Abstract
In the past, laboratory tests were considered of limited value in Crohn’s disease (CD). In the era of biologics, laboratory tests have become essential to evaluate the inflammatory burden of the disease (C-reactive protein, fecal calprotectin) since symptoms-based scores are subjective, to predict the response to pharmacological options and the risk of relapse, to discriminate CD from ulcerative colitis, to select candidates to anti-tumor necrosis factors [screening tests looking for hepatitis B virus and hepatitis C virus status and latent tuberculosis], to assess the risk of adverse events (testing for thiopurine metabolites and thiopurine-methyltransferase activity), and to personalize and optimize therapy (therapeutic drug monitoring). Pharmacogenetics, though presently confined to the assessment of thiopurineme methyltransferase polymorphisms and hematological toxicity associated with thiopurine treatment, is a promising field that will contribute to a better understanding of the molecular mechanisms of the variability in response to the drugs used in CD with the attempt to expand personalized care and precision medicine strategies.
Keywords: Crohn’s disease, laboratory tests, inflammatory bowel disease
Introduction
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory diseases of the bowel, whose etiology is unknown and pathogenesis is attributed to the interaction of genetic susceptibility, environmental factors (smoking, diet, infections), and the gut microbiota, which results in an uncontrolled immune response leading to mucosal damage. Both diseases are characterized by a peak incidence in young adults (20–30 years), chronic relapsing course, and the presence of intestinal (diarrhea with mucus and/or blood, abdominal pain, perianal fistulas, bowel obstruction), constitutional (weight loss, fever) symptoms, and complications and extraintestinal manifestations. While UC is confined to the mucosa of the colon, CD can affect any segment of the gastrointestinal tract from mouth to anus and is characterized by focal, asymmetric, transmural, and, occasionally, granulomatous inflammation. The assessment of disease activity in UC involves mainly clinical and endoscopic findings integrated by laboratory tests, while it is more complex in CD where imaging is a major tool and symptoms are related not only to inflammation but also to fibrosis and the confounding irritable bowel syndrome (IBS) component.
Laboratory markers have been investigated in CD, with the aim of objectively assessing disease activity, since clinical indices are subjective, and, if possible, to avoid repeat invasive diagnostic tests.
In the past, laboratory markers were underestimated because of their low specificity. The development of biological drugs has renewed the interest in biomarkers, especially C-reactive protein (CRP), in the attempt to select patients potentially responding to these drugs.
An ideal test should be technically simple, low cost, and reproducible. It should be sensitive and specific enough to discriminate a disease from differential diagnoses. It should help in monitoring disease activity and treatment efficacy and predicting relapse and disease course. Unfortunately, these expectations are unmet by available markers.
Biomarkers used in CD are either acute-phase reactants or alterations of blood cells linked to active inflammation or alterations of parameters related to malnutrition [serum proteins and electrolytes, iron, ferritin, total iron-binding capacity (TIBC), vitamin B12, vitamin D].1–4
Renal function tests, liver enzymes, and stool cultures should also be considered at onset or relapse to monitor drug safety or to discriminate intercurrent infections.5,6 The presence of antibodies, such as anti-Saccharomyces cerevisiae, can be used for differentiating CD from UC.
Lactoferrin and fecal calprotectin are neutrophil-derived proteins considered specific surrogate markers of gut inflammation.7,8
In this review, we will report most of the laboratory tests used in patients with CD both in clinical and research settings, critically evaluating their accuracy and limits (Table 1).
Table 1.
Role of laboratory tests used in the clinical management of Crohn’s disease.
| LABORATORY TEST | ROLE | COMMENT |
|---|---|---|
| CRP and ESR | Assessment of disease activity Monitoring disease activity Predicting disease course Monitoring response to therapy |
Used in clinical practice Objective measure of inflammation Cheap Low specifity |
|
Other acute phase-reactant: Orosomucoids Beta2microglobulin Sialic acid |
Assessment of disease activity | Not routinely available Potentially useful to integrate CRP |
| Fecal calprotectin | Assessment of disease activity Monitoring disease activity Predicting disease course Monitoring response to therapy |
A surrogate marker of intestinal inflammation in IBD related to endoscopy activity Discriminating IBD from irritable bowel syndrome Can be used to avoid invasive procedures Accuracy demonstrated in the assessment of response to anti-TNF therapy Increasingly use in clinical trials of novel therapies |
| Fecal lactoferrin | Assessment of disease activity Monitoring disease activity Predicting disease course Monitoring response to therapy |
A surrogate marker of intestinal inflammation in IBD Used in the assessment of response to anti-TNF therapy Less stable at room temperature, used in research |
| Complete blood cell count (WBC, Hb, platelets) | Assessment of disease activity Monitoring disease activity Identifying complications |
Routine evaluation of recurrence Diagnosis of intercurrent infections Diagnosis of anemia Monitoring of drug safety (thiopurines) |
| Serum iron, TIBC, transferrin, ferritin, Albumin, Vitamin B12, folate | Assessment of nutrional status Identifying complications |
Diagnosis of iron-deficiency anemia Evaluation of malnutrition and selective deficiencies |
| Anti-Saccharomyces cerevisiae antibodies (ASCA) Anti-ompc Anti-pseudomonas 12 Anti-flagellin |
Antibodies- anti serum microbial antigens more specific for Crohn’s disease | Discriminating Crohn’s disease from ulcerative colitis in colonic IBD High titres related to early surgery and complications |
| Intestinal fatty acid binding proteins (FABPs) | Plasma and urine marker that indicates intestinal damage | Evidence from research Not used in clinical practice |
| PGRN antibodies (PGRN-Abs) | Proinflammatory effects | Evidence from research Not used in clinical practice |
|
Virology studies: HBV Markers Anti-HCV Anti-HIV EBV status CMV-DNA Tuberculin Skin Test (TST) Quantiferon-TB |
Screening candidates to biologics and thiopurines Assessment of refractory colitis |
Recommended by clinical practice guidelines to prevent viral infections and latent TB reactivations CMV superinfection related to refractoriness of acute colitis EBV negative subjects can develop EBV-related lymphoproliferative dysorders |
| Anti-TNF trough levels and antibodies Thiopurine metabolites |
Therapeutic drug monitoring | Approach to personalized therapy and safety and costs optimization strategy |
| TPMT (thiopurine polymorphisms) testing | Selecting candidates to thiopurines | Pharmacogenomic approach Use in clinical practice debated |
CRP, Erythrocyte Sedimentation Rate, and Acute- Phase Reactants
CRP is a 224-residue protein synthesized by the liver in low concentrations (0.1 mg/L), whose name derives from its ability to precipitate the C-polysaccharide of Streptococcus pneumoniae.2 Although CRP concentrations increase in response to many physiological conditions, it usually correlates with inflammation, being the most used acute-phase reactant.9–11
CRP binds specifically to a wide range of substances derived both from damaged autologous cells and from microorganisms.12–15 CRP has a half-life of 19 hours, shorter than other acute-phase reactants; it then increases quickly after the inflammatory insult and rapidly decreases after resolution. CD is characterized by a strong CRP response caused by massive interleukin (IL)-6 release. A serum CRP ≥ 5 mg/L has a relatively high specificity for the detection of endoscopic disease activity in patients with an established diagnosis of IBD. However, the sensitivity is poor, and a negative test does not exclude the presence of active inflammation. About 15% of patients fail to mount a CRP response, and disease subtype also affects the sensitivity of the test.16,17 In CD, CRP production correlates with the anatomic location (high expression in patients with ileitis)18 and the degree of endoscopic and histological severity.19,20
Erythrocyte sedimentation rate (ESR), expressing the rate at which erythrocytes migrate through the plasma, depends on plasma concentration and on the number and size of erythrocytes. In fact, diseases affecting the red cell lineage (anemia, polycythemia, thalassemia) influence the ESR values.21 Increase of ESR with age has been described.22 ESR values slowly increase as compared to CRP, and take several days to normalize even if the inflammation resolves.
In the study by Shine et al23 in pediatric patients with CD submitted to colonoscopy, 100% of patients had increased levels of CRP as compared to children with polyps or a normal examination. In the same cohort, ESR was positive only in 85% of patients. A larger study on 203 patients with nonspecific gastrointestinal symptoms showed that CRP was an accurate marker for the differential diagnosis between IBD and IBS.24
CRP and ESR are used to monitor disease activity. Patients with severe disease more often have increased markers of inflammation, as compared with patients in remission or mild disease. This has been shown in a prospective study by Tromm et al,25 who investigated biomarkers such as ESR, serum albumin, a1 proteinase inhibitor, blood cholinesterase, CRP, and hematocrit, and correlated these markers with endoscopic activity. The correlation was dependent on the disease site, ESR being often linked to CD of the small bowel.26,27 Fagan et al28 showed that both CRP and ESR correlated well with disease activity but the correlation was better for CRP. This has been confirmed in many other studies.28 However, there is a significant overlap in CRP values among mild, moderate, and severe disease. Since the range of CRP values is wide, in clinical practice it is more useful to perform serial testing to demonstrate the efficacy of a given therapy.
CRP has been shown to have a prognostic role. Brignola et al, in a prospective study, followed up 41 patients with CD in remission (Crohn’s Disease Activity Index [CDAI] < 150) for six months, using a panel of inflammatory markers (ESR, white blood cells (WBCs), hemoglobin, albumin, alpha-2 macroglobulin, serum iron, CRP, alpha-1-acid glycoprotein, and alpha-2-antitrypsin)29 and showed an increased recurrence rate at two years in those who had higher CRP values. Recently, Louis et al30 demonstrated that laboratory indices, including CRP <5 mg/L, hemoglobin level >145 g/L, WBC count <6.0 × 109/L, and fecal calprotectin <300 g/g, can be used to select patients with CD in stable remission with combined therapy with infliximab (IFX) and an antimetabolite, in whom IFX can be safely stopped with a low risk of relapse.
A decrease of the CRP value during therapy is an indicator of efficacy. On the other hand, a persistently high value indicates treatment failure. Recent trials on biologics have increasingly used baseline-elevated CRP level as an inclusion criterion to ensure that the enrolled patients have active inflammation. This practice largely stems from results of the Phase II induction studies of certolizumab published in the mid-2000s.31–33 The study by Schreiber et al31 demonstrated a high placebo response rate in subgroups of patients with low CRP and, in post hoc analysis, a significant effect of therapy only in the subgroup of patients with baseline CRP > 10 mg/L. In the further certolizumab induction trial by Sandborn et al32 and in the maintenance trial by Schreiber et al,33 patients were stratified according to their baseline CRP level. Among patients with a baseline CRP level of at least 10 mg/L, 37% of patients in the certolizumab group had a response at week 6, as compared with 26% in the placebo group (P = 0.04). The clinical response was maintained through week 26 in 62% of patients with a baseline high CRP.
Fifty percent of patients enrolled to the active ulcerative colitis (ACT) 1 and ACT 2 trials had elevated CRP at enrollment, but changes were not reported as a marker of response to treatment.34 Other smaller studies on conventional drugs have also included CRP measurement.35 Other acute-phase reactants such as sialic acid, alpha1-acid glycoprotein, ororosomucoid, fibrinogen, lactoferrin, β-2-microglobulin, serum amyloid A, α-2-macroglobulin, and α-2-antitrypsin have been investigated in IBD with conflicting results. For sure, mainly due to the longer half-life of these proteins, they have a lower accuracy than CRP and are not used in routine practice.36,37
Finally, β-2-microglobulin is a low molecular weight protein released by activated T- and B-lymphocytes, with an estimated half-life of two hours. A few studies have shown good correlation between β-2-microglobulin and disease activity,38,39 although other authors could not confirm these results.40
Hematologic Tests
The components of the complete blood cell count can indicate disease activity and iron or vitamin deficiency. An elevated WBC count is common in patients with active IBD and does not necessarily mean infection. High leukocyte count is also common in patients taking steroids due to drug-induced mobilization of marginated neutrophils. Anemia is common, either as anemia of chronic disease [normal mean corpuscular volume (MCV) or as iron-deficiency anemia (low MCV)]. Elevated MCV (macrocytosis) occurs in patients taking azathioprine (AZA) or 6-mercaptopurine (6-MP). Platelet count is frequently elevated because of active inflammation or iron deficiency. The accuracy of platelet count to monitor disease activity has been found low.
Vitamin B12 and folic acid levels often need to be evaluated as expressions of selected deficiency. Vitamin B12 deficiency can occur in patients who have extensive terminal ileum disease or in patients submitted to terminal ileum resection. Folate deficiency may occur in patients on sulfasalazine, which is a folate reductase inhibitor, and is common in patients taking methotrexate. Folate deficiency is related to increased homocysteine levels and thromboembolic complications. The assessment of nutritional status includes serum albumin, prealbumin, ferritin, and transferrin levels. Hypoalbuminemia may reflect malnutrition due to poor oral intake or protein-losing enteropathy and can be considered a negative acute-phase reactant since decreased levels may be found during inflammation.
Fecal Calprotectin and Other Fecal Markers
Stool samples are routinely collected in CD patients to test the presence of WBCs, routine pathogens, ova, parasites, and Clostridium difficile toxin to rule out superinfections during relapses and before the initiation of immunomodulators.41 Recently, a number of neutrophil-derived proteins present in stools have been studied searching for a gut-selective biomarker of inflammation, including fecal lactoferrin, lysozyme, elastase, myeloperoxidase, and calprotectin. Fecal calprotectin, a 36-kDa calcium- and zinc-binding protein, is the most promising fecal marker and has been proposed as a noninvasive surrogate marker of intestinal inflammation.42 Calprotectin represents 60% of cytosolic proteins in activated neutrophils, and its presence in stools can be seen as an expression of neutrophil migration to the gut. Although calprotectin is a very sensitive marker for the detection of gut inflammation, it is not specific enough since increased levels are also found in colorectal carcinoma, infections, and polyps. Fecal calprotectin is stable for more than one week at room temperature and is resistant to degradation. This protein can be measured using commercially available enzyme-linked immunosorbent assay (ELISA) or more recently developed quantitative rapid tests, although the latter are considered less accurate.43 Early studies in IBD have shown a good correlation with indium-labeled leukocyte excretion and intestinal permeability.44 Fecal calprotectin levels increase upon exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) as well as with older age.45
More recently, fecal calprotectin was shown to predict the relapse of CD.46–48 In a patient with high pretest probability of endoscopically active disease (eg, 80%), a positive fecal marker could predict that disease is present, and endoscopy could be avoided. A negative result in the same patient would not confidently rule out active disease (ie, 50% posttest probability). Conversely, in a patient with a lower clinical suspicion of active disease (eg, 25% pretest probability), a positive test might provide enough evidence to proceed with further investigations (ie, 50% posttest probability), whereas a negative test almost completely rules out the possibility of active disease.49,50 In the study by Tibble et al, calprotectin levels predicted the risk of relapse. At 50 mg/L, the sensitivity and specificity of calprotectin for predicting relapse in patients with IBD were 90% and 83%, respectively.45,51,52 A meta-analysis of eight studies, including 394 pediatric IBD cases and 321 non-IBD controls by Henderson et al,53 exploring the possibility to reduce colonoscopy rate, found that fecal calprotectin has a high sensitivity but modest specificity for diagnosing IBD in children.
Fecal lactoferrin is another fecal marker. It is an iron-binding glycoprotein expressed by activated neutrophils. In contrast to CRP and fecal calprotectin, the use of fecal lactoferrin has been mainly limited to research, probably because of its shorter stability at room temperature.54 Fecal calprotectin and fecal lactoferrin have been demonstrated to have better sensitivity than CRP. Langhorst et al55 investigated fecal lactoferrin, fecal calprotectin, fecal PMN-elastase, and serum CRP in patients with IBD and found evidence that all of them were able to differentiate active IBD from inactive IBD as well as from IBS. None of these three stool markers is consistently superior in its ability to detect endoscopic inflammation, but all three are superior to CRP in their diagnostic accuracy. Schroder et al56 evaluated the role of fecal markers in identifying gut inflammation and found calprotectin to be superior to lactoferrin and PMN-elastase. Shitrit et al57 confirmed calprotectin to be the most reliable predictor of abnormal colonic pathology in 72 consecutive patients undergoing colonoscopy.
We have recently assessed58 the role of fecal calprotectin in the evaluation of patients with chronic diarrhea. Patients were selected carefully, and potential false-positive subjects were excluded, ie, patients with gastrointestinal bleeding, known colorectal or gastric cancer, polyposis syndromes, active infections, recent use of NSAIDs or aspirin, pregnant women, and alcoholics. Three-hundred and forty-six consecutive patients were submitted to a thorough investigation including serum CRP, ESR, stool cultures, and colonoscopy with biopsies. Of the 346 enrolled patients, 242 (69.9%) had a normal colonoscopy, 82 patients (23.7%) had IBD, 1 had ischemic colitis (0.3%), 10 had polyps (2.9%), and 11 had diverticular disease (3.2%). Calprotectin levels were significantly higher in patients with an abnormal colonoscopy (P-value < 0.0001). When we focused on histologic changes alone, calprotectin levels were found to be significantly higher in the patients with histologic inflammation (P-value < 0.001). Using a cutoff of 150 μg/g of stool, fecal calprotectin had 75.4% sensitivity, 88.3% specificity, and 81% and 83% positive and negative predictive values, respectively.
In a meta-analysis of 30 studies, von Roon et al59 found calprotectin to have a sensitivity of 0.95 [95% confidence interval (CI) 0.93–0.97], specificity of 0.91 (95% CI 0.86–0.91), and area under the curve of 0.95 for the diagnosis of IBD.
Calprotectin can also be used to assess disease activity in patients with already diagnosed IBD.60–62 In patients with CD, calprotectin did not correlate with clinical disease activity but significantly correlated with endoscopic activity.
In the era of biologics, there is evidence that supports the use of fecal calprotectin for monitoring the response to anti-tumor necrosis factor (anti-TNF) therapy. Sipponen et al63 demonstrated a drop in the mean fecal calprotectin levels after therapy with anti-TNF agents and found a moderate correlation between change in the fecal calprotectin levels and change in endoscopic activity using the Crohn’s disease endoscopic index of severity. Another study failed to demonstrate any significant change in fecal calprotectin levels in patients who responded to medical therapy;64 however, these studies had a limited sample size. Fecal calprotectin is increasingly used as an objective measure of inflammation in clinical trials of novel therapeutic agents.65,66
There are data supporting the use of fecal calprotectin to predict relapse in CD. In a prospective multicentre study, Gisbert et al67 demonstrated that CD patients who relapsed within one year had significantly higher fecal calprotectin levels at baseline with a low sensitivity (28%) but high specificity.63
Other Serological Markers
Anti-S. cerevisiae antibodies (ASCA) have been detected in patients with CD. Positive ASCA and negative perinuclear antineutrophil antibody (pANCA) in patients with colonic inflammation help in discriminating CD from UC.68 ASCA-positivity is associated with a higher risk of surgery. A greater titer of ASCA is also related to a greater risk of complications such as strictures and fistulas.69,70 Additional serum antibodies to microbial antigens have been described in CD, such as Escherichia coli anti-OmpC (outer membrane porin C), detected in about 50% of CD cases, anti-Pseudomonas fluorescens associated sequence 12 (anti-12), and anti-flagellin-like antigen (anti-Cbir1), the last being associated with small bowel fistulizing and stenosing disease. A meta-analysis evaluated the stratification powers of serum antibodies to microbial antigens in characterizing progression of CD and found that anti-OmpC had the highest power for the risk of both complications and surgery.70
Sarikaya et al71 demonstrated the role of Intestinal fatty acid-binding proteins (FABPs), a plasma and urine marker that indicates intestinal damage. In a preliminary study, on 74 patients with CD (41 active and 33 in remission) and 37 healthy controls, the levels of serum I-FABP of patients with active disease were statistically higher as compared to patients in the remission and control groups (P = 0.012 and P = 0.038, respectively). No statistically significant difference was observed among patients in remission and healthy controls (P = 0.145).
Thurner et al72 investigated the occurrence of progranulin-antibodies (PGRN-Abs) in IBD. PGRN is a secreted protein with strong anti-inflammatory effects, believed to be mediated by the direct inhibition of TNF receptors. PGRN-Abs were found in the sera of 23/141 CD patients (16.31%).
Microbiology
The role of stool cultures and search for ova and parasites and C. difficile toxins A and B to rule superinfections has been already reported;73 especially, C. difficile must be searched in any patient hospitalized with a flare of colitis. Diagnostic tests looking for cytomegalovirus (CMV) [antigenemia or CMV-DNA by polymerase chain reaction (PCR)] should be carried out in cases of acute colitis refractory to steroids to rule out CMV infection.74
Laboratory Tests in Screening for Biologics
A panel of laboratory tests [markers for hepatitis B virus (HBV) and hepatitis C virus (HCV), HIV, and tuberculine tests] is recommended by current guidelines before starting biologics.73 Viral markers should be searched to prevent viral reactivation, which, in the case of hepatitis B, can lead to fulminant liver failure. Chronic HBV is the most common chronic viral infection of the liver, and the prevalence of chronic HBV carriers the general population is about 2–7%.75 The European Crohn’s and Colitis Organization (ECCO) Guidelines report that “all IBD patients should be tested for HBV (HBsAg, anti-HBAbs, anti-HBcAb). In patients with positive HBsAg, viremia (HBV-DNA) should also be quantified. Since patients with positive HBcAb and negative HBsAg may have occult HBV infection, and reactivation of occult HBV rarely occurs with immunosuppressive therapy, viremia (HBV DNA) should be assessed every 2–3 months but antiviral therapy is not recommended unless HBV-DNA is detected”.
Screening for HCV using antibody testing is also recommended. If positive, it should be confirmed by the detection of HCV RNA. This is important because of the potential risk of worsening liver function as a result of immunosuppressive therapy, concomitant infection with other viruses, (HBV/HIV), or by the potentiating the effects of hepatotoxic medications.
ECCO recommends also testing for HIV in adolescent and adult patients with IBD, based on reports of increased risk and severity of HIV-related infection in patients receiving immunomodulator therapy.73
Screening for tuberculosis (TB) by tuberculin skin test (TST) or interferon-γ release assay (IGRA) aims at reducing the occurrence of TB reactivation in patients treated with TNF-α inhibitors.76 The additive value of IGRA in TB screening remains unclear because, though reducing the rate of false-positive results, it has higher costs.77 However, ECCO guidelines on opportunistic infections in IBD recommend “TB skin text or, better, interferon-γ assays (Quantiferon-TB) in all patients candidates to biologics”.68,78
In the Western world, the estimated prevalence of latent TB is about 4.8%.79 CD patients treated with anti-TNF-α have a 4- to 20-fold increased risk of reactivation of TB, with a mortality rate as high as 14%.80–84
TST has a high rate of false-positive results due to the nonspecific nature of purified protein derivative and the booster phenomenon of serial TST testing.85,86 In a recent meta-analysis of nine studies comparing TST with different IGRAs, Shahidi et al76 showed a modest to strong agreement between TST and IGRA. Concordance was affected by the Bacillus Calmette–Guerin (BCG) vaccination status, which increases the false-positive rate of TST. Thus, IGRA may have an additive value in IBD patients who are BCG-vaccinated. A recent cost-effectiveness analysis shows that among the multiple screening strategies—TST alone, IGRA alone, or both TST and IGRA—the use of IGRA in BCG-vaccinated individuals is the most cost-effective screening strategy.87
Unfortunately, Helwig et al88 demostrated that the result of Quantiferon-TB Gold testing in IBD patients is affected by corticosteroids and immunomodulators; in their study, nearly 28.9% had an indeterminate result of the Quantiferon test and the main predictor was combination therapy.
As far as thiopurines are concerned, Epstein–Barr virus (EBV) antibody status should be checked before starting therapy, and EBV-negative patients should not be treated because of the risk of EBV-related lymphoproliferative disorders.73
Therapeutic Drug Monitoring
TNF-α inhibitors have dramatically changed the therapeutic scenario in IBD. IFX is a chimeric IgG1 monoclonal antibody consisting of human constant and murine variable regions, while adalimumab (ADA) is a recombinant fully human IgG1 monoclonal antibody; both are indicated for the induction and maintenance of clinical remission in patients with moderate to severely active CD.34,89–91 Anti-TNF’s benefits include reduced rates of hospitalization and surgery and improvement of the quality of life.92,93 Unfortunately, 13% of patients/year for IFX and 20% for ADA lose response (secondary nonresponders), and up to 30% are primary nonresponders.94 Loss of response (LOR) can be due to multiple factors, the most important being the appearance of anti-drug antibodies, a phenomenon known as immunogenicity, which increases drug clearance and ultimately contributes to treatment failure.
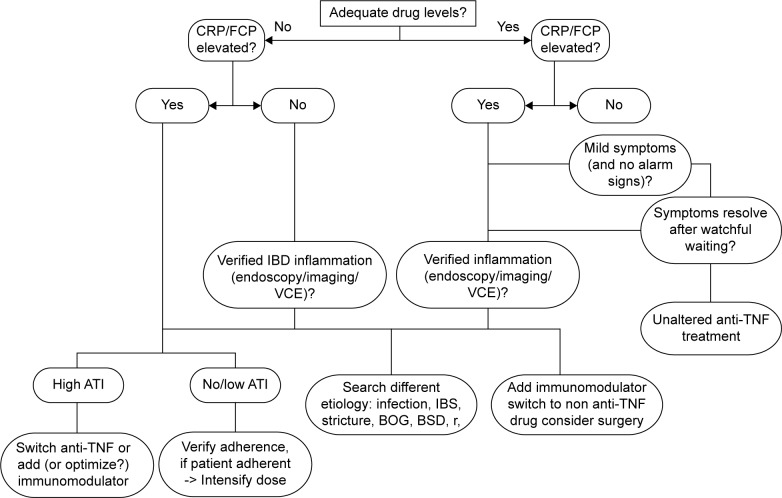
Monitoring of drug levels and anti-drug antibodies may allow the identification of patients in whom dose escalation or intensification is warranted and patients in whom a therapeutic switch within the class or out of class (also called swap; Fig. 1) is necessary, thus leading to a more appropriate and cost-effective management strategy.95 Consensus on trough level cutoffs related to clinical response is lacking. Most researchers agree that a level of 3 μg/mL for IFX is a marker of efficacy,96 and some report that trough levels of 3–7 μg/mL97 and 5–10 μg/mL98 are the target levels for maintenance of remission. Trough levels can predict clinical response. In a subgroup analysis of the ACCENT 1 study, postinduction trough levels of IFX were related to sustained clinical response at week 54.99 A serum level of IFX of 12.0 μg/mL four weeks after the last infusion and an ADA cutoff drug level of 5.85 μg/mL have been proved as having high sensitivity, specificity, and positive likelihood ratio for the prediction of clinical response.100
Figure 1.
A TDM based algorithm for management of loss of response to TNFα inhibitors. Adapted by permission from Macmillan Publishers Ltd: Nat Rev Gastroenterol Hepatol. Ben-Horin S and Chowers Y. Tailoring anti-TNF therapy in IBD: drug levels and disease activity. 11:243–255 (2014).148
There are several methods to measure IFX and ADA trough levels, the most used being a solid-phase, double-antigen ELISA. Though simple, reproducible, and relatively cheap, this method has some pitfalls, such as its limited sensitivity in the presence of IFX in the serum and the inability to detect immunoglobulin 4 (IgG 4) ATI. Inconclusive results are not uncommon in the routine practice as well in the research setting (72% in the SONIC trial).101 Other methods are a fluid-phase radioimmunoassay and a recent homogeneous mobility shift assay using high-performance liquid chromatography which has higher sensitivity and specificity for all immunoglobulin subclasses.102,103 Both techniques permit the measurement of ATI, even in the presence of IFX, but on direct comparison of these new methods with standard ELISA techniques, an improved performance could not be demonstrated. A modified ELISA, employing the anti-human λ antigen detection antibody (AHLC), was found more accurate in the detection of ATI in the presence of IFX, regardless of Ig subtypes.104–107 In patients with IBD, the negative effect that antibodies to IFX (ATI) and antibodies to ADA (ATA) have on clinical outcomes has been well established,106–110 although some studies yielded discrepant results.65,111–114 The discrepancy may be due to the different sensitivities of the employed assays but also to the presence of non-neutralizing antibodies and alternative ways of anti-TNF clearance.115 In addition, the appearance of ATI may start with low titers not affecting the drug trough levels and progressively increase to high titers, leading to drug clearance. Also, the timing of investigation of ATI in the literature is different, from weeks before LOR to tests carried on after LOR has manifested.116,117 Moreover, transient ATI are frequently detected (up to 28% of patients). Their appearance is unpredictable and has no significant impact on LOR-free survival.118,119
In a landmark study, Baert et al120 studied the immunogenicity of anti-TNF in a group of patients with CD on an episodic maintenance schedule. They found that 61% had detectable ATI after the fifth infusion, which did not increase after subsequent infusions. They found that those patients with ATI > 8.0 μg/mL before an IFX infusion had a shorter duration of response and a higher risk of infusion reactions.
Afif et al121 looked at the clinical utility of measuring IFX levels and ATI in patients with LOR or an incomplete response to IFX. They found that most patients with ATI did not respond to IFX dose escalation, but those with no ATIs and subtherapeutic concentrations did benefit from a higher dose. With ADA, the experience is more limited. In an observational study, Karmiris et al110 found that lower ADA serum trough levels were associated with drug discontinuation and that the presence of ATA was associated with lower ADA levels. Recent studies have found that detectable ATA and ADA levels of <5 μg/mL are associated with higher CRP serum levels, increased endoscopic inflammatory activity, and the use of steroids. Another recent study measuring ADA trough levels by ELISA showed similar results.110 The concomitant use of immunomodulators122 (AZA, MP, or methotrexate) or hydrocortisone123 increases anti-TNF trough levels. This can be explained by the reduction of immunogenicity and combined effect on the burden of inflammation.124
Thiopurine Metabolites
Thiopurines (6-MP and AZA) are the most common immunomodulators used in IBD. After absorption, AZA is converted to 6-MP by a nonenzymatic pathway (Fig. 2). Measuring thiopurines metabolites has two main goals: to optimize dose and treatment efficacy and to reduce the risk of adverse events (Table 2). Metabolites that can be tested are 6-TGN and 6-MMP. 6-TGN has been associated with response to treatment. A 6-TGN level >230 pmol/8 × 108 Red blood cells (RBCs) has been correlated with clinical response and remission in both adults and children with IBD.125 Increasing the drug dosage has been recommended in patients without clinical response and subtherapeutic 6-TGN levels to improve the response.126 Unfortunately, another study including only adult patients failed to confirm this result.127
Figure 2.
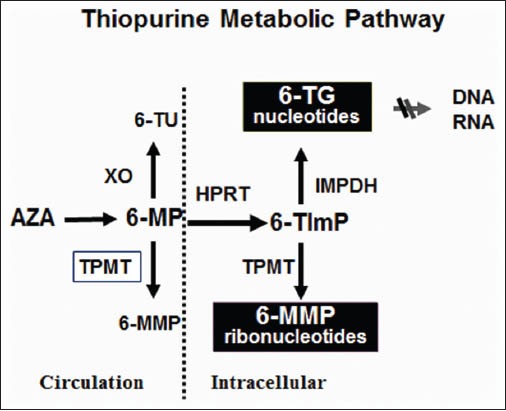
Thiopurine metabolic pathway.
Table 2.
Metabolite profiles, clinical interpretation and management recommendations in thiopurine non-responders.
| METABOLITE PROFILE | CLINICAL INTERPRETATION | MANAGEMENT |
|---|---|---|
| Negligible or undetectable 6-TGN and 6-MMP | Non-adherence | Patient education |
| Low 6-TGN Low 6-MMP |
Underdosing | Dose escalation |
| Low 6-TGN High 6-MMP |
Thiopurine “resistant” |
Unlikely to respond Switch therapeutic class |
| High 6-TGN High 6-MMP |
Thiopurine “refractory” |
Switch therapeutic class |
Another indication for metabolite measurement is the evaluation of adherence to therapy, which is a main issue in long-term treatments.128,129 Thiopurine methyltransferase (TPMT) enzyme activity can also be measured, and a relationship with response to thiopurines has been suggested. The cutoff value of 35 pmol/h/mg correlated with the clinical response rate (81% vs 43%; P < 0.001). In an additional study, TPMT activity below 15.3 U/mL was significantly associated with a sixfold higher response rate to AZA.130,131 The risk of resistance is increased in patients with higher TPMT activity (OR, 0.21; 95% CI, 0.06–0.71; P = 0.009). Patient nonresponders with normal TPMT activity and high 6-TGN levels should be classified as refractory to thiopurines, and treatment should be discontinued.132
AZA metabolite testing can also be used to prevent drug-related adverse events. Higher 6-MMP levels are related to hepatotoxicity, and levels above 5700 pmol/8 × 108 RBC have a threefold increased risk. However, positive and negative predictive values are not high enough since not all patients with high 6-MMP levels will experience an increase of liver enzymes, and having a low 6-MMP level does not preclude the development of liver damage.131 As for 6-MMP, 6-TGN levels have limited accuracy in predicting the development of myelotoxicity.
Thiopurine metabolite levels should be tested two to three weeks after starting therapy or after dose optimization; they should be reassessed in case of LOR or adverse events or when drug interactions are suspected (concomitant therapy with 5-ASA or allopurinol). In addition, it would be useful to assess the metabolite levels twice a year to check adherence.133 Measuring 6-TGN and 6-MMP levels, however, cannot replace monitoring of liver enzymes and blood count. This and the conflicting results of published studies explain the limited use of thiopurine metabolites in real life, confined to tertiary referral centers.
Genetic testing for TPMT polymorphism has been also suggested before starting thiopurines. Adverse events to AZA and 6-MP might develop suddenly and unpredictably. Thus, screening the patients for genetic susceptibilities to predict the risk of toxicity has aroused considerable interest. Genetic polymorphisms that account for reduced (heterozygote) or absent (homozygote) TPMT activity have been confirmed. Approximately 89% of the population has wild-type TPMT, which is associated with normal or high TPMT enzyme activity, while 11% are heterozygous and have low TPMT enzyme. One in 300 (0.3%) of the population is homozygous for mutations of TPMT, having negligible activity, which produces high levels of 6-TGN and consequently bone marrow suppression. Intermediate and normal metabolizers can have up to a threefold difference in initial target doses of AZA and 6-MP to achieve therapeutic 6-TG concentrations. In a meta-analysis published in 2010,134 IBD patients with TPMT polymorphisms were confirmed more likely to experience adverse events, in particular bone marrow toxicity, but not hepatotoxicity and pancreatitis. The overall concordance rate between the genetic and phenotypic tests for TPMT was 71.6%. These pitfalls, together with the ethnic differences in polymorphisms and clinical observations that adverse events which can also be attributed to concurrent viral infections or drug interaction (aminosalycilates, allopurinol), limit the clinical relevance of genetic testing, and practice guidelines do not recommend its routine use.
Future Biomarkers
In the field of fecal biomarkers, a limitation to their routine use in clinical practice is their cost. A novel fecal marker, Lipocalin 2 (Lcn-2), could be a cost-effective substitute of calprotectin or lactoferrin. Lcn-2 and its human counterpart neutrophil gelatinase-associated lipocalin (NGAL)135,136 belong to a family of small secreted proteins expressed by a variety of cells, the richest source being neutrophils. Previous studies have shown systemic upregulation of Lcn-2 (also known as siderocalin, uterocalin, and 24p3) in various murine models of colitis. In mice, fecal Lcn-2 increases 10-fold in response to dextran sodium sulfate (DSS)-induced low-grade/subclinical inflammation and 10,000-fold in response to DSS concentrations that induced histopathologically evident colitis.
In addition, human NGAL has been reported to be increased in patients with UC. The pro-inflammatory transcription factor NF-κB transactivates Lcn-2 expression by binding to the consensus motif within its promoter. Intestinal epithelial cells are one of the cell types in which Lcn-2 is highly induced, and in these cells most Lcn-2 is secreted apically (ie, luminally), so fecal Lcn-2 might also be a noninvasive marker of intestinal inflammation. As sample acquisition and preparation is very simple and stable, and all requisite reagents for fecal Lcn-2 assay via sandwich ELISA are commercially available at low cost, fecal Lcn-2 can be competitive when compared to other fecal markers such as calprotectin or lactoferrin.
New anti-glycan antibodies,137 anti-GP2, and anti-GM-CSF are currently investigated for their impact on diagnosis, prognosis, and prediction of therapeutic ressponses. Anti-glycan, anti-GP2, and anti-GM-CSF Ab are especially associated with CD and seem to be correlated with complicated disease phenotypes, progression to a more severe disease course, and a higher risk for IBD-related surgery. Anti-GP2 Ab could particularly contribute to better stratify cases of pouchitis.
Moreover, it is likely that future biomarkers will result from emerging molecular biology approaches, such as genomics, metagenomics, and transcriptomics, as already observed in other immune-mediated diseases, where they have directly impacted clinical care such as gene expression profiling of peripheral blood in heart transplant patients in monitoring for rejection138 and proteomic profiling of blood plasmacytoid dendritic cells in systemic sclerosis patients.139
In CD, polymorphisms in FOXO3 have been associated with more severe disease, but not with disease onset140 and pharmacogenetics, though presently confined to the assessment of TPMT polymorphisms, is a promising field that will contribute to a better understanding of the molecular mechanisms of variability in response to drugs.
MicroRNAs (miRNAs)141,142 are short, endogenous, noncoding single-stranded RNAs that have been highly conserved throughout evolution, and are involved in the control of gene expression at the post-transcriptional level. Their role has been recognized in the regulation of cell cycle, differentiation, proliferation, apoptosis, fibrosis, and immune function. MiRNAs have been found in tissues, serum, plasma and other body fluids. Serum levels are stable and reproducible, making them attractive as biomarkers. Distinct profiles of miRNA143–146 expression have been identified in IBD patients versus healthy subjects, as well as between UC versus CD, and between patients with active or inactive disease; serum levels have been also related to tissue levels. MiRNAs have been implicated in the modulation of pharmacological response, and a prospective clinical study has found a relationship between miRNAs and glucocorticoid response in patients with IBD. MiR-19-3p has been indicated as a potential circulating marker of stricturing CD. A suite of miRNAs, including miR-31-5p, miR-215, miR-223-3p, miR-196b-5p, and miR-203, could stratify patients with CD according to disease behavior and serve as reliable prognostic markers to drive therapeutic intervention. These findings suggest a potential translation of epigenetics and miRNA technology into clinical practice.
Conclusions
This review has explored the features, diagnostic accuracy, relevance, limits, costs, and future applications of biomarkers in CD. Besides offering a comprehensive and updated panoramics, the authors’ aim was to stress that clinical scores and endoscopic and imaging procedures, in the era of treat to target strategy and of the emerging concepts of personalized care and precision medicine,147 need to be supplemented by serum and fecal biomarkers both in the diagnostic workup of CD and in the assessment of disease activity, determining prognosis and monitoring therapeutic response. The ultimate goal of using surrogate markers is also the avoidance of excessive invasive procedures. The challenge of innovative biomarkers is to obtain a molecular characterization of the patient with CD, thus allowing stratification of patients according to disease phenotype and risk of complications.
Footnotes
ACADEMIC EDITOR: Melpakkam Srinivas, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 421 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: MC and GCM. Analyzed the data: MC and GCM. Wrote the first draft of the manuscript: MC and GCM. Contributed to the writing of the manuscript: MC and GCM. Agree with manuscript results and conclusions: MC and GCM. Jointly developed the structure and arguments for the paper: MC and GCM. Made critical revisions and approved final version: MC and GCM. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Mazlam MZ, Hodgson HJ. Peripheral blood monocyte cytokine production and acute phase response in inflammatory bowel disease. Gut. 1992;33:773–778. doi: 10.1136/gut.33.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederau C, Backmerhoff F, Schumacher B, et al. Inflammatory mediators and acute phase proteins in patients with Crohn’s disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90–107. [PubMed] [Google Scholar]
- 3.Pepys MB, Druguet M, Klass HJ, et al. Immunological studies in inflammatory bowel disease. In: Porter R, Knight J, editors. Immunology of the Gut, Ciba Foundation Symposium. Amsterdam/North Holland: Elsevier/ExcerptaMedica; 1977. pp. 283–297. [DOI] [PubMed] [Google Scholar]
- 4.Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stange EF, Travis SP, Vermeire S, et al. European Crohn’s and Colitis Organisation European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55(suppl 1):i1–i15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99(11):2235–2241. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 7.Kallel L, Ayadi I, Matri S, et al. Fecal calprotectin is a predictive marker of relapse in Crohn’s disease involving the colon: a prospective study. Eur J Gastroenterol Hepatol. 2010;22(3):340–345. doi: 10.1097/MEG.0b013e32832bab49. [DOI] [PubMed] [Google Scholar]
- 8.Sidhu R, Wilson P, Wright A, et al. Faecal lactoferrin—a novel test to differentiate between the irritable and inflamed bowel. Aliment Pharmacol Ther. 2010;31(12):1365–1370. doi: 10.1111/j.1365-2036.2010.04306.x. [DOI] [PubMed] [Google Scholar]
- 9.Siemons L, Ten Klooster PM, Vonkeman HE, van Riel PL, Glas CA, van de Laar MA. How age and sex affect the erythrocyte sedimentation rate and c-reactive protein in early rheumatoid arthritis. BMC Musculoskelet Disord. 2014;15:368. doi: 10.1186/1471-2474-15-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136:138–146. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 11.Wunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, Birkhäuser MH. Serum leptin and c-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. Eur J Endocrinol. 2006;155:137–142. doi: 10.1530/eje.1.02178. [DOI] [PubMed] [Google Scholar]
- 12.Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1:653–657. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- 13.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- 14.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23:118–124. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- 15.Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J Autoimmun. 2002;19:147–154. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- 16.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 17.Kathiresan S, Larson MG, Vasan RS, et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum c-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 19.Kiss LS, Papp M, Lovasz BD, et al. High-sensitivity c-reactive protein for identifi cation of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification. Inflamm Bowel Dis. 2012;18:1647–1654. doi: 10.1002/ibd.21933. [DOI] [PubMed] [Google Scholar]
- 20.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of c-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RD, Westengard JC, Hay KL, Bull BS. Calibration and validation for erythrocyte sedimentation tests. Role of the International Committee on Standardization in Hematology reference procedure. Arch Pathol Lab Med. 1993;117:719–723. [PubMed] [Google Scholar]
- 22.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 23.Shine B, Berghouse L, Jones JE, et al. C-reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin Chim Acta. 1985;148:105–109. doi: 10.1016/0009-8981(85)90219-0. [DOI] [PubMed] [Google Scholar]
- 24.Poullis AP, Zar S, Sundaram KK, et al. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409–412. doi: 10.1097/00042737-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Tromm A, Tromm CD, Huppe D, et al. Evaluation of different laboratory test-sand activity indices reflecting the inflammatory activity of Crohn’s disease. Scand J Gastroenterol. 1992;27:774–778. doi: 10.3109/00365529209011182. [DOI] [PubMed] [Google Scholar]
- 26.Sachar DB, Smith H, Chan S, et al. Erythrocytic sedimentation rate as a measure of clinical activity in inflammatory bowel disease. J Clin Gastroenterol. 1986;8:647–650. doi: 10.1097/00004836-198612000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Sachar DB, Luppescu NE, Bodian C, et al. Erythrocyte sedimentation as a measure of Crohn’s disease activity: opposite trends in ileitis versus colitis. J Clin Gastroenterol. 1990;12:643–646. doi: 10.1097/00004836-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 29.Brignola C, Campieri M, Bazzocchi G, et al. A laboratory index for predicting relapse in asymptomatic patients with Crohn’s disease. Gastroenterology. 1986;91:1490–1494. doi: 10.1016/0016-5085(86)90206-4. [DOI] [PubMed] [Google Scholar]
- 30.Louis E, Mary J-Y, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebocontrolled trial of certolizumabpegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumabpegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. PRECISE 2 Study Investigators Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 35.Romano C, Famiani A, Comito D, Rossi P, Raffa V, Fries W. Oral beclomethasone dipropionate in pediatric active ulcerative colitis: a comparison trial with mesalazine. J Pediatr Gastroenterol Nutr. 2010;50:385–389. doi: 10.1097/MPG.0b013e3181bb3457. [DOI] [PubMed] [Google Scholar]
- 36.Jensen KB, Jarnum S, Koudahl G, Kristensen M. Serum orosomucoid in ulcerative colitis: its relation to clinical activity, protein loss, and turnover of albumin and IgG. Scand J Gastroenterol. 1976;11:177–183. [PubMed] [Google Scholar]
- 37.Andre C, Descos L, Landais P, et al. Assessment of appropriate laboratory measurements to supplement the Crohn’s disease acitivity index. Gut. 1981;22:571–574. doi: 10.1136/gut.22.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Descos L, Andre C, Beorghia S, et al. Serum levels of beta-2-microglobulin—new marker of activity in Crohn’s disease. N Engl J Med. 1979;301:440–441. doi: 10.1056/NEJM197908233010826. [DOI] [PubMed] [Google Scholar]
- 39.Zissis M, Afroudakis A, Galanopoulos G, et al. B2 microglobulin: is it a reliable marker of activity in inflammatory bowel disease? Am J Gastroenterol. 2001;96:2177–2183. doi: 10.1111/j.1572-0241.2001.03881.x. [DOI] [PubMed] [Google Scholar]
- 40.Ricci G, D’Ambrosi A, Resca D, et al. Comparison of serum total sialic acid, C-reactive protein, alpha 1-acid glycoprotein and beta 2-microglobulin in patients with non-malignant bowel diseases. Biomed Pharmacother. 1995;49:259–262. doi: 10.1016/0753-3322(96)82632-1. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(2):196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 42.Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41(1):56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Vestergaard TA, Nielsen SL, Dahlerup JF, Hornung N. Fecal calprotectin: assessment of a rapid test. Scand J Clin Lab Invest. 2008;68:343. doi: 10.1080/00365510701576198. [DOI] [PubMed] [Google Scholar]
- 44.Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 45.Tibble JA, Sigthorsson G, Foster R, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362–366. doi: 10.1136/gut.45.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecalcal protectin, a novel granulocyte marker protein. Digestion. 1997;58:176–180. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 47.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 48.Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung-Faye G, Sandhu K, Logan RP, et al. Fecal calproctectin is strongly predictive of clinical disease activity and histological severity in inflammatory bowel disease. Gastroenterology. 2011;1:S421. [Google Scholar]
- 50.Chamouard P, Richert Z, Meyer N, et al. Diagnostic value of c-reactive protein for predicting activity level of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:882–887. doi: 10.1016/j.cgh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Halme L, von Smitten K, Stenman S, Turpeinen U, Stenman UH. Concentrations of pancreatic secretory trypsin inhibitor (PSTI), acute phase proteins, and neopterin in Crohn’s disease. Comparison with clinical disease activity and endoscopical findings. Scand J Clin Lab Invest. 1993;53:359–366. doi: 10.3109/00365519309086628. [DOI] [PubMed] [Google Scholar]
- 52.D’Inca R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse in inflammatory bowel disease? Gastroenterology. 2005;128(suppl):A307. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 53.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(5):637–645. doi: 10.1038/ajg.2013.131. [DOI] [PubMed] [Google Scholar]
- 54.Silberer H, Kuppers B, Mickisch O, et al. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–126. [PubMed] [Google Scholar]
- 55.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 56.Schroder O, Naumann M, Shastri Y, et al. Prospective evaluation of fecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Ailment Pharmacol Ther. 2007;26:1035–1042. doi: 10.1111/j.1365-2036.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 57.Shitrit AB, Braverman D, Stankiewics H, Shitrit D, Peled N, Paz K. Fecal calprotectin as a predictor of abnormal colonic histology. Dis Colon Rectum. 2007;50:2188–2193. doi: 10.1007/s10350-007-9038-x. [DOI] [PubMed] [Google Scholar]
- 58.Licata A, Randazzo C, Cappello M, et al. Fecal calprotectin in clinical practice: a non-invasive screening tool for patients with chronic diarrhea. J Clin Gastroenterol. 2012;46:504–508. doi: 10.1097/MCG.0b013e318248f289. [DOI] [PubMed] [Google Scholar]
- 59.Von Roon A, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 60.Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091. doi: 10.3109/00365521.2011.584897. [DOI] [PubMed] [Google Scholar]
- 61.Schoepfer A, Beglinger C, Straumann A, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, CRP, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 62.Jones J, Loftus E, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNFalpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 64.Wagner M, Peterson CGB, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol. 2008;14:5584–5589. doi: 10.3748/wjg.14.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 66.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gisbert JP, Bermejo F, Perez-Calle J-L, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 68.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 69.Sandler RS, Loftus EV. Epidemiology of inflammatory bowel disease. In: Sartor RB, Sandborn WJ, Kirsner JB, editors. Kirsner’s Inflammatory Bowel Diseases. 6th ed. Edinburgh: Saunders; 2004. pp. 245–262. [Google Scholar]
- 70.Xiong Y, Wang GZ, Zhou JQ, Xia BQ, Wang XY, Jiang B. Serum antibodies to microbial antigens for Crohn’s disease progression: a meta-analysis. Eur J Gastroenterol Hepatol. 2014;26(7):733–742. doi: 10.1097/MEG.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 71.Sarikaya M, Ergül B, Doğan Z, Filik L, Can M, Arslan L. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn’s disease: a preliminary study. Clin Lab. 2015;61(1–2):87–91. doi: 10.7754/clin.lab.2014.140518. [DOI] [PubMed] [Google Scholar]
- 72.Thurner L, Stoger E, Fadle N, et al. Proinflammatory progranulin antibodies in inflammatory bowel diseases. Dig Dis Sci. 2014;59(8):1733–1742. doi: 10.1007/s10620-014-3089-3. [DOI] [PubMed] [Google Scholar]
- 73.Rahier JF, Magro F, Abreu C, et al. Second European evidence based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 74.World Gastroenterology Organisation (WGO) Inflammatory Bowel Disease: A Global Perspective. Munich, Germany: World Gastroenterology Organisation (WGO); 2009. World Gastroenterology Organisation Global Guideline. [Google Scholar]
- 75.Alter MJ. Epidemiology of hepatitis B in Europe and in worldwide. J Hepatol. 2003;39(suppl II):S64–S69. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 76.Shahidi N, Fu Y-T, Qian H, et al. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:2034–2042. doi: 10.1002/ibd.22901. [DOI] [PubMed] [Google Scholar]
- 77.Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–1772. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 78.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 79.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 80.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;16:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Reino JJ, Carmona L, Valverde VR, et al. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk—a multicentre active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 83.Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52:1986–1992. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- 84.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 85.Pal M, Zwerling A, Menzies D, et al. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion and reversion. Am J Respir Crit Care Med. 1999;159:15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- 87.Marra F, Marra CA, Sadatsafavi M, et al. Cost-effectiveness of new interferon-based bloodassay, QuantiFERON-TB Gold, in screening tuberculosis contacts. Int J Tuberc Lung Dis. 2008;1:1414–1424. [PubMed] [Google Scholar]
- 88.Helwig U, Müller M, Hedderich J, Schreiber S. Corticosteroids and immunosuppressive therapy influence the result of QuantiFERON TB Gold testing in inflammatory bowel disease patients. J Crohns Colitis. 2012;6(4):419–424. doi: 10.1016/j.crohns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 90.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 91.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128:862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 93.Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–109. doi: 10.2147/ceg.s4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebocontrolled trials. Clin Gastroenterol Hepatol. 2008;6(644):653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 95.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63(6):919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 96.Feagan BG, Singh S, Lockton S, et al. Novel infliximab (IFX) and antibody-to-infliximab (ATI) assays are predictive of disease activity in patients with Crohn’s disease (CD) Gastroenterology. 2012;142:S114. [Google Scholar]
- 97.VandeCasteele N, Compernolle G, Ballet V, et al. Individualised infliximab treatment using therapeutic drug monitoring: a prospective controlled Trough level Adapted InfliXImabTreatment (TAXIT) trial. J Crohns Colitis. 2012;6:S6. [Google Scholar]
- 98.Vaughn BP, Martinez-Vasquez M, Pattwardhan V, et al. Prospective therapeutic drug monitoring to optimizing infliximab (IFX) maintenance therapy in patients with inflammatory bowel disease. Gastroenterology. 2014;146:5, S-54. [Google Scholar]
- 99.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein levelare associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721–1727. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paul S, Moreau AC, Del Tedesco E, et al. Pharmacokinetics of adalimumab in inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20(7):1288–1295. doi: 10.1097/MIB.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 101.Mazor Y, Kopylov U, Ben Hur D, et al. Evaluating adalimumab drug and antibody levels as predictors of clinical and laboratory response in Crohn’s disease patients. Gastroenterology. 2013;144:S778. doi: 10.1111/apt.12869. [DOI] [PubMed] [Google Scholar]
- 102.Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal. 2013;7(8–79):39–44. doi: 10.1016/j.jpba.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 103.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–188. doi: 10.1016/j.jim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18(9):1628–1633. doi: 10.1002/ibd.21919. [DOI] [PubMed] [Google Scholar]
- 105.Steenholdt C, Ainsworth MA, Tovey M, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit. 2013;35:530–538. doi: 10.1097/FTD.0b013e31828d23c3. [DOI] [PubMed] [Google Scholar]
- 106.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47. doi: 10.1038/ajg.2012.363. quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamada A, Sono K, Hosoe N, et al. Monitoring functional serum antitumor necrosis factor antibody level in Crohn’s disease patients who maintained and those who lost response to anti-TNF. Inflamm Bowel Dis. 2010;16:1898–1904. doi: 10.1002/ibd.21259. [DOI] [PubMed] [Google Scholar]
- 108.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–666. doi: 10.1016/j.cgh.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 109.Krieckaert CL, Nair SC, Nurmohamed MT, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis. 2015;74:361–368. doi: 10.1136/annrheumdis-2013-204101. [DOI] [PubMed] [Google Scholar]
- 110.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–1640. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 111.Vande Casteele N, Compernolle G, Ballet V, et al. OP11 individualised infliximab treatment using therapeutic drug monitoring: a prospective controlled Trough level Adapted infliXImab Treatment (TAXIT) trial. J Crohns Colitis. 2012;6:S6. [Google Scholar]
- 112.Jürgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:421–427. doi: 10.1016/j.cgh.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 113.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 114.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 115.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 116.Ben-Horin S. Loss of response to anti-tumor necrosis factors: what is the next step? Dig Dis. 2014;32(4):384–388. doi: 10.1159/000358142. [DOI] [PubMed] [Google Scholar]
- 117.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 118.Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63:1258–1264. doi: 10.1136/gutjnl-2013-305259. [DOI] [PubMed] [Google Scholar]
- 119.VandeCasteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 120.Baert F, De Vos M, Louis E, Vermeire S, Belgian IBD Research Group Immunogenicity of infliximab: how to handle the problem? Acta Gastroenterol Belg. 2007;70(2):163–170. [PubMed] [Google Scholar]
- 121.Afif W, Loftus EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105(5):1133–1139. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 123.Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology. 2003;124:917–924. doi: 10.1053/gast.2003.50145. [DOI] [PubMed] [Google Scholar]
- 124.Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–226. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 125.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 126.Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X. Review article: the benefits of pharmacogenetics for improving thiopurinetherapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:15–36. doi: 10.1111/j.1365-2036.2011.04905.x. [DOI] [PubMed] [Google Scholar]
- 127.Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047–1053. doi: 10.1053/j.gastro.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 128.Stocco G, Londero M, Campanozzi A, et al. Usefulness of the measurement of azathioprine metabolites in the assessment of non-adherence. J Crohns Colitis. 2010;4(5):599–602. doi: 10.1016/j.crohns.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 129.Lowry PW, Franklin CL, Weaver AL, et al. Measurement of thiopurinemethyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut. 2001;49:665–670. doi: 10.1136/gut.49.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dubinsky MC, Yang H, Hassard PV, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904–915. doi: 10.1053/gast.2002.32420. [DOI] [PubMed] [Google Scholar]
- 131.Shaye OA, Yadegari M, Abreu MT, et al. Hepatotoxicity of 6-mercaptopurine (6-MP) and azathioprine (AZA) in adult IBD patients. Am J Gastroenterol. 2007;102:2488–2494. doi: 10.1111/j.1572-0241.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 132.Roblin X, Peyrin-Biroulet L, Phelip JM, Nancey S, Flourie B. A 6-thioguanine nucleotide threshold level of 400 pmol/8 × 10(8) erythrocytes predicts azathioprine refractoriness in patients with inflammatory bowel disease and normal TPMT activity. Am J Gastroenterol. 2008;103:3115–3122. doi: 10.1111/j.1572-0241.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 133.Seidman EG. Clinical use and practical application of TPMT enzyme and 6-mercaptopurine metabolite monitoring in IBD. Rev Gastroenterol Dis. 2003;3(suppl 1):S30–S38. [PubMed] [Google Scholar]
- 134.Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms andthiopurine toxicity in treatment of inflammatory boweldisease. World J Gastroenterol. 2010;16(25):3187–3195. doi: 10.3748/wjg.v16.i25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Playford RJ, Belo A, Poulsom R, et al. Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology. 2006;131:809–817. doi: 10.1053/j.gastro.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 136.Nielsen OH, Gionchetti P, Ainsworth M, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999;94:2923–2928. doi: 10.1111/j.1572-0241.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 137.Bonneau J, Dumestre-Perard C, Rinaudo-Gaujous M, et al. Systematic review: new serological markers (anti-glycan, anti-GP2, anti-GM-CSF Ab) in the prediction of IBD patient outcomes. Autoimmun Rev. 2015;14:231–245. doi: 10.1016/j.autrev.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 138.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 139.van Roon JAG, Tesselaar K, Radstake TR. Proteome-wide analysis and CXCL4 in systemic sclerosis. N Engl J Med. 2014;370:1563–1564. doi: 10.1056/NEJMc1402401. [DOI] [PubMed] [Google Scholar]
- 140.Lee JC, Espéli M, Anderson CA, et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 2013;155:57–69. doi: 10.1016/j.cell.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu F, Zhang S, Dassopoulos T, et al. Identification of microRNAs associated with ileal andcolonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:187–193. doi: 10.1002/ibd.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Iudicibus S, Lucafò M, Martelossi S, Pierobon C, Ventura A, Decorti G. MicroRNAs as tools to predict glucocorticoid response in inflammatory bowel diseases. World J Gastroenterol. 2013;19:7947–7954. doi: 10.3748/wjg.v19.i44.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lewis A, Mehta S, Hanna LN, et al. Low serum levels of MicroRNA-19 are associated with a Stricturing Crohn’s disease phenotype. Inflamm Bowel Dis. 2015;21(8):1926–1934. doi: 10.1097/MIB.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 145.Wu F, Guo NJ, Tian H, et al. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peck BCE, Weiser M, Lee SE, et al. MicroRNAs classify different disease behavior phenotypes of Crohn’s disease and may have prognostic utility. Inflamm Bowel Dis. 2015;21:2178–2187. doi: 10.1097/MIB.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ben-Horin S, Chowers Y. Tailoring anti-TNF therapy in IBD: drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 2014;11:243–255. doi: 10.1038/nrgastro.2013.253. [DOI] [PubMed] [Google Scholar]