Abstract
Background: The management of hyperglycemia in the intensive care unit has been a controversial topic for more than a decade, with target ranges varying from 80–110 mg/dL to <200 mg/dL. Multiple insulin infusion protocols exist, including several computerized protocols, which have attempted to achieve these targets. Importantly, compliance with these protocols has not been a focus of clinical studies.
Methods: GlucoCare™, a Food and Drug Administration (FDA)-cleared insulin-dosing calculator, was originally designed based on the Yale Insulin Infusion Protocol to target 100–140 mg/dL and has undergone several modifications to reduce hypoglycemia. The original Yale protocol was modified from 100–140 mg/dL to a range of 120–140 mg/dL (GlucoCare 120–140) and then to 140 mg/dL (GlucoCare 140, not a range but a single blood glucose [BG] level target) in an iterative and evidence-based manner to eliminate hypoglycemia <70 mg/dL. The final modification [GlucoCare 140(B)] includes the addition of bolus insulin “midprotocol” during an insulin infusion to reduce peak insulin rates for insulin-resistant patients. This study examined the results of these protocol modifications and evaluated the role of compliance with the protocol in the incidence of hypoglycemia <70 mg/dL.
Results: Protocol modifications resulted in mean BG levels of 133.4, 136.4, 143.8, and 146.4 mg/dL and hypoglycemic BG readings <70 mg/dL of 0.998%, 0.367%, 0.256%, and 0.04% for the 100–140, 120–140, 140, and 140(B) protocols, respectively (P < 0.001). Adherence to the glucose check interval significantly reduced the incidence of hypoglycemia (P < 0.001). Protocol modifications led to a reduction in peak insulin infusion rates (P < 0.001) and the need for dextrose-containing boluses (P < 0.001).
Conclusion: This study demonstrates that refinements in protocol design can improve glucose control in critically ill patients and that the use of GlucoCare 140(B) can eliminate all significant hypoglycemia while achieving mean glucose levels between 140 and 150 mg/dL. In addition, attention to the timely performance of glucose levels can also reduce hypoglycemic events.
Introduction
There has been intense interest in the management of hyperglycemia in the intensive care unit (ICU) over the past decade and a half. Multiple observational studies have demonstrated an association between hyperglycemia and adverse outcomes, including mortality.1–5 An early randomized clinical trial from Van den Berghe et al. revolutionized the role of intensive glucose management in critically ill patients6 and led to the adoption of the 80–110 mg/dL target range for BG control for this patient population at many centers. Despite this initial enthusiasm, however, multiple subsequent trials failed to find the same benefits.7–9 In fact, the largest of its kind, the multicenter NICE-SUGAR study suggested a possible increase in mortality in patients who were more aggressively targeted to achieve a glucose range of 80–110 mg/dL versus the more conservative 140–180 mg/dL.8 This finding, combined with the much higher incidence of severe hypoglycemia (defined as <40 mg/dL) with more stringent glucose control strategies, led to a reassessment of optimal glycemic targets in the ICU. More recently, there has been increasing concern about the potential link between even moderate hypoglycemia (defined as <70 mg/dL) and adverse events.10–12
As a result of this controversy, medical societies have developed guidelines for the target range for glycemic control in the critically ill. The American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) advise a target range of 140–180 mg/dL.13 The Society of Critical Care Medicine (SCCM) recommends targeting a range of <150 mg/dL.14 Combining these two societal recommendations might lead to a reasonable composite target of 140–150 mg/dL.
Most importantly, all the studies referenced above used paper-based protocols to guide insulin infusion adjustments. Compliance with the paper protocols and the quality of the overall protocol itself have not been emphasized or presented formally.
Previously, our group reported on the use of GlucoCare™, an FDA-cleared proprietary insulin-dosing calculator based on the original “Yale Insulin Infusion Protocol” (whose target range was 100–140 mg/dL), and analyzed the causes of hypoglycemia that occurred during the initial implementation.15 Since then, we have used the results of this study to further modify and refine the original Yale 100–140 mg/dL protocol to develop protocols with higher target ranges [GlucoCare 120–140, GlucoCare 140, and GlucoCare 140(B); the latter two being the first protocols to target a specific BG level instead of a range]. The sole difference between the 140 and 140(B) protocols is the use of “midprotocol boluses,” defined as additional insulin boluses while patients are on the insulin infusion in those circumstances when BG levels are increasing despite insulin administration. (The standard Yale protocol only recommends an insulin bolus initially when the infusion is being started.) The purpose of adding the midprotocol insulin boluses was to reduce overall continuous insulin infusion rates for insulin-resistant patients. We herein report the results of these efforts, with specific focus on the incidence and potential causes of moderate hypoglycemia, defined as <70 mg/dL.
Materials and Methods
In 2008, the Yale protocol-based GlucoCare IGC System (GlucoCare) was 510k cleared by the FDA, with a target range of 100–140 mg/dL. Additional protocols were subsequently developed [GlucoCare 120–140, GlucoCare 140, and GlucoCare 140(B)], each developed iteratively, after our evaluation of the possible causes of hypoglycemia.
Clinical use of GlucoCare began in January 2009, and the data for this study were gleaned from multiple institutions and analyzed in aggregate. As mentioned above, we hypothesized after the results of our clinical experience with the computerized Yale 100–140 mg/dL protocol study that increasing the lower target from 100 mg/dL to achieve a target range of 120–140 mg/dL would further mitigate the risk of hypoglycemia. This led to the development of the 120–140 mg/dL protocol. Operationally, this protocol was identical to the original, except that the infusion rate began to be reduced once the 120 mg/dL cutpoint was achieved, instead of allowing the BG to fall toward the 100 mg/dL range. A similar analysis was undertaken after the use of GlucoCare 120–140, which led to the development of the GlucoCare 140 protocol. In this version, adjustments to the infusion were made whenever the BG level deviated from the 140 mg/dL target. In other words, there was not a target range but a single target. Finally, concern about the high level of continuous insulin infusion rates in some insulin-resistant patients, and the time to reach glucose levels <180 mg/dL in some patients who were substantially hyperglycemic, led to the inclusion of midprotocol boluses in the 140(B) protocol. See Table 1 for details of each protocol.
Table 1.
(A–D) Blood Glucose (BG) Measurements and Bolus Protocol Recommended to Reduce Hypoglycemia
| Table 1A below represents the column formats from the original Food and Drug Administration-cleared 100–140 mg/dL protocol and the newly created modified protocols. The subsequent table is recreated identically to the original Yale column format, with the exception of the 75–99 mg/dL column on the far left. There has been no change to the calculations for the recommendations. The only change is the glucose level, which triggers the calculation. | |||||
| Table 1A | |||||
| Original 100–140 | BG 75–99 mg/dL | BG 100–139 mg/dL | BG 140–199 mg/dL | BG ≥200 mg/dL Instructions | |
| 120–140 | BG 75–99 mg/dL | BG 100–119 mg/dL | BG 120–140 mg/dL | BG 141–200 mg/dL | BG >200 mg/dL Instructions |
| 140 | BG 75–99 mg/dL | BG 100–139 mg/dL | BG 140 mg/dL | BG 141–200 mg/dL | BG >200 mg/dL Instructions |
| Table 1B | |||||
| BG ↑ >50 mg/dL/h | BG ↑ | ↑Infusion by “2Δ” (see Table 1C) | |||
| BG ↑ by >25 mg/dL/h | BG ↑1–50 mg/dL/h or BG unchanged | BG unchanged or BG ↓1–25 mg/dL/h | ↑ Infusion by “Δ” (see Table 1C) | ||
| See belowa | BG ↑ | BG ↑ by 1–25 mg/dL/h, BG unchanged, or BG ↓1–25 mg/dL/h | BG ↓ by 1–50 mg/dL/h | BG ↓ by 26–75 mg/dL/h | No infusion change |
| BG unchanged or BG ↓ by 1–25 mg/dL/h | BG ↓26–50 mg/dL/h | BG ↓ by 51–75 mg/dL/h | BG ↓ by 76–100 mg/dL/h | ↓ Infusion by “Δ” (see Table 1C) | |
| BG ↓ by >25 mg/dL/h (see belowa) | BG ↓ by >50 mg/dL/h | BG ↓ by >75 mg/dL/h | BG ↓ by >100 mg/dL/h | Hold insulin infusion. Check BG in 30 min. If greater than lower target, restart infusion at a rate ↓ by “2Δ” (see Table 1C) If BG lower than lower target, see belowa | |
| aD/C Insulin infusion: check BG every 30 min until ≥90 and then every hour; when BG ≥ lower target mg/dL, restart infusion at 75% of most recent rate. | |||||
| Table 1C: Changes in infusion rate (“Δ”) are determined by the current rate | |||||
| Current rate (U/h) | Δ = Rate change (U/h) | 2Δ = 2 × Rate change (U/h) | |||
| <3.0 | 0.5 | 1 | |||
| 3.0–6.0 | 1 | 2 | |||
| 6.5–9.5 | 1.5 | 3 | |||
| 10–14.5 | 2 | 4 | |||
| 15–19.5 | 3 | 6 | |||
| 20–24.5 | 4 | 8 | |||
| ≥25 | ≥5 | 10 (consult MD) | |||
| Table 1D: Bolus protocol | |||||
| BG | BG increase | Bolus | |||
| 141–160 | >20 | Δ | |||
| 161–180 | >20 | 2Δ | |||
| >180 | ≥0 | 2Δ | |||
| When following the paper protocol, users perform a BG measurement and find which column corresponds to the glucose reading. They then would have to calculate the change in glucose reading from the prior glucose reading. Once this is determined, the user chooses the row that corresponds to the glucose reading and the rate/direction of change. The far right column of that row combined with Table 1C is then used to determine the adjustment. Table 1D is the bolus protocol. | |||||
In brief, GlucoCare is installed on a hospital server or accessed through the “cloud.” After a point-of-care BG result is obtained (typically a bedside capillary BG meter), the result is entered by the nurse into GlucoCare. As this is a retrospective study from multiple hospitals, no standardization of glucose meters or method of obtaining glucose readings occurred. After the system calculates the insulin infusion rate change, this recommendation is displayed for the nurse, who then confirms that the change was made. An audible alert occurs when the next glucose reading is due, typically in 1 h. Frequency of glucose checks varies from 15 min when patients are at risk for hypoglycemia (rapidly falling BG levels or BG levels below the target range) to up to every 4 h in those patients who have demonstrated glycemic stability. Users have the ability to decline or override all recommendations allowing for clinical judgment. All such protocol deviations along with time delays for glucose readings are recorded and available in real time to clinical and administrative staff.
Statistical methods
Glycemic data for all patients were monitored from December 2008 to January 2016. Analyses were restricted to glucose readings recorded after initiating the insulin infusion, unless otherwise stated (i.e., glucose readings performed before initiation of an insulin infusion were excluded). Means and standard errors were calculated from a repeated measures mixed model. The incidence of hypoglycemia was reported for levels <70 mg/dL (moderate hypoglycemia) and <40 mg/dL (severe hypoglycemia). The influence of nonadherence to the GlucoCare-directed glucose check interval (typically, 1 h) was examined by evaluating the influence of delayed glucose readings as a cause of hypoglycemia, with delays defined as a percentage past the check time (100% delay = >1 h late and 50% delay = 30 min late). Delayed reading time frames were determined by evaluating the preceding glucose reading's time stamp in relation to the suggested glucose check time. Frequencies of hypoglycemia, stratified by protocol, were evaluated with chi-square tests, or Fisher's exact test, as appropriate. As it is not uncommon for individual patients to have insulin infusion protocols initiated and then stopped more than once during an admission, separate protocol use periods for the same patient were considered as unique, and the data will be displayed by protocol use periods instead of “by patient.” This does not apply to when an insulin infusion is held for hypoglycemia or rapidly falling BG levels but only when the user stops the protocol and then subsequently restarts a new instance of the protocol. All statistical analyses were considered significant at the P ≤ 0.05 level and evaluated with SAS version 9.4 (SAS Institute, Cary, NC).
Glucose levels clearly entered in error were excluded (e.g., BG of 1, associated with a note that stated “real level was 129”).
Results
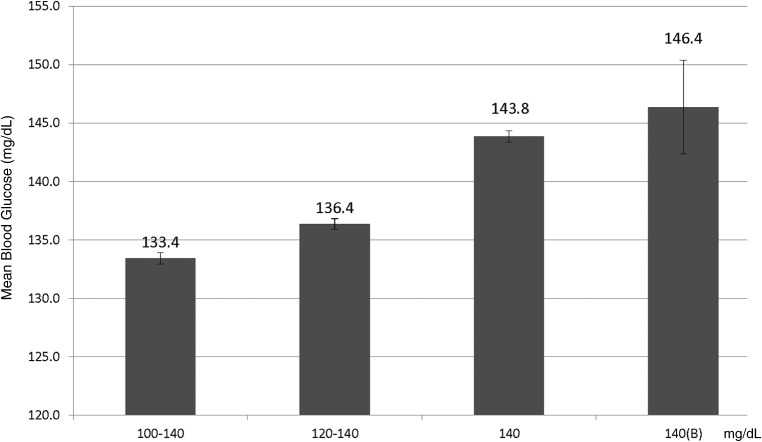
The results are stratified into four protocol-specific target protocols: Yale 100–140 (n = 2924 completed protocols and n = 74,728 glucose readings), GlucoCare 120–140 (n = 3150 completed protocols and n = 92,489 glucose readings), GlucoCare 140 (n = 2620 completed protocols and n = 87,856 glucose readings), and GlucoCare 140(B) (n = 105 completed protocols and n = 2531 glucose readings). Figure 1 shows the mean BG levels each protocol achieved once glucose levels were reduced below 180 mg/dL. Time from initiation of the protocol to achieving a BG level ≤180 mg/dL did not vary significantly between the protocols (range: 5.6–6.4 h, data not shown).
FIG. 1.
Mean blood glucose levels achieved after achieving a blood glucose level <180 mg/dL.
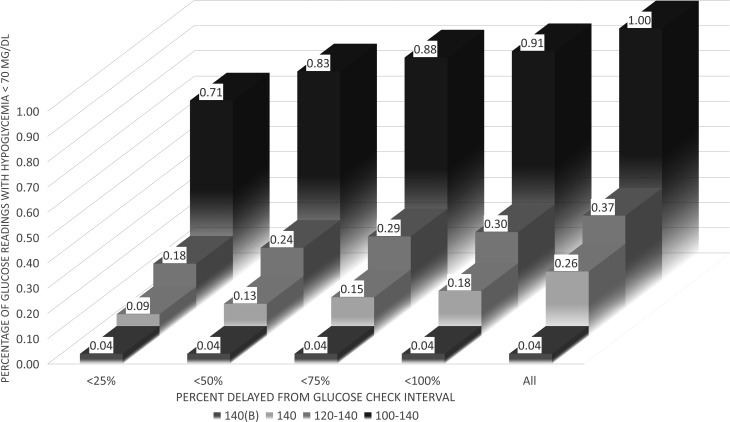
The incidence of hypoglycemia <70 mg/dL stratified by timing of glucose readings is displayed in Figure 2. The incidence of hypoglycemia <40 mg/dL was extremely low for all groups [0 in the 140(B) protocol], and modifications to the protocol did not significantly impact this endpoint (P = 0.10, data not shown), except when eliminating glucose levels >25% (15 min) late (P = 0.046). A steady decrease, however, in the incidence of hypoglycemia <70 mg/dL was found when moving from the original 100–140 mg/dL protocol to the modified protocols (P < 0.00l). Figure 3 displays the incidence of hypoglycemia <70 mg/dL stratified by protocol use period (as explained above) and revealed a decreased incidence of hypoglycemia between the different protocols (P = 0.03). This decrease was even more pronounced when considering delays in glucose readings, where hypoglycemia is virtually eliminated when glucose levels are performed within 25% (15 min late) of the scheduled glucose interval check (P < 0.001) (Figs. 2 and 3). Of note, the one glucose level <70 mg/dL that was encountered with the GlucoCare 140(B) protocol was 69 mg/dL.
FIG. 2.
Percentage of blood glucose readings <70 mg/dL stratified by protocol and glucose check interval compliance. For typical glucose check intervals of 60 min: <25% late = 15 min, <50% = 30 min, <75% = 45 min, and <100% = 60 min.
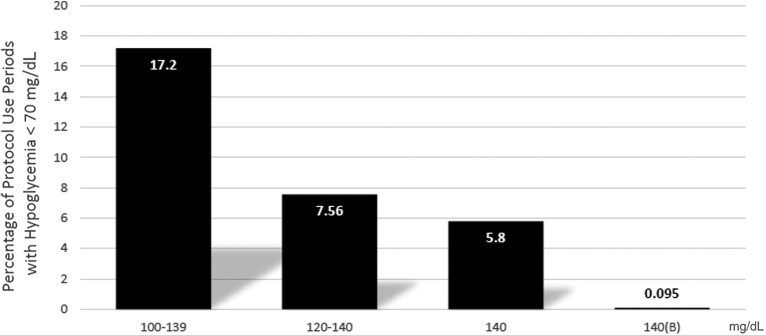
FIG. 3.
Percentage of protocol use periods with hypoglycemia <70 mg/dL. A protocol use period is defined as the unit of time where a patient's insulin dosing is recommended by GlucoCare. Each individual protocol use, including repeated use in the same patient at different times during an admission, is counted separately.
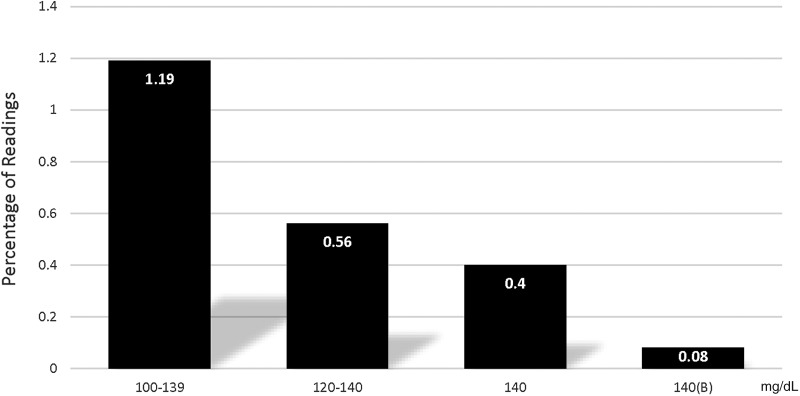
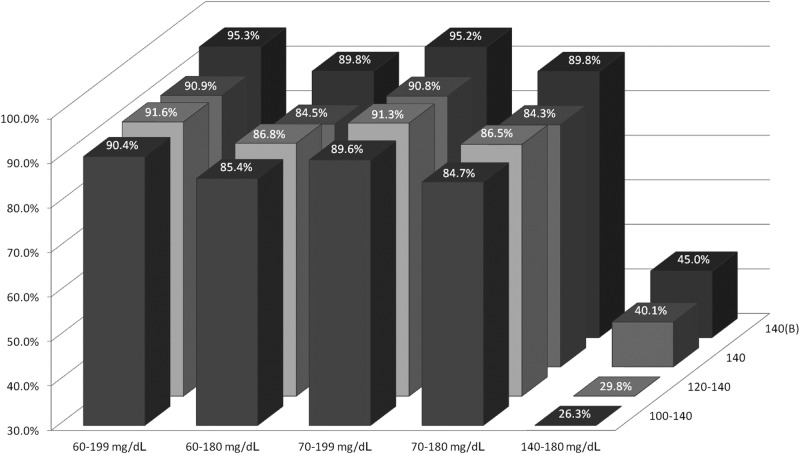
The need to administer dextrose-containing solutions (D50) to treat hypoglycemia decreased progressively with the modified protocols (P < 0.001) (Fig. 4). In addition, overall continuous insulin infusion rates were lower with the GlucoCare 140(B) protocol compared to the others (P < 0.001) with 64.8% of infusions <5 U/h compared to 44.4% for the GlucoCare 140 protocol. Overall glycemic control was improved with the modified protocols, with percentage of glucose readings within specified desirable ranges increasing with protocol modifications (Fig. 5, P < 0.001).
FIG. 4.
Percentage of readings requiring D50 boluses.
FIG. 5.
Percentage of glucose readings within the designated glucose range stratified by protocol.
Discussion
This study is a follow-up to our original analysis of the causes of hypoglycemia with the use of the Yale protocol-based GlucoCare IGC System (100–140 mg/dL target).15 In that study, we proposed that nearly two thirds of hypoglycemic events <70 mg/dL may have been caused by inherent protocol features, such as continuing the insulin infusion, although at a lower rate, when BG levels fell below 100 mg/dL in the setting of relatively stable glucose levels (see Table 1 for details), as well as expanding the glucose value acquisition interval from hourly to every 2–4 h once glucose control had been stabilized. Of note, other investigators have demonstrated that hourly BG readings are in fact optimal for reducing the incidence of both hypoglycemia and hyperglycemia in patients on continuous insulin infusions.14,16 Another finding of our original study was that nearly one in six hypoglycemic events <70 mg/dL was correlated with protocol deviations, including late glucose checks. As a result of these findings, further modifications to the original 100–140 mg/dL protocol were pursued to enhance patient safety.
This report confirms that first the incidence of hypoglycemia can be reduced while maintaining society-recommended glucose control by increasing the lower protocol range from 100 to 140 mg/dL as we did to develop GlucoCare 140. By raising the lower target from 100 to 120 mg/dL (GlucoCare 120–140), we reduced the incidence of hypoglycemia <70 mg/dL from 0.998% to 0.367%. Raising the target further to 140 mg/dL (GlucoCare 140) reduced the incidence to 0.256%. Adding the additional boluses [GlucoCare 140(B)] reduced the incidence to 0.04% (Fig. 2). Of note, the original results reported with the use of the Yale Infusion Protocol demonstrated an incidence of hypoglycemia <40 and <60 mg/dL of 0.045% and 0.3% of glucose readings, respectively.17 The GlucoCare 140(B) modifications completely eliminated hypoglycemia <60 mg/dL (including <40 mg/dL) while achieving a mean BG level between 140 and 150 mg/dL, precisely within the range advocated by combining the AACE/ADA and SCCM recommended target ranges.
Second, protocol compliance with the glucose data interval is critical for optimal glucose control and plays a significant role in the risk of hypoglycemia. When BG levels are performed with a <25% delay (typically within 15 min of indicated time), compared to >100% delayed (typically >1 h delayed), hypoglycemic events were reduced significantly in all groups, except the GlucoCare 140(B) as there was only 1 episode of 69 mg/dL (Figs. 2 and 3). This metric was not formally evaluated in the randomized studies of intensive glycemic control. Of note, the glucose check interval in the NICE-SUGAR trial was 2.5 h.8 It is possible that this interval contributed to hypoglycemic events in this study.
When considering the NICE-SUGAR study, the incidence of moderate hypoglycemia in the control group was 15.8%. Our original unmodified Yale 100–140 mg/dL protocol achieved a similar result (17.2% <70 mg/dL), but GlucoCare 120–140, GlucoCare 140, and GlucoCare 140(B) achieved 7.56%, 5.8%, and 0.10%, respectively, a >99.4% reduction. Importantly, the NICE-SUGAR investigators noted a 40% increase in the risk of death with moderate hypoglycemia. Our study demonstrates that with the use of GlucoCare 140(B), BG control in the range between 140 and 150 mg/dL, with no severe hypoglycemia, no hypoglycemia <60 mg/dL, and nearly no moderate hypoglycemia (1 instance of 69 mg/dL), can be achieved (mean BG = 146 mg/dL).
Third, the addition of midprotocol boluses (n = 105 protocol use periods) to the GlucoCare 140 protocol further improved overall glycemic control, reduced the need for D50 boluses, and lowered overall continuous insulin infusion requirements. These results appear promising but will require a larger sample size to confirm the results.
These results have important implications for clinical research in the field and suggest that lower targets than those currently recommended by the AACE/ADA can be achieved safely. Of course, whether those lower targets might result in better outcomes is not yet clear. Moreover, future trials of insulin infusion therapy in the critical care setting should ideally account for the often overlooked factors of protocol quality, implementation, and adherence when evaluating either biochemical or clinical outcomes.
Conclusion
This study demonstrates that refinements in protocol design can improve glucose control in critically ill patients. The use of GlucoCare 140(B), a protocol developed and modified from the original Yale Infusion Protocol, eliminated all significant hypoglycemia while achieving mean glucose levels between 140 and 150 mg/dL. In addition, attention to the timely performance of glucose levels can also reduce hypoglycemic events. Future studies should consider these factors in the design of clinical trials.
Author Disclosure Statement
The authors M.R.M. and B.J.B. are cofounders of Pronia Medical Systems, LLC, the developer of GlucoCare, and have a financial interest in the company.
References
- 1.Krinsley JS: Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–1478 [DOI] [PubMed] [Google Scholar]
- 2.Zerr KJ, et al. : Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997;63:356–361 [DOI] [PubMed] [Google Scholar]
- 3.Umpierrez GE, et al. : Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 4.Baker EH, et al. : Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 2006;61:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furnary AP, et al. : Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352–360; discussion 360–362 [DOI] [PubMed] [Google Scholar]
- 6.van den Berghe G, et al. : Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, et al. : Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 8.Finfer S, et al. : Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 9.Griesdale DE, et al. : Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009;180:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finfer S, et al. : Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–1118 [DOI] [PubMed] [Google Scholar]
- 11.Krinsley JS, et al. : Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 2011;15:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egi M, et al. : Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010;85:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moghissi ES, et al. : American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009;15:353–369 [DOI] [PubMed] [Google Scholar]
- 14.Jacobi J, et al. : Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012;40:3251–3276 [DOI] [PubMed] [Google Scholar]
- 15.Marvin MR, Inzucchi SE. Besterman BJ: Computerization of the Yale insulin infusion protocol and potential insights into causes of hypoglycemia with intravenous insulin. Diabetes Technol Ther 2013;15:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krinsley JS: Glycemic control in the critically ill: what have we learned since NICE-SUGAR? Hosp Pract (1995) 2015;43:191–197 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg PA, et al. : Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004; 27:461–467 [DOI] [PubMed] [Google Scholar]