Abstract
PURPOSE
We encountered a case where an infection with group A streptococcus (GAS; ie, Streptococcus pyogenes) initially caused primary peritonitis and then subsequently caused streptococcal toxic shock syndrome. The patient’s life was likely saved by an emergency laparotomy followed by extensive peritoneal lavage and drainage.
CASE PRESENTATION
A 40-year-old woman was admitted to the Emergency Department for lower abdominal pain and numbness in the extremities. She presented with systemic inflammatory response syndrome. An emergency laparotomy was performed, and ascites that resembled pus and general peritonitis were noted. Peritoneal lavage and drainage were performed, and GAS was isolated from peritoneal fluid. Gram staining of cervical polyp specimens revealed Gram-positive bacteria.
CONCLUSIONS
The patient was diagnosed with streptococcal toxic shock syndrome due to an ascending GAS infection originating from vagina.
Keywords: ascending infection, emergency laparotomy, group A streptococcus, peritoneal lavage and drainage, streptococcal toxic shock syndrome
Introduction
Streptococcal toxic shock syndrome (STSS) is a condition that suddenly and rapidly progresses as a result of an infection with group A streptococcus (GAS; ie, Streptococcus pyogenes). This condition can lead to septic shock, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), or multiple organ failure, which accounts for its high fatality rate. As reported, we encountered a case where GAS initially caused primary peritonitis and subsequently caused STSS. In the case in question, the patient’s life was likely saved by performing an emergency laparotomy followed by extensive peritoneal lavage and drainage.
Case Report
A 40-year-old woman was admitted to the Emergency Department of Aomori Prefectural Central Hospital for lower abdominal pain and numbness in the extremities that developed 14 hours prior. She was on day 6 of her menstrual cycle, had a temperature of 37.9 °C, a blood pressure of 92/46 mmHg, a heart rate of 97 beats/min, and an oxygen saturation of peripheral artery (SpO2) of 96%. No abnormal skin findings were noted. The lower abdomen was tender, and peristalsis had diminished slightly. Blood test results were as follows: white blood cell (WBC) count of 8100/µL, hemoglobin (Hb) of 6.2 g/dL, platelet (PLT) count of 46.2 × 104/µL, C-reactive protein (CRP) of 9.61 mg/dL, blood urea nitrogen (BUN) of 20.1 mg/dL, and creatinine of 1.52 mg/dL. Uterine fibroids, ascites, and thickening of the peritoneum were suggested, and intraabdominal hemorrhage was also a possibility, but computed tomography (CT) scan failed to reveal any obvious sources of bleeding. A pelvic infection was suspected, so the patient was admitted and azithromycin (AZM) 500 mg/d was started. However, 29 hours after initial presentation, abdominal pain intensified and the patient’s temperature increased to 38.4 °C. The patient continued to exhibit hypotension, breathing difficulties, and SpO2 of 90% or lower. An intraabdominal hemorrhage or formation of a pelvic abscess was suspected after 32 hours of initial presentation, and the decision was made to perform an emergency laparotomy. Blood test results just prior to surgery were WBC count of 700/µL, Hb of 7.8 g/dL, PLT count of 10.2 × 104/µL, CRP of 25.5 mg/dL, BUN of 35.2 mg/dL, and creatinine of 2.12 mg/dL. Systemic inflammatory response syndrome and renal dysfunction were manifested. A large volume of ascites that resembled pus and general peritonitis were manifested in the peritoneal cavity. Other than uterine fibroids, lesions were not evident in the uterus, ovaries, or fallopian tubes. The intestines were somewhat edematous, but no gastrointestinal problems, such as a gastrointestinal perforation and cholangitis, were noted. However, the greater omentum was very congested (Fig. 1). Peritoneal lavage and drainage was performed along with a partial omentectomy and an appendectomy. A peritoneal drain was placed and the abdomen was closed.
Figure 1.

Surgical findings. The greater omentum was very congested. Peritoneal lavage and drainage was performed along with a partial omentectomy and an appendectomy.
While she was managed on a ventilator, 1 g of meropenem three times a day (tid) was added to AZM. The total volume of transfusions from surgery until discharge from the intensive care unit was 24 units of packed red blood cells, 22 units of fresh frozen plasma, and 60 units of platelets, continuous hemodiafiltration was performed, direct hemoperfusion was performed with a polymyxin B-immobilized column, an immunoglobulin preparation was administered, and hemodynamics was managed with continuous intravenous infusion of noradrenaline. Blood test results about 10 hours after surgery revealed the following: WBC count of 21,000/µL, Hb of 10.1 g/dL, PLT count of 4.6 × 104/µL, CRP of 21.5 mg/dL, BUN of 36.4 mg/dL, creatinine of 2.15 mg/dL, total bilirubin of 1.83 mg/dL, aspartate aminotransferase of 699 IU/L, alanine aminotransferase of 226 IU/L, fibrin degradation product of 93.3 µg/mL, fibrinogen of 204 mg/dL, and prothrombin time of 24.6 seconds. Chest X-rays revealed infiltrated in the fields of both lungs, and ARDS, DIC, and multiple organ failure were manifested. On day 4 after initial presentation, GAS was isolated in an intraoperative peritoneal fluid culture, resulting in the diagnosis of STSS. The bacteria isolated in blood cultures, vaginal culture, or urine culture after administration of AZM were not the same. In addition, 900 mg of clindamycin tid and four million units of penicillin G once a day (sid) were administered. On day 4 after initial presentation, a rash resembling erythema developed primarily in both lower extremities and necrosis developed in the distal extremities due to poor peripheral circulation. She was discharged from the intensive care unit on day 24 after initial presentation.
There was no improvement in the necrosis in the distal extremities (Fig. 2). On day 75 after initial presentation, necrotic tissue in the distal extremities was debrided. Pedunculated tumors protruding from the cervical canal were resected transvaginally (Fig. 3A). Histopathology indicated necrotic tissue, and Gram staining revealed Gram-positive bacteria (Fig. 3B). They have a potential for the existence of GAS. Thus, she was surmised to have an ascending GAS infection originating from vagina. The patient was discharged on day 106 after initial presentation.
Figure 2.
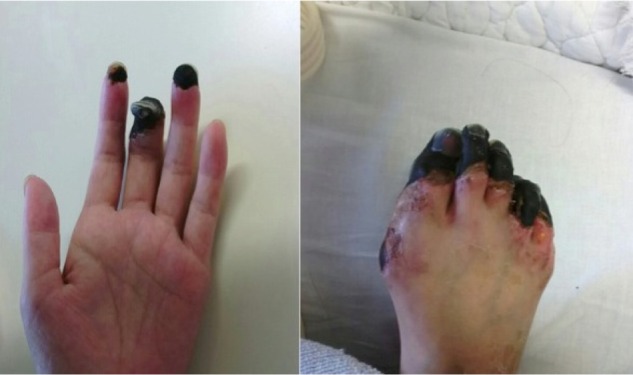
Necrosis in the distal extremities. Necrotic tissue in the distal extremities was debrided under spinal anesthesia.
Figure 3.

(A) Pedunculated tumors protruding from the cervical canal. (B) Gram staining of pedunculated tumors protruding from the cervical canal. Gram staining revealed Gram-positive bacteria (*). Magnification × 200.
Discussion
STSS is a reemerging infectious disease that was initially reported in the US in 1987.1 STSS has a fatality rate of 40% or higher.1 GAS infection has long been known to cause pharyngitis, tonsillitis, scarlet fever, and sequelae, such as rheumatic fever and acute glomerulonephritis. Initial manifestations of STSS include pain in the extremities, swelling, a fever, and hypotension; soon after developing STSS (within 24–48 hours), it can cause acute renal failure, ARDS, DIC, and soft tissue necrosis, and it can even lead to death.2 The virulence factors of GAS are far more varied than those of other bacteria, and the various exotoxins produced by GAS stimulate cytokine release and act as superantigens.3 A study of STSS isolates has suggested that specific genetic mutations allow GAS to evade elimination by the host’s neutrophils, causing a highly pathogenic disease characterized by fulminant infection.4
According to a systematic review of 32 cases of primary peritonitis due to STSS, the median age for the development of STSS was 38 years (range: 23–87 years), female patients outnumber male patients at a ratio of 4:1, and 91% of patients were healthy with no underlying illness.5 According to the same systematic review, 16% of patients developed STSS from an ascending infection originating from vagina, 6% of patients developed it from pharyngitis, 9% of patients developed it from a droplet infection, and 69% of patients developed it via an unknown route.5
In the current case, the patient was transported to the Emergency Department 14 hours after developing symptoms. When the patient was seen, she had a slight fever, and her vital signs stabilized, so STSS would have been an unlikely differential diagnosis. There is a room to discuss the appropriateness of performing an emergency laparotomy, while the patient had a WBC count as low as 700/µL. Nonetheless, purulent ascites were noted, and peritoneal lavage and drainage were performed. Peritoneal fluid was tested for bacteria about 70 hours after initial presentation, and the results revealed the presence of GAS. The patient was treated with antibacterials that GAS was susceptible to. This approach saved the patient’s life, so performing surgery was the correct choice in this case. However, necrosis in the distal extremities failed to improve. It may be a cause that Fasciitis due to gas gangrene irreversibly occurred.
Routes of infection have often cited an ascending infection originating from vagina.5 In the current case, infection occurred during menses, and a pedunculated tumor stained positive for Gram-positive bacteria, so the patient presumably had an ascending infection originating from vagina. If a patient complaining of lower abdominal pain has a fever or elevated inflammatory markers, further testing (which includes vaginal culture) must be performed prior to the administration of an antibiotic. This point was underscored by the current case. A recent report demonstrated a 6-year-old girl who initially presented with presumed viral gastrointestinal infection for a week and later developed catastrophic primary peritonitis and septic shock requiring resuscitation and emergency exploratory laparotomy due to group A streptococci.6 The incidence of STSS has increased over the past few years, so STSS must be kept in mind as potential diagnosis if a patient has abdominal symptoms that abruptly worsen.
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 523 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the article: MYokoyama, YY. Contributed to the writing of the article: MYokoyama, AM, YY. Agreed the study results and conclusions: FO, AI, MYokota, DM, ST, TK, YN, TO. Jointly developed the structure and arguments for the article: MYokoyama, AM, YY. Made the critical revisions and approved the final version: FO, AI, MYokota, DM, ST, TK, YN, TO. All authors reviewed and approved the final article.
REFERENCES
- 1.Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317(3):146–9. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 2.Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321(1):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 3.Byrne JL, Aagaard-Tillery KM, Johnson JL, Wright LJ, Silver RM. Group A streptococcal puerperal sepsis: initial characterization of virulence factors in association with clinical parameters. J Reprod Immunol. 2009;82(1):74–83. doi: 10.1016/j.jri.2009.06.126. [DOI] [PubMed] [Google Scholar]
- 4.Hirose Y, Shibuya H, Okazaki E, et al. Toxic shock-like syndrome with flu-like prodrome: a possible role of ‘enhancing tissue focus’ for streptococcal toxic shock. J Infect. 2001;42(3):195–200. doi: 10.1053/jinf.2001.0820. [DOI] [PubMed] [Google Scholar]
- 5.Westwood DA, Roberts RH. Management of primary group A streptococcal peritonitis: a systematic review. Surg Infect. 2013;14(2):171–6. doi: 10.1089/sur.2012.038. [DOI] [PubMed] [Google Scholar]
- 6.Patel RV, Kumar H, More B, Rajimwale A. Primary group A streptococcal septic shock syndrome simulating perforated appendicitis in a previously healthy girl. BMJ Case Rep. 2013;2013:bcr2013009502. doi: 10.1136/bcr-2013-009502. [DOI] [PMC free article] [PubMed] [Google Scholar]


