IV X 1 | Over a 15 to 30 minute period |
| rFVIIa (39) | *Activation of -Factor X -Thrombin - Thrombin activatable fibrinolytic inhibitor -Factor XIII |
*Postpartum hemorrhage (off label) |
90 mcg/kg IV (The dose can be repeated in 30-60 minutes if there is no improvement) |
To have the optimum effect of rFVII, there should be an optimal hemostatic environment |
| Four factor concentrate |
Factors II, VII, IX, X | *Rapid reversal of vitamin K antagonist |
Individualized dosage/ IV |
Additional use of 5-10 mg of IV vitamin K is recommended (48) |
| Three factor concentrate |
Factors II, IX, X | *Rapid reversal of vitamin K antagonist |
Individualized dosage/ IV |
Additional use of 5-10 mg of IV vitamin K is recommended (48) |
| Cryoprecipitate | Fibrinogen, FVIII, vWF, FXIII |
*Hemophilia *Von Willebrand Disease *Afibrinogenemia *Postpartum hemorrhage/DIC |
Individualized Dosage/ IV |
One pool (5 units) is expected to raise fibrinogen levels by 50 mg/dl |
| Fibrinogen | Factor I | *Afibrinogenemia *Hypofibrinogenemia *Postpartum hemorrhage |
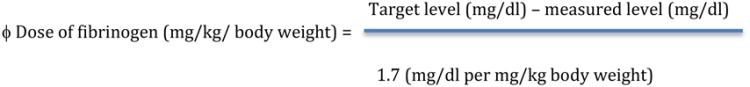
*70 mg/kg (When fibrinogen level is unknown) ϕ |
|
| ||||
Therapies in Development
Anti-Hepcidin agents: A promising therapy for iron deficiency anemia resistant to IV iron and eryhtropoietin
Hepcidin, a liver-derived hormone with a central role in iron metabolism, acts by preventing the export of iron flows into plasma (54). The mechanisms of this action include the degradation of iron-exporting channel ferroportin on duodenal enterocytes leading to decreased iron absorption, also inhibition of the release of recycled iron from reticuloendothelial cells and inhibition of mobilization of iron from hepatic stores (54,55). It is elevated in inflammatory diseases such as chronic infections, autoimmune diseases, malignancies and as well as in chronic kidney disease and was suggested to be responsible for treatment resistance to erythropoietin stimulating agents and also iv iron in iron deficiency anemia (56). Certain inflammatory cytokines (IL-6, IL-1 and IL-22) are suggested to be responsible for the increased synthesis in inflammatory diseases, whereas its decreased clearance is suggested to be the main mechanism in chronic kidney disease (54).
Several approaches for Hepcidin inhibition are under development (57). Lexaptepid, which is a non-natural mirror image of monomethoxy-polyethylene-glycol, inactivates hepcidin in a similar conception with antibodies (55) and it has been shown to correct anemia in animal models (58-60). In a recent study it was tested in humans and found to be effective in prevention of serum iron decrease in experimental human endotoxemia. Anti-hepcidin therapies seem to be promising for utilization of iron stores especially in patients who refuse blood transfusion.
Hemoglobin based oxygen carriers
Oxygen carriers augment oxygen carrying capacity in anemic patients. Two types of oxygen carriers have been developed: hemoglobin based oxygen carriers (HBOCs) and Perfluorocarbons (PFCs). The latter is actually not a true oxygen carrier, but a solvent with the ability to absorb gases. PFCs are not currently commercially available.
HBOCs are derived from animals (bovine) or human outdated red blood cells. The development process includes obtainment of hemoglobin molecules through ultrafiltration and purification followed by cross-linking as well as polymerization to prevent dissociation of hemoglobin into its globin chains and to form high molecular weight molecules to prevent its filtration through renal glomeruli (61). Currently, most of the research for developing an optimal oxygen carrier is on hold due to safety issues. However, these agents should still be considered in situations where benefits outweigh the risks as when a severely anemic patient refuses blood transfusion. At this point, the question is when to administer these agents. In light of the physiology and mortality reports of Jehovah’s Witness patients, it is logical to start infusion before Hb levels decreases to 5-6 g/dl in healthy individuals and 7-8 g/dl in patients with comorbid conditions. In a study in which HBOC is administered to patients with Hb level of < 8 g/dl, mortality was found to be increased proportional to duration from onset of anemia until administration of HBOC-201 (Hemopure) (62). The drug is not available in the market and requires specific permissions from the Food and Drug Administration (FDA) and Institutional Review Board (IRB) of the hospitals. These steps include contacting manufacturer, obtaining an FDA investigational new drug (IND) and IRB approval, and obtaining the informed consent from the patient or surrogate. This process takes approximately 24-48 hours. Once it is obtained, the infusion can be started immediately but slowly (60 ml/hr) to avoid the vasoconstrictive effects. Hemopure has some advantageous properties over pRBCs, as it does not require ABO screening and can be stored in room temperature for 3 years without any need for refrigeration. One unit includes 32.5 gram of polymerized bovine hemoglobin in approximately 225 to 250 ml of a solution and increases the plasma hemoglobin level 0.63 g/dl with a half-life of 19 hours (63).
The side effects that causes concern about HBOCs include myocardial infarction, stroke, increase in blood pressure, acute renal failure, increase in liver enzymes, methemoglobinemia and death (64,65). These side effects are mainly attributed to nitric oxide scavenging properties of extracellular hemoglobin and increased methemoglobin levels (66-68). If hypertension develops, methemoglobin levels exceed 10% or cyanosis or shortness of breath occurs, the infusion should be held.
Surgical considerations
In the Jehovah’s Witness patient, every effort should be made to pursue minimally invasive approaches. These include vaginal, laparoscopic, robotic, and radiologically-guided procedures such as uterine artery embolization and Magnetic Resonance guided focused ultrasound (MRgFUS). Surgically, vaginal approach should be the first choice, followed by laparoscopic or robotic approaches. These approaches are proved to decrease blood loss compared to open surgery (69). Uterine artery embolization and MRgFUS are successfully used with minimal blood loss for uterine fibroids (70,71). Uterine artery embolization has also been used in selected cases to preserve fertility in postpartum hemorrhage (72).
Intraoperative blood salvage also known as ‘cell saver’ should also be considered in a Jehovah’s Witness patient when significant blood loss is expected during a surgical procedure. The technique provides the advantage of autologous blood transfusion to the patient and time saving as it is done in the operating room without involvement of the transfusion service. The procedure includes two main components: collection (aspiration) and processing of blood (centrifugation, washing, reinfusion) (73).
Collection is done via a suction system through negative pressure aspiration. As shear stress might increase the risk of hemolysis, lowest tolerable suction pressure (80-120 torr) should be applied (ref). Heparin is the most commonly used anticoagulant in a carrier (e.g. normal saline) at a dose of 30,000 units/L and this solution is added automatically to the aspirated blood at a rate of 15 mL per 100 mL of collected blood (74). Citrate should be the preferred anticoagulant if there is concern about heparin-induced thrombocytopenia. When the blood reaches to a sufficient amount (375-750 mL), the processing starts in the reservoir. Centrifugation allows the high-density red blood cells to be separated from plasma, platelets and waste products. Washing with an isotonic solution eliminates lighter debris from the blood and washed-blood is then re-infused to the patient. A 40-micron micro-aggregate filter is used between re-infusion line and the patient. A leukocyte depletion filter may also be used as an additional safety measure. Cell saver instruments can process the whole reservoir blood in three minutes. A full reservoir salvaged blood includes > 17 g/dl hemoglobin (55-225 mL) (74) The American College of Obstetricians and Gynecologists recommends its use in high risk patients (e.g. placenta accreta) (75).
Ethical Considerations
From an ethical perspective, a patient’s decision to forego whole blood transfusion is an potential example of a case of “informed refusal,” which requires clinicians to assess the appropriateness of that refusal. Patients with decision-making capacity may refuse any and all treatments; however, to be considered ethically appropriate, this refusal must be informed. The assessment of whether a patient’s refusal is informed or not requires careful consideration of the patient’s narrative, decision-making capacity, values, and religious beliefs so that treatment options may be selected that align with the individual patient’s goals. Interpreting the comments made by the patient in the introduction reveals at least two potentially inappropriate responses. First, physicians should avoid assuming that this patient’s refusal to accept blood transfusion means that she is choosing death, refusing care, or indicating a lack of interest in alternative options. Failing to further discuss with the patient the meaning of her refusal and the values that underlie it denies her the possibility of a genuine therapeutic relationship. Physicians have a fiduciary duty to create a trusting relationship with their patients. This relationship is harmed by failing to disclose alternative treatment options based on the assumption that a patient would simply refuse them. Second, physicians should avoid the tendency to stereotype Jehovah’s Witness patients based on this religious denomination’s well-known position on blood transfusions. Religious identity or affiliation does not entail full endorsement of that group’s positions. Failing to treat each patient as an individual can be a sign of lack of respect for them as persons and lack of respect for their autonomy.
Autonomy
Respect for autonomy, also commonly understood as the right to self-determination or to choose for oneself, entails presenting the preservation of life through aggressive interventions as one of many values within a “hierarchy of goods,” (76) resisting the temptation to subordinate the patients’ values to one’s professional values, and acknowledging that the goods provided by medicine are to be judged relative to how the individual patient envisions the good life. That being said, respect for autonomy also requires that physicians avoid assuming that these beliefs about the sanctity of blood are firmly held by every Jehovah’s Witness patient and that they are not able to balance refusing blood with the consequences of disfellowshipping and disassociation (77). Furthermore, respecting autonomy requires designing a decision-making process that does not preclude other potential treatments (78). In cases where physicians’ and patients’ values conflict, respect for autonomy is often expressed as a recognition of the right of decisionally-capacitated adults to decline treatment. This right ordinarily trumps other considerations even when the treatment offered is life-sustaining or will preserve vital physiological functions or functional abilities. The right to refuse is well-enshrined in the bioethics literature, codified in a number of state laws, hospital policies, and has been expressed in a number of court decisions. However, to truly honor this right, physicians must engage in a dialogical relationship with their patients and seek to understand the nature of their beliefs, especially as concerns the value of continued biological life in comparison to the prospect of spiritual rebirth after death.
Communication
Several time points during a patient’s care present opportunities to ask crucial questions. For an outpatient prior to delivery, the conversation should begin to clarify patient wishes. At this time, preventive measures, such as iron supplementation, can be instituted and subsequent plans for hospital admission be discussed. This conversation and plan indicates to the patient that the physician is taking a proactive role in creating a situation where blood supplementation is unnecessary. As noted above, when a patient’s hemoglobin levels are starting to show signs of a downward trajectory, a conversation about alternative treatment options becomes necessary. Some physicians rule out further fruitful discussions with patients by asking the wrong question at the beginning of the conversation. For example, asking the patient, “Will you accept a blood transfusion?” may be interpreted as an ultimatum that places the patient in the role of a passive recipient of treatment rather than an active partner in the decision-making process. Furthermore, assuming that an outright rejection of blood transfusions should be interpreted as a desire to reject all care often leads to a refusal to treat and a missed opportunity to engage patients. What is missing from these interactions is an effort to empathize with the patient and to avoid stereotyping her. These efforts should include conversations that address her expectations for what may happen if the moral obligations that follow from her religious beliefs are not met as well as the effect that her decision may have on her family.
In terms of practical suggestions for improving patient-physician communication, an ongoing record of the conversation is important. Documenting each successive conversation in the medical chart will help other providers understand how each patient interprets their spiritual connection to blood and blood products. For example, notes in the chart might indicate how the patient has interpreted the blood refusal card, any conversations that have been had with family members, arrangements for the care of children, and a detailed set of guidelines regarding treatment options that the patient is willing to consider (79). Some institutions with large Jehovah’s Witness populations have developed an automatic alert that appears in the electronic medical record once the religious preferences of the JW patient are noted in the electronic medical record (77). This alert notifies physicians who have ordered whole blood products that the patient has noted religious objections to these products. Ideally, this notification should be interpreted as a prompt for a conversation about the patient’s treatment preferences. Adopting this practice more widely might help to set the tone of mutual respect and recognition of the important role that nonmedical values play in the lives of patients.
Legal Considerations
In general, the body of American case law supports the right of competent adults to refuse treatment for themselves. The most straightforward cases involve attempts by physicians or hospitals to override the right of a competent adult without dependent children to refuse blood transfusion. Most of these requests have been denied or overturned on appeal. However, in a case where a decisionally incapacitated adult JW patient lacked an advance directive or other written documentation of her religious beliefs, the Supreme Court of New Jersey determined that “…[w]hen the hospital and staff are thus involuntary hosts and their interests are pitted against the belief of the patient, we think it reasonable to resolve the problem by permitting the hospital and its staff to pursue their functions according to their professional standards. The solution sides with life, the conservation of which is, we think, a matter of State interest.” (80). The State’s interest in preserving life is generally viewed as a prima facie obligation, as is the physician’s obligation to practice medicine within the current standard of care. In most cases, these two obligations will not conflict.
Some of the legal issues related to physicians’ assessments of benefits and risks emerge in Mercy Hospital v Jackson. The plaintiff, Mercy Hospital, attempted to override the wishes of a competent JW patient, who was pregnant and seeking a cesarean delivery without blood transfusions. Mercy Hospital declined her request, citing an unfavorable risk-benefit ratio and sought to appoint a guardian for Mrs. Jackson in order to consent to the surgery with blood transfusions. The guardianship application was denied and the surgery was performed without blood transfusions. Mrs. Jackson and her child survived. Mercy Hospital appealed the court’s ruling. The Court of Special Appeals of Maryland agreed with the lower court’s opinion that “a competent, pregnant adult does have the paramount right to refuse a blood transfusion in accordance with her religious beliefs, where such decision is made knowingly and voluntarily and will not endanger the delivery, survival or support of the fetus.” Given the existing case law cited above, the presumption is that if the fetus’s life was in danger, the State would likely cite a compelling interest to intercede on its behalf. Mothers who have recently given birth and whose treatment refusal will not affect the physical health of the child are judged by a slightly different standard.
Conclusions
Management of the Jehovah’s Witness patient with severe anemia can present challenging clinical and ethical issues for physicians. All obstetricians and gynecologists should be familiar with alternatives and ‘less invasive’ options for patients who refuse blood transfusions. Respecting religious and ethical values of patients is critical.
Learning Objectives.
After completing this activity, the learner will be better able to:
Explain the oxygen delivery systems and response to tissue hypoxia mechanisms in humans.
Recognize options to correct anemia in Jehovah’s Witness patients.
Recognize agents that are used in certain clinical scenarios in obstetrics and gynecology setting to reduce blood loos in patients who are refusing blood transfusion
Identify ethical/legal considerations for Jehovah’s Witness patients
Footnotes
Authors have no financial conflicts of interest to disclose.
References
- 1.Anon The Watchtower. 1945 [Google Scholar]
- 2.Bodnaruk ZM, Wong CJ, Thomas MJ. Meeting the clinical challenge of care for Jehovah’s Witnesses. Transfus Med Rev. 2004;18:105–116. doi: 10.1016/j.tmrv.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Tuman KJ. Tissue oxygen delivery: the physiology of anemia. Anesthesiol Clin North Am. 1990;9:451–69. [Google Scholar]
- 4.Barcroft J. The respiratory function of the blood. Part I: Lessons from high altitudes. Cambridge University Press; New York: 1925. [Google Scholar]
- 5.Hebert PC, Hu LQ, Biro GP. Review of physiologic mechanisms in response to anemia. Can Med Assoc J. 1997;156:27–40. [Google Scholar]
- 6.Wilson P. Jehovah’s Witness children: when religion and the law collide. Paediatr Nurs. 2005;17:34–37. doi: 10.7748/paed2005.04.17.3.34.c978. [DOI] [PubMed] [Google Scholar]
- 7.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–21. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 8.Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, Watson JJ, Hopf HW, Toy P. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646–52. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–8. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 10.Viele MK1, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah's Witnesses. Transfusion. 1994 May;34(5):396–401. doi: 10.1046/j.1537-2995.1994.34594249050.x. [DOI] [PubMed] [Google Scholar]
- 11.Beliaev AM1, Marshall RJ, Smith W, Windsor JA. Mortality risk stratification in severely anaemic Jehovah's Witness patients. Intern Med J. 2012 Mar;42(3):e1–3. doi: 10.1111/j.1445-5994.2011.02699.x. [DOI] [PubMed] [Google Scholar]
- 12.Werner E, Kaltwasser JP, Ihm P. [Oral iron treatment: intestinal absorption and the influence of a meal (author's transl)] Dtsch Med Wochenschr. 1977;102:1061. doi: 10.1055/s-0028-1105464. [DOI] [PubMed] [Google Scholar]
- 13.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 14.Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfus Med Rev. 2013 Oct;27(4):221–34. doi: 10.1016/j.tmrv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bircher AJ, Auerbach M. Hypersensitivity from intravenous iron products. Immunol Allergy Clin North Am. 2014;34:707. doi: 10.1016/j.iac.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron herapy in reducing requirement for allogeneic blood transfusion systematic review and meta-analysis of randomized clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrier SL, Auerbach M. Mentzer WC, Tirnauer JS, editors. Treatment of iron deficiency anemia in adults. Uptodate, Feb 11, 2016. [Google Scholar]
- 18.Corwin HL, Gettinger A, Rodriguez RM, et al. Efficacy of recombinant human erythropoietin in the critically ill patient: A randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350. doi: 10.1097/00003246-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 19.De Andrade JR, Jove M, Landon G, et al. Baseline hemoglobin as a predictor of risk of transfusion and response to epoietin alfa in orthopedic surgery patients. Am J Orthop. 1996;25:533–542. [PubMed] [Google Scholar]
- 20.Ball AM, Winstead PS. Recombinant human erythropoietin therapy in critically ill Jehovah's Witnesses. Pharmacotherapy. 2008 Nov;28(11):1383–90. doi: 10.1592/phco.28.11.1383. [DOI] [PubMed] [Google Scholar]
- 21.Coleman T, Brines M. Recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care. 2004;8:337–41. doi: 10.1186/cc2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brines M, Cerani A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–50. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 24.Royston D. Aprotinin versus lysine analogues: the debate continues. Annals of Thoracic Surgery. 1998;65(4):S9–19. doi: 10.1016/s0003-4975(98)00071-x. [DOI] [PubMed] [Google Scholar]
- 25.Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfusion Medicine Reviews. 1998;12(3):206–25. doi: 10.1016/s0887-7963(98)80061-8. [DOI] [PubMed] [Google Scholar]
- 26.Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 27.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD001886. doi: 10.1002/14651858.CD001886.pub3. doi: 10.1002/14651858.CD001886.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Matteson KA, Rahn DD, Wheeler TL, 2nd, Casiano E, Siddiqui NY, Harvie HS, et al. Non surgical management of heavy menstrual bleeding: a systematic review. Obstet Gynecol. 2013 Mar;121(3):632–43. doi: 10.1097/AOG.0b013e3182839e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breau RH, Kokolo MB, Punjani N, Cagiannos I, Beck A, Niznick N, et al. The effects of lysine analogs during pelvic surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014 Jul;28(3):145–55. doi: 10.1016/j.tmrv.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing && mortality in emergency and urgent surgery. Cochrane Database Syst Rev. 2013;1:CD010245. doi: 10.1002/14651858.CD010245.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015 Jun 16;6:CD007872. doi: 10.1002/14651858.CD007872.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 33.Crescenzi G, Landoni G, Biondi-Zoccai G, et al. Desmopressin reduces transfusion needs after surgery: a meta-analysis of randomized clinical trials. Anesthesiology. 2008;109:1063–1076. doi: 10.1097/ALN.0b013e31818db18b. [DOI] [PubMed] [Google Scholar]
- 34.Monroe DM, Hoffman M, Oliver JA, et al. Platelet activity of high-dose factor VIIa is independent of tissue factor. British Journal of Haematology. 1997;99:542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 35.Wolberg AS, Allen GA, Monroe DM, et al. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. British Journal of Haematology. 2005;131:645–655. doi: 10.1111/j.1365-2141.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- 36.Hedner U, Ezban M. Tissue factor and factor VIIa as therapeutic targets in disorders of hemostasis. Annual Review of Medicine. 2008;59:29–41. doi: 10.1146/annurev.med.59.061606.095605. [DOI] [PubMed] [Google Scholar]
- 37.Grottke O1, Henzler D, Rossaint R. Activated recombinant factor VII (rFVIIa) Best Pract Res Clin Anaesthesiol. 2010 Mar;24(1):95–106. doi: 10.1016/j.bpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Mercier FJ, Bonnet MP. Use of clotting factors and other prohemostatic drugs for obstetric hemorrhage. Curr Opin Anaesthesiol. 2010 Jun;23(3):310–6. doi: 10.1097/ACO.0b013e32833835a2. [DOI] [PubMed] [Google Scholar]
- 39.Franchini M, Franchi M, Bergamini V, Montagnana M, Salvagno GL, Targher G, Lippi G. The use of recombinant activated FVII in postpartum hemorrhage. Clin Obstet Gynecol. 2010 Mar;53(1):219–27. doi: 10.1097/GRF.0b013e3181cc4378. [DOI] [PubMed] [Google Scholar]
- 40.Hsia CC, Chin-Yee IH, McAlister VC. Use of recombinant activated factor VII in patients without hemophilia: a meta-analysis of randomized control trials. Ann Surg. 2008;248:61–68. doi: 10.1097/SLA.0b013e318176c4ec. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15:201. doi: 10.1186/cc9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riess HB, Meier-Hellmann A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;121:9–16. doi: 10.1016/j.thromres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Gill R. Practical management of major blood loss. Anaesthesia. 2015 Jan;70(Suppl 1):54–7. doi: 10.1111/anae.12915. [DOI] [PubMed] [Google Scholar]
- 44.Wilson MD, Davis JE. Antithrombotic reversal agents. Emerg Med Clin North Am. 2014 Aug;32(3):715–25. doi: 10.1016/j.emc.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Leissinger CA, Blatt PM, Hoots WK, et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83(2):137–43. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein J, Marrero M, Masrur S, et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol. 2010;67(8):965–9. doi: 10.1001/archneurol.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein JN, Refaai MA, Milling TJ Jr, Lewis B, Goldberg-Alberts R, Hug BA, Sarode R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomized trial. Lancet. 2015 May 23;385(9982):2077–87. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holbrook A1, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e152S–84S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collis RE, Collins PW. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70:78–86. doi: 10.1111/anae.12913. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed S, Harrity C, Johnson S, Varadkar S, McMorrow S, Fanning R, et al. The efficacy of fibrinogen concentrate compared with cryoprecipitate in major obstetric haemorrhage--an observational study. Transfus Med. 2012;22:344–9. doi: 10.1111/j.1365-3148.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- 51.Fibrinogen concentrate (Human) RiaSTAP. Available at: http://www.fda.gov/downloads/Biolog…ionatedPlasmaProducts/ucm094006.pdf.
- 52.James AH, McLintock C, Lockhart E. Postpartum hemorrhage: when uterotonics and sutures fail. American Journal of Hematology. 2012;87:16–22. doi: 10.1002/ajh.23156. [DOI] [PubMed] [Google Scholar]
- 53.Collins PW, Lilley G, Bruynseels D, et al. Fibrin-based clot formation an early and rapidly biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014;124:1727–36. doi: 10.1182/blood-2014-04-567891. [DOI] [PubMed] [Google Scholar]
- 54.Nemeth E. Anti-hepcidin therapy for iron-restricted anemias. Blood. 2013;122:2929–31. doi: 10.1182/blood-2013-08-522466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 56.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 57.Ganz T, Nemeth E. The hepcidin-ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematology Am Soc Hematol Educ Program. 2011;2011(1):538–542. doi: 10.1182/asheducation-2011.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 59.Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30(7):917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 60.Theurl I, Schroll A, Sonnweber T, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118(18):4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackenzie CF, Moon-Massat PF, Shander A, Javidroozi M, Greenburg AG. When blood is not an option: factors affecting survival after the use of a hemoglobin-based oxygen carrier in 54 patients with life-threatening anemia. Anesth Analg. 2010 Mar 1;110(3):685–93. doi: 10.1213/ANE.0b013e3181cd473b. [DOI] [PubMed] [Google Scholar]
- 63.Jahr JS, Mackenzie C, Pearce LB, et al. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 2008;64:1484–97. doi: 10.1097/TA.0b013e318173a93f. [DOI] [PubMed] [Google Scholar]
- 64.Estep T, Bucci E, Farmer M, Greenburg G, Harrington J, Kim HW, Klein H, Mitchell P, Nemo G, Olsen K, Palmer A, Valeri CR, Winslow R. Basic science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working group Workshop, March 1, 2006. Transfusion. 2008;48:776–82. doi: 10.1111/j.1537-2995.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 65.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;299:2324–32. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jan KM, Chien S. Effect of hematocrit variations on coronary hemodynamics and oxygen utilization. Am J Physiol. 1977;233:H106–13. doi: 10.1152/ajpheart.1977.233.1.H106. [DOI] [PubMed] [Google Scholar]
- 67.Cain SM. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol. 1977;42:228–34. doi: 10.1152/jappl.1977.42.2.228. [DOI] [PubMed] [Google Scholar]
- 68.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multi-center, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 69.Orady M, Aslanova R, Fidela Paraiso M. Minimally invasive hysterectomy for benign indications. Minerva Ginecol. 2014 Feb;66(1):13–21. [PubMed] [Google Scholar]
- 70.Panagiotopoulou N, Nethra S, Karavolos S, Ahmad G, Karabis A, Burls A. Uterine-sparing minimally invasive interventions in women with uterine fibroids: a systematic review and indirect treatment comparison meta-analysis. Acta Obstet Gynecol Scand. 2014 Sep;93(9):858–67. doi: 10.1111/aogs.12441. [DOI] [PubMed] [Google Scholar]
- 71.Singh SS, Belland L. Contemporary management of uterine fibroids: focus on emerging medical treatments. Curr Med Res Opin. 2015 Jan;31(1):1–12. doi: 10.1185/03007995.2014.982246. [DOI] [PubMed] [Google Scholar]
- 72.Hunter LA. Exploring the role of uterine artery embolization in the management of postpartum hemorrhage. J Perinat Neonatal Nurs. 2010 Jul-Sep;24(3):207–14. doi: 10.1097/JPN.0b013e3181e8c994. [DOI] [PubMed] [Google Scholar]
- 73.Waters JH. Intraoperative blood recovery. ASAIO J. 2013;59:11–17. doi: 10.1097/MAT.0b013e31827b5187. [DOI] [PubMed] [Google Scholar]
- 74.Avidan M, Silvergleid AJ, Heath M. Kleinman S, Mark JB, editors. Surgical blood conservation: Blood salvage. Uptodate, Sep 14, 2015. [Google Scholar]
- 75.American College of Obstetricians and Gynecologists ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–1047. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 76.Feigenbaum F, Sulmasy DP, Pellegrino ED, Henderson FC. Spondyloptotic Fracture of the Cervical Spine in a Pregnant, Anemic Jehovah's Witness: Technical and Ethical Considerations. Case Report. J Neurosurg. 1997;87:458–63. doi: 10.3171/jns.1997.87.3.0458. [DOI] [PubMed] [Google Scholar]
- 77.Shaner DM, Prema J. Conversation and the Jehovah's Witness Dying from Blood Loss. Narrat Inq Bioeth. 2014;4:253–61. doi: 10.1353/nib.2014.0071. [DOI] [PubMed] [Google Scholar]
- 78.Gyamfi C, Berkowitz RL. Responses by Pregnant Jehovah's Witnesses on Health Care Proxies. Obstet Gynecol. 2004;104:541–4. doi: 10.1097/01.AOG.0000135276.25886.8e. [DOI] [PubMed] [Google Scholar]
- 79.Gyamfi CM, Gyamfi M, Berkowitz RL. Ethical and Medicolegal Considerations in the Obstetric Care of a Jehovah's Witness. Obstet Gynecol. 2003;102:173–80. doi: 10.1016/s0029-7844(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 80.John F. Kennedy Memorial Hospital v. Heston 58 N.J. 576; 279 A.2d 670; 1971 N.J [Google Scholar]
- 81.Kriplani A, Mahey R, Dash BB, Kulshreshta V, Agarwal N, Bhatla N. Intravenous iron sucrose therapy for moderate to severe anaemia in pregnancy. Indian J Med Res. 2013;138:78–82. [PMC free article] [PubMed] [Google Scholar]
- 82.Drugs. Available at: https://online.epocrates.com/drugs.


