Abstract
Adolescents account for 40% of new HIV infections, and HIV testing strategies to increase uptake of testing are needed. A community-based adolescent and youth HIV and health testing campaign was conducted in seven slum neighborhoods of Port-au-Prince, Haiti, from December 2014 to September 2015. Community health workers provided community sensitization and recruited 10- to 24-year-olds to test for HIV, syphilis, gonorrhea/chlamydia, and to screen for tuberculosis (TB) and pregnancy. HIV-infected individuals were escorted to the GHESKIO HIV clinic for same-day enrollment in care. Among 3425 individuals eligible for testing, 3348 (98%) accepted an HIV test. HIV prevalence was 2.65% (n = 89). Median age was 19 [interquartile range (IQR) 17–20]; 73% were female. HIV prevalence was 0.6–7.4% across slum neighborhoods. All HIV-infected individuals enrolled in care the same day as testing; median CD4 was 529 cells/μL [IQR 363–761]. Syphilis prevalence was 2.60% (65/2536) and gonorrhea/chlamydia prevalence was 6.25% (96/1536). Among 168 (5%) individuals who reported TB symptoms, 7.7% (13/168) had microbiologically confirmed disease. One hundred twenty-nine females (5% of all females) were pregnant. This community-based testing campaign identified an adolescent and youth population with an HIV prevalence six times higher than the estimated national adolescent HIV prevalence (0.4%) in Haiti, including perinatally infected adolescents. This type of community-based campaign for HIV testing within a package of services can serve as a model for other resource-poor settings to identify high-risk adolescents and youth, and curb the global HIV epidemic among adolescents.
Introduction
Adolescents account for 40% of new HIV infections every year, and AIDS-related deaths among adolescents have increased by 50% over the past 10 years.1,2 Nearly 5 million adolescents and youth aged 10–24 years are living with HIV globally and an additional 900,000 adolescents aged 10–19 years are newly infected each year, putting them at the epicenter of the HIV epidemic.3 Yet, only 9% of adolescent females and 4% of adolescent males know their HIV status.4 Unknown HIV status results in failure to initiate antiretroviral (ART), increased HIV mortality, and continued high-risk sexual activity and HIV transmission.5 Interventions to increase HIV testing to identify HIV-infected adolescents are urgently needed.
Reported challenges to HIV testing include stigma, lack of transportation to testing facilities, and inconvenient clinic hours.6,7 Additional barriers specific to adolescent testing include low perception of risk, heightened stigma of HIV, and clinic hours conflicting with school hours.8,9 Moreover, provider-initiated HIV testing among adolescents is often underutilized due to perceived lack of skills among healthcare workers to discuss HIV with younger adolescents.10 The WHO recommends community-based approaches to increase HIV testing among adolescents and youth as it may remove barriers such as stigma associated with visiting an HIV clinic, lack of convenience, and low perception of risk.11 Data on the feasibility and effectiveness of community-based HIV testing among adolescents and youth in resource-poor settings are limited.12
We conducted a community-based adolescent and youth health testing campaign, including HIV testing, in urban Port-au-Prince, Haiti, from December 2014 to September 2015. The campaign included testing for HIV, sexually transmitted infections (STIs), and tuberculosis (TB) and pregnancy screening. The objective of this analysis is to report the results of this campaign.
Methods
Study setting and population
Haiti is the poorest country in the Western Hemisphere with the highest adult HIV prevalence in the region (1.9%).3 An estimated 10,000 adolescents aged 10–19 years and 14,000 youth aged 20–24 years are currently living with HIV in Haiti.4 The United Nations estimates that the HIV prevalence in Haiti is 0.4% among adolescents aged 10–19 years and 1.5% among youth aged 20–24 years (0.7% among male youth and 2.1% among female youth).3,13 The striking prevalence among female youth highlights this group as an important high-risk population for testing.
GHESKIO is the largest provider of integrated services for HIV, TB, STIs, and preventive healthcare services in Haiti. In December of 2014, GHESKIO implemented a community-based testing campaign to increase HIV testing among adolescents and youth aged 10–24 years living in the slum neighborhoods surrounding GHESKIO. Data collected for routine clinical purposes in the campaign were extracted for this retrospective analysis.
Before the community-based HIV testing campaign, HIV testing was only conducted via voluntary counseling and testing. In 2014, ∼7700 adolescents self-presented for HIV testing at GHESKIO. Adolescents and young adults self-presented to a known HIV clinic and all care was provided in the clinical setting. The community-based testing campaign involved implementing active recruitment of adolescents in the community. Community health workers (CHWs) went out into the community to recruit adolescent and young adults and either escorted them back to the community HIV clinic or gave them a referral card.
The slum neighborhoods included Village de Dieu, Cite l'Eternel/Cite Plus, Martissant, Cite Soleil, Delmas, Carrefour, and Carrefour Feuille. The conditions of these communities include abject poverty and extreme violence; the United Nations once declared these slums as “the most dangerous place in the world.”14 We estimate the population to be at least 600,000 persons based on GHESKIO's work in these slums with cholera vaccination in 2010.15,16
Community sensitization and recruitment
The campaign included pretesting community sensitization by trained CHWs, many of whom live in the slum communities. Meetings were held with community, school, and religious leaders to answer questions and encourage adolescents and youth to get tested for HIV and other diseases. CHWs also met with advocacy leaders of high-risk groups, including commercial sex workers and men who have sex with men, to provide information on HIV testing.
Subsequently, CHWs conducted daily recruitment by visiting schools, community centers, Internet cafes, and other areas where adolescents and youth frequented to encourage testing. Adolescents aged ≤16 years required parent/guardian consent for testing as per national guidelines and were instructed to bring their guardian for testing. Adolescents and youth >16 years were individually escorted or given a referral card to the community testing center. The community testing center was located in a nonclinical building near GHESKIO with a separate entrance and no identification on the building to indicate it was an HIV testing center or clinic.
Comprehensive testing package
Adolescents and youth were offered a comprehensive package of health testing services, including an HIV rapid test (Alere Determine), syphilis rapid test (SD Bioline) beginning in February 2015, and a urine GeneXpert test for gonorrhea and chlamydia beginning in May 2015, all performed at the testing visit. Females were offered pregnancy testing with a urine rapid test. All individuals were screened for symptoms of TB and those reporting symptoms received a sputum GeneXpert test for TB.17 Participants were encouraged to test for all diseases but could opt out of any. All testing and counseling were provided by CHWs. Testing was provided from December 2014 through September 2015.
Linkage of patients to ongoing services
Participants who tested HIV positive were physically escorted to the GHESKIO HIV clinic, on the same day as testing, by the CHW who performed their testing. As per clinical procedures at GHESKIO, individuals ≤19 years were escorted to the adolescent clinic, individuals >19 were escorted to the adult clinic, and pregnant adolescents were escorted to the PMTCT (prevention of mother-to-child transmission) clinic. At each respective clinic, routine clinical intake procedures included confirmatory HIV testing, followed by a point-of-care CD4 cell test, and treatment and counseling for any coinfections. Participants who tested positive for syphilis or gonorrhea/chlamydia were linked to care at the STI clinic. Participants who screened positive for symptoms of TB received a physical examination, chest radiograph, and a sputum GeneXpert test, and those with positive tests were linked to care at the TB clinic. Participants who had a positive pregnancy test were linked to the GHESKIO maternal health clinic.
Data collection and analysis
Each individual who received testing completed a routine demographic survey, including age, sex, education level, and residence zone, as well as sexual risk behaviors and other symptoms. Survey data were entered by CHWs into a central electronic database. Participants were categorized by age at HIV testing: 10–15 years (younger adolescents), 16–19 years (older adolescents), and 20–24 years (youth). Adolescents and youth identified their address at time of testing and were categorized into one of the seven major slum neighborhoods in Port-au-Prince. Participants residing in Port-au-Prince in a designated non-slum neighborhood or participants residing outside of Port-au-Prince in another Province were categorized as “Non-slum/Province.” Statistical comparisons between the age categories with regard to sociodemographic characteristics and sexual risk behaviors were made using Pearson's chi-squared and Wilcoxon rank sum tests. Proportions and prevalence of HIV and syphilis were calculated for each of the slum communities.
Ethics
Institutional Review Boards at Weill Cornell Medical College and GHESKIO approved this analysis.
Results
Testing campaign results
Among 3483 individuals recruited for testing, 26 (<1%) were less than 16 years of age without a guardian to provide consent, and 32 (<1%) presented on a day when violence in the slum prohibited testing services. Among the remaining 3425, acceptance of HIV testing was 98% (3348/3425). Among those who received an HIV test, 76% were female and median age was 19 years [interquartile range (IQR) 17–20]. The majority of testing was among adolescents and youth, 16 years and older. Nearly three-quarters (74%) of those tested reported having completed some or all secondary schooling and nearly all participants were unmarried (98%). The primary reason for testing was to determine HIV status (92%) (Table 1).
Table 1.
Sociodemographic Characteristics of Adolescents and Youth Testing for HIV (n = 3348)
| Total tested n = 3348 (%) | |
|---|---|
| Sex | |
| Female | 2551 (76) |
| Male | 797 (24) |
| Age | |
| Median [IQR] | 19 [17–20] |
| 10–15 | 191 (6) |
| 16–19 | 1857 (55) |
| 20–24 | 1300 (39) |
| Education | |
| Completed primary | 678 (20) |
| Completed secondary or more | 2484 (74) |
| Missing | 186 (6) |
| Marriage status | |
| Single/separated | 3292 (98) |
| Married/living with partner | 56 (2) |
| Reason for seeking HIV testing | |
| Nonexclusive partner | 15 (0) |
| Seeking HIV status | 3064 (92) |
| Unprotected sex | 22 (1) |
| Symptom of STI | 47 (1) |
| Other | 118 (4) |
| Missing | 82 (2) |
IQR, interquartile range; STI, sexually transmitted infection.
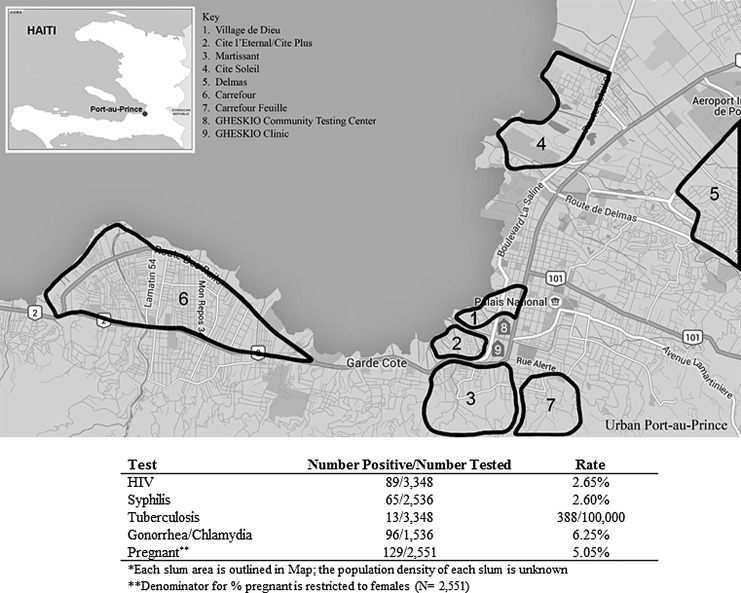
HIV prevalence was 2.65% (89/3348) among those who accepted testing. Syphilis prevalence was 2.60% (65/2536), and gonorrhea/chlamydia prevalence was 6.25% (96/1536). A total of 368 females accepted pregnancy testing, of whom 129 (5% of females in the testing campaign) were found to be pregnant. Among those who presented for HIV testing, 168 reported symptoms of TB and among symptomatic participants, 5% (13/168) tested sputum positive for TB disease (rate of 338/100,000 persons among all screened) (Fig. 1).
FIG. 1.
Map of slum areas and testing campaign results.
Results by slum neighborhood
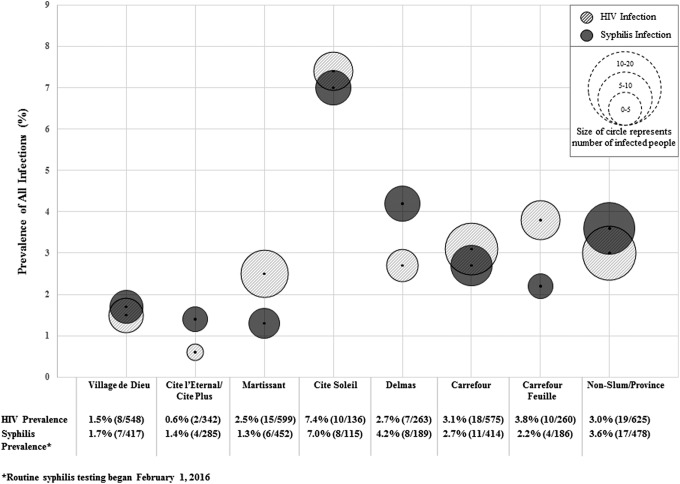
The total number of participants tested in each slum neighborhood varied from 136 in Cite Soleil to 599 in Martissant. HIV prevalence ranged from 0.6% (2/342) in Cite l'Eternel to 7.4% (10/136) in City Soleil. Five slums had an HIV prevalence higher than the national adult prevalence (1.9%), and the national prevalence among female youth (2.1%) was as follows: Cite Soleil (7.4%), Martissant (2.5%), Delmas (2.7%), Carrefour (3.1%), and Carrefour Feuille (3.8) (Fig. 2).
FIG. 2.
HIV and syphilis prevalence by slum area.
Syphilis prevalence ranged from 1.3% (6/452) in Martissant to 7.0% (8/115) in Cite Soleil. Twelve percent of HIV-infected adolescents and youth were coinfected with syphilis. Gonorrhea and/or chlamydia prevalence ranged from 2.4% (4/170) in Village de Dieu to 8.1% (7/86) in Cite Soleil. Prevalence of HIV and syphilis appears to track together by neighborhood (Fig. 2). Cite Soleil had the highest prevalence of HIV (7.4%, 10/136) and syphilis (7.0%, 8/115). Slums with the lowest prevalence of HIV and syphilis included Village de Dieu with an HIV prevalence of 1.4% (8/548,) and syphilis prevalence of 1.7% (7/417) and Cite l'Eternel/Cite Plus with an HIV prevalence of 0.6% (2/342) and syphilis prevalence of 1.4% (4/285).
In the slum neighborhoods with an HIV prevalence higher than the national average, the demographic group with the highest prevalence of all infections was older adolescent girls (aged 16–19 years). In Cite Soleil, the slum with the highest HIV prevalence (7.4%), the HIV prevalence among older adolescent girls was 6.9%. Syphilis prevalence was also the highest in Cite Soleil (7.0%), and among older adolescent girls in this slum, the syphilis prevalence was 4.1%. Among all older adolescent girls who received a test for gonorrhea and/or chlamydia, the prevalence was 5.4%—higher than all other demographic groups.
HIV-infected adolescents and youth
Among the 89 adolescents and youth who tested HIV positive, 73% were female and median age was 19 [IQR 17–20]. The HIV prevalence ranged between age groups: 7.3% (14/191) among younger adolescents (aged 10–15 years), 2.1% (39/1857) among older adolescents (aged 16–19 years), and 2.7% (36/1300) among youth (aged 20–24 years). The parent or guardian of all HIV-infected younger adolescents reported to the CHW that the adolescent was infected at birth but had not disclosed to the teen until now.
More than 75% of all HIV-infected teens reported being sexually active with a median age of first intercourse of 16 [IQR 14–17]. Only 5% of participants reported using a modern family planning method and 6% reported regular condom use.
Several sociodemographic characteristics and sexual risk behaviors differed between the three age groups (Table 2). Half of HIV-positive younger adolescents (aged 10–15 years) were boys compared to older participants, in which less than a quarter was male. Fewer younger adolescents were sexually active (21% compared to 87% of older adolescents and 83% of youth). Family planning uptake among older adolescents and youth who reported sexual activity was extremely low at 3% among older adolescents and 8% among youth. Similarly, condom use was low—among older adolescents 3% reported using a condom regularly, 62% sometimes, and 18% never, and among youth, 11% reported using a condom regularly, 36% sometimes, and 33% never.
Table 2.
Sociodemographic Characteristics and Risk Behaviors by Age Group Among HIV-Positive Participants
| Total HIV positive (n = 89) (%) | Age 10–15 (n = 14) (%) | Age 16–19 (n = 39) (%) | Age 20–24 (n = 36) (%) | p Value | |
|---|---|---|---|---|---|
| Sex | 0.10 | ||||
| Female | 65 (73) | 7 (50) | 30 (77) | 28 (78) | |
| Male | 24 (27) | 7 (50) | 9 (23) | 8 (22) | |
| Education | 0.09 | ||||
| Completed primary | 29 (33) | 8 (57) | 15 (38) | 6 (17) | |
| Completed secondary | 54 (61) | 6 (43) | 23 (59) | 25 (69) | |
| Refused answer | 6 (7) | 0 (0) | 1 (3) | 5 (14) | |
| Sexually active | <0.01 | ||||
| Yes | 67 (75) | 3 (21) | 34 (87) | 30 (83) | |
| Ever been raped | 0.29 | ||||
| Yes | 2 (2) | 0 (0) | 2 (5) | 0 (0) | |
| Age at first intercourse | |||||
| Median [IQR] | 16 [14–17] | 14 [13–14] | 15 [14–16] | 16 [14–17] | |
| No. of sexual partners in past 6 months | <0.01 | ||||
| ≤1 | 33 (37) | 1 (7) | 14 (36) | 18 (50) | |
| 2–5 | 18 (20) | 1 (7) | 9 (23) | 8 (22) | |
| ≥6 | 3 (3) | 0 (0) | 3 (3) | 0 (0) | |
| Refused answer | 35 (39) | 12 (86) | 13 (33) | 10 (28) | |
| Sexual partner has suspected current STI | 0.04 | ||||
| Yes | 7 (8) | 0 (0) | 0 (0) | 7 (19) | |
| No | 27 (30) | 1 (7) | 16 (41) | 10 (28) | |
| Do not know | 29 (33) | 2 (14) | 15 (38) | 12 (33) | |
| Refused answer | 26 (29) | 11 (79) | 8 (21) | 7 (19) | |
| Sexual partner has HIV | 0.31 | ||||
| Yes | 1 (1) | 0 (0) | 0 (0) | 1 (3) | |
| No | 28 (31) | 2 (14) | 23 (59) | 13 (36) | |
| Do not know | 24 (27) | 1 (7) | 9 (23) | 14 (39) | |
| Refused answer | 26 (29) | 11 (79) | 7 (18) | 8 (22) | |
| Currently uses modern family planning method | 0.07 | ||||
| Yes | 4 (4) | 0 (0) | 1 (3) | 3 (8) | |
| Used condom within past 3 months | 0.08 | ||||
| Always | 5 (6) | 0 (0) | 1 (3) | 4 (11) | |
| Sometimes | 38 (43) | 3 (21) | 24 (62) | 13 (36) | |
| Never | 14 (16) | 0 (0) | 7 (18) | 12 (33) | |
| Refused answer | 32 (36) | 11 (79) | 7 (18) | 7 (19) | |
IQR, interquartile range; STI, sexually transmitted infection.
The only differences between HIV-infected and HIV-uninfected teens were education and condom use. A higher proportion of HIV-uninfected than HIV-infected adolescents reported having completed some or all of high school (75% vs. 61%). A higher proportion of HIV-uninfected compared to HIV-infected adolescents and youth reported using condoms regularly (17% vs. 6%) (data not shown).
Linkage of HIV-infected individuals to care, CD4 results, and ART initiation
All 89 HIV-infected participants were enrolled in HIV care at GHESKIO on the same day as HIV testing. Among 89 adolescents who tested HIV positive, the median CD4 count at testing was 529 [IQR 363–761]. Thirty-eight (43%) adolescents were eligible for ART, based on the current national guidelines,18 and among those eligible, 24 (65%) initiated ART the same day and 13 (35%) initiated ART a median of 12 days from enrollment [IQR 6–44].
Adverse events or social harms
There were no needle stick injuries, specimen transport accidents, or social harms reported by testing staff or adolescents, youth, or their families. Adolescents and young adults were asked in the routine pretest survey about fears of loss of confidentiality of their HIV status and none reported concern.
Discussion
This large community-based comprehensive health testing campaign demonstrates that targeting adolescents and youth in slum neighborhoods is both feasible and effective in identifying HIV-infected adolescents and youth and linking them to ongoing care. The HIV testing approach reached more than 3000 individuals for testing to identify a group with an HIV prevalence over six times the national Haitian estimate for adolescent HIV prevalence—2.65% versus 0.4%. Our results characterize the HIV adolescent and youth epidemic in urban Port-au-Prince as being primarily composed of older adolescent girls aged 16–19 years with low levels of education. Our results also suggest possible geographic clustering of HIV and syphilis in certain slums, which could identify higher risk communities needing additional testing and HIV prevention programs. This community-based HIV testing campaign can serve as a model to other resource-poor settings where adolescents are driving the global HIV epidemic.
Reasons for the testing campaign's high HIV testing acceptance of 96% are multifactorial. A key element of the testing approach was packaging HIV testing within a larger bundle of health services, including STI, pregnancy, and TB screening. Bundling testing may have normalized and de-stigmatized HIV testing. Similar to other research supporting the integration of HIV care with other services, the testing approach used an “opt-out” strategy for HIV testing, which has been associated with increased uptake of HIV testing in other populations.19 Finally, providing testing in the community, rather than clinic, increased access to teens who may not have otherwise visited an HIV clinic, either due to stigma, lack of convenience, or low perceived risk. A study in Zimbabwe reported that adults who received HIV testing at a community-based site perceived themselves at higher behavioral risk of HIV infection, compared to adults who refused testing.6 This testing approach also provided increased access to guardians who were seeking a venue for facilitating disclosure to perinatally infected teens.
Combining community-based testing with prompt linkage to care is essential for optimizing engagement in care and ART initiation. Early testing and linkage to care are particularly important in adolescents as they have better treatment outcomes than adults following ART initiation, despite the additional barriers adolescents face in accessing HIV care.20 This campaign provided same-day linkage to care based on lessons learned from prior studies on home-based and community-based HIV testing for adults that were not effective in linking persons found to be HIV positive to care.21,22 All HIV-infected teens received same-day CD4 cell count tests, and among eligible participants with CD4 ≤500, 65% (25/38) initiated ART the same day as HIV testing, and the remaining 13 initiated ART a median of 12 days from testing [IQR 6–44]. Prompt linkage from HIV testing to care is essential for scale-up of WHO guidelines recommending universal treatment for adolescents in resource-poor settings.23
No adolescents or youth reported adverse events of social harms, inadvertent disclosure, or any other negative effects from testing such as increased stigma or bullying from peers. Lack of present guardian for consent prevented testing among a small proportion (<1%) of individuals younger than 16 years, but many participants younger than 16 years reported that they did not live with or have a parent or guardian and thus consent was not a major barrier. While violent activity in the slums did impede testing at times, employing CHWs with strong ties to community leaders enabled the campaign to operate safely and effectively.
Similar to other studies in sub-Saharan Africa, the majority of HIV-infected adolescents were older females with high sexual activity and low uptake of family planning and condom use.24,25 Among all participants tested, nearly 70% were younger than 18 and 23% were younger than 15 at time of first sexual activity. National estimates of age at first sexual activity in Haiti report 18 years for women and 16 years for men aged 20–49 years, but our results suggest a younger age of sexual debut among this population.4,26 Our results underscore the urgent need for access to and uptake of family planning methods. These results also indicate that human papillomavirus (HPV) vaccination in this population should be a priority for cervical cancer prevention.27
A unique group of adolescents identified and in need of testing are young adolescents with perinatal infection who were not aware of their status. This subpopulation has been identified in other studies as a challenging group to reach, given the requirement of parental consent, lack of provider training in disclosure to younger adolescents, and lack of guidance on timing of disclosure, and whether responsibility lies with guardians or healthcare workers.28–31 Our results suggest that community-based testing is an effective approach to identify this group. Some parents and guardians of adolescents with perinatally acquired infection shared with the CHWs that they viewed the community-based testing campaign as an opportunity for disclosure with the assistance of CHWs. While data on the reasons these adolescents were not previously linked to care are not available, studies have shown that parents and guardians may withhold knowledge of adolescents' HIV status due to heightened stigma of HIV; community-based testing campaigns can provide an environment for CHWs to assist with disclosure.32
Interestingly, the prevalence of HIV and syphilis appears to cluster in some slum neighborhoods. For example, Cite Soleil and Carrefour had the highest prevalence of both HIV and syphilis, and Village de Dieu had the lowest. Reasons for this clustering could be chance, given that CHWs targeted a convenience sample of participants from each neighborhood or reflect that high-risk youth recruited friends within their sexual networks for testing. Alternatively, these data may reflect some communities may have higher microepidemics of HIV than others based on activities in that community and prior testing among youth in that community. For example, Cite Soleil and Carrefour have the largest nightlife scene of bars and clubs and also have the highest gang activity and violence. In contrast, Village de Dieu is geographically closest to the GHESKIO clinic and the community that CHWs have extensively engaged with, resulting in higher HIV testing rates in the past. Knowledge of HIV status is associated with lower sexual risk behaviors, which may have reduced transmission of HIV and other STIs in these communities.33 Additional testing among a random sample of adolescents and youth in each slum is warranted to better understand if, and the extent to which, clustering is present. However, even among a nonrandom sample, identifying clustering of HIV and other infections in specific slum neighborhoods can inform programmatic interventions for prevention and treatment as well as help target future testing.34
A limitation of our study is that we report on a convenience sample of adolescent and youth who received testing from the slum communities. This is not a random sample, and thus, findings may not be generalizable for the entire slum community. Moreover, Haiti does not have national census data for these communities.
Community-based HIV counseling and testing, which incorporates community sensitization and active recruitment of adolescents and youth, bundles HIV testing without other health services, and provides same-day linkage to care, can effectively identify HIV-infected adolescents and youth. Increased detection of new HIV infections among this high-risk group and prompt ART initiation, coupled with reduced risk behavior, are needed to curb the adolescent HIV epidemic.
Acknowledgments
Funding for this study was provided by NIH/NIAID 5K24AI098627, MACAIDS Foundation, Flora Family Foundation, NIH/FIC 5D43TW009606, and UNICEF.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. Young People and HIV. New York: UNAIDS, 2012 [Google Scholar]
- 2.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults—seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep 2014;63:1097–1103 [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Available at: www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf (Last accessed April27, 2016)
- 4.UNICEF. UNICEF Global HIV and AIDS Databases (September 2015) Based on MICS, DHS, AIS and Other Nationally Representative Household Surveys, 2010–2014. Available at: http://data.unicef.org/hiv-aids/global-trends.html (Last accessed April27, 2016)
- 5.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: A transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved 2013;24:359–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: Same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr 2006;41:218–224 [DOI] [PubMed] [Google Scholar]
- 7.Meyerson B, Barnes P, Emetu R, Bailey M, Ohmit A, Gillespie A. Institutional and structural barriers to HIV testing: Elements for a theoretical framework. AIDS Patient Care STDS 2014;28:22–27 [DOI] [PubMed] [Google Scholar]
- 8.Peralta L, Deeds BG, Hipszer S, et al. Barriers and facilitators to adolescent HIV testing. AIDS Patient Care STDS 2007;21:400–408 [DOI] [PubMed] [Google Scholar]
- 9.Dorjgochoo T, Noel F, Deschamps MM, et al. Risk factors for HIV infection among Haitian adolescents and young adults seeking counseling and testing in Port-au-Prince. J Acquir Immune Defic Syndr 2009;52:498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: A mixed methods study. PLoS Med 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. HIV and Adolescents: Guidance for HIV Testing and Counselling and Care for Adolescents Living with HIV. Available at: http://apps.who.int/iris/bitstream/10665/94334/1/9789241506168_eng.pdf?ua=1 (Last accessed April27, 2016)
- 12.Wachira J, Ndege S, Koech J, et al. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. J Acquir Immune Defic Syndr 2014;65:e58–e66 [DOI] [PubMed] [Google Scholar]
- 13.Andrews BE. Prevalence and correlates of HIV testing among Caribbean youth. Int J STD AIDS 2011;22:722–726 [DOI] [PubMed] [Google Scholar]
- 14.Weiss D. Five Years Later: Haiti's Progress, Before and After the Earthquake. Huffington Post, January 9, 2015. Available at: www.huffingtonpost.com/david-weiss/five-years-later-haitis-progress_b_6437418.html (Last accessed January20, 2016)
- 15.Pape JW, Rouzier V. Embracing oral cholera vaccine—Shifting response to cholera. N Engl J Med 2014;370:2067–2069 [DOI] [PubMed] [Google Scholar]
- 16.Pape JW, Severe PD, Fitzgerald DW, et al. The Haiti research-based model of international public health collaboration: The GHESKIO Centers. J Acquir Immune Defic Syndr 2014;65 Suppl 1:S5–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocheretina O, Escuyer VE, Mabou MM, et al. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: Investigation of cases with discrepant susceptibility results. PLoS One 2014;9:e90569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral drugs: Evidence and Implementation. 2013. Available at: www.who.int/hiv/pub/journal_articles commentary_ARV_operational_guidance/en/ (Last accessed June26, 2016)
- 19.Peck R, Fitzgerald DW, Liautaud B, et al. The feasibility, demand, and effect of integrating primary care services with HIV voluntary counseling and testing: Evaluation of a 15-year experience in Haiti, 1985–2000. J Acquir Immune Defic Syndr 2003;33:470–475 [DOI] [PubMed] [Google Scholar]
- 20.Shroufi A, Gunuwo H, Dixon M, et al. HIV-Infected adolescents in southern Africa can achieve good treatment outcomes: Results from a retrospective cohort study. AIDS 2013;27:1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker LA, Jobanputra K, Rusike L, et al. Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Trop Med Int Health 2015;20:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): A randomised study. Lancet Infect Dis 2011;11:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Consolidated Guidelines on HIV Testing Services. 2015. Available at: http://apps.who.int/iris/bitstream/10665/179870/1/9789241508926_eng.pdf?ua=1&ua=1 (Last accessed April27, 2016) [PubMed]
- 24.UNICEF. Opportunity in Crisis: Preventing HIV from Early Adolescence to Young Adulthood. 2011. Available at: www.unicef.org/publications/files/Opportunity_in_Crisis-Report_EN_052711.pdf (Last accessed April27, 2016)
- 25.Ajah LO, Onubogu ES, Anozie OB, et al. Adolescent reproductive health challenges among schoolgirls in southeast Nigeria: Role of knowledge of menstrual pattern and contraceptive adherence. Patient Prefer Adherence 2015;9:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carver JW, Dévieux JG, Gaston SC, et al. Sexual risk behaviors among adolescents in Port-au-Prince, Haiti. AIDS Behav 2014;18:1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Weekly Epidemiological Record: Human Papillomavirus Vaccines: WHO Position Paper, October 2014. Available at: www.who.int/wer/2014/wer8943.pdf?ua=1 (Last accessed April27, 2016)
- 28.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis 2010;51:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: A review of emerging challenges. Lancet Infect Dis 2014;14:627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandason T, Langhaug LF, Makamba M, et al. Burden of HIV among primary school children and feasibility of primary school-linked HIV testing in Harare, Zimbabwe: A mixed methods study. AIDS Care 2013;12:1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, Ying R, Tarr G, et al. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015;528:S77–S85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busza J, Dauya E, Bandason T, et al. “I don't want financial support but verbal support.” How do caregivers manage children's access to and retention in HIV care in urban Zimbabwe? J Int AIDS Soc 2014;17:18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toska E, Cluver LD, Hodes R, et al. Adolescents, safer sex and HIV-status disclosure in South Africa. AIDS Care 2015;27 Suppl 1:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramjee G, Wand H. Geographical clustering of high risk sexual behaviors in “Hot-Spots” for HIV and sexually transmitted infections in Kwazulu-Natal, South Africa. AIDS Behav 2014;18:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]