Abstract
Friedreich's ataxia (FRDA) represents a rare neurodegenerative disease caused by expansion of GAA trinucleotide repeats in the first intron of the FXN gene. The number of GAA repeats in FRDA patients varies from approximately 60 to <1000 and is tightly correlated with age of onset and severity of the disease symptoms. The heterogeneity of Friedreich's ataxia stresses the need for a large cohort of patient samples to conduct studies addressing the mechanism of disease pathogenesis or evaluate novel therapeutic candidates. Herein, we report the establishment and characterization of an FRDA fibroblast repository, which currently includes 50 primary cell lines derived from FRDA patients and seven lines from mutation carriers. These cells are also a source for generating induced pluripotent stem cell (iPSC) lines by reprogramming, as well as disease-relevant neuronal, cardiac, and pancreatic cells that can then be differentiated from the iPSCs. All FRDA and carrier lines are derived using a standard operating procedure and characterized to confirm mutation status, as well as expression of FXN mRNA and protein. Consideration and significance of creating disease-focused cell line and tissue repositories, especially in the context of rare and heterogeneous disorders, are presented. Although the economic aspect of creating and maintaining such repositories is important, the benefits of easy access to a collection of well-characterized cell lines for the purpose of drug discovery or disease mechanism studies overshadow the associated costs. Importantly, all FRDA fibroblast cell lines collected in our repository are available to the scientific community.
Introduction
According to the National Institutes of Health, a rare disease is defined as affecting less than 200,000 people in the United States. Currently, more than 7,000 rare diseases have been identified, totaling approximately 30 million patients.1 Studies on rare disorders are frequently hampered by a lack of appropriate models to test the effects of potential treatments or decipher the mechanism of pathology. Moreover, diverse phenotypes observed with these diseases require a larger number of samples to be analyzed to obtain statistically informative results.
Friedreich's ataxia (FRDA, FA, OMIM 229300) belongs to a group of more than 30 diseases caused by expansion of short tandem repeats.2,3 It is a rare neurodegenerative ataxia inherited in an autosomal recessive manner. Expansion of trinucleotide GAA repeats located in intron 1 of the FXN gene above the threshold of approximately 65 repeats leads to transcriptional silencing of FXN expression.4,5 Frataxin is a ubiquitously expressed mitochondrial protein functioning predominantly as a chaperone in the synthesis of iron–sulfur clusters.6,7 FRDA is observed with a prevalence of approximately 1 in 30,000–50,000 individuals; however, due to its recessive inheritance, the prevalence of asymptomatic carriers is much greater and reaches approximately 1 in 100 individuals.3,5
Although classified as a neurodegenerative disease, Friedreich's ataxia presents with a plethora of clinical symptoms, including ataxia, sensory abnormalities, heart dysfunction, diabetes, and auditory and visual loss.8–10 These clearly indicate the multisystem (multiorgan) character of this disorder. FRDA typically manifests in preadolescents and adolescents; however, the size of GAA expansion, particularly of the shorter expanded allele, correlates tightly with the age of onset.11 The variable number of GAA repeats among FRDA patients contributes to the clinical variability of the disease.12,13
Because the FRDA phenotype varies significantly among patients, appropriate selection of patient subcohorts and access to samples representing the entire phenotypic spectrum of FRDA patients are essential for elucidation of molecular mechanisms of the disease. Moreover, studies conducted using a limited number of samples can lead to confounding and sometimes contradictory results. Instead of advancing the field, such studies can lead to a loss of resources.14 Thus, development of comprehensive disease-oriented cell line and tissue repositories is critical.
In the case of Friedreich's ataxia and other repeat expansion diseases, especially those caused by a massive increase in the number of tandem repeats (>400 repeats), genetic instability of the repeating sequences renders generating animal models a difficult task. Additionally, the presence of human-specific genomic regulatory elements underlines the necessity for conducting experiments in human-derived cell lines. Recent advances in reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) allow us to create cell lineages directly relevant to the pathogenesis of FRDA, such as neurons, cardiomyocytes, and pancreatic cells. Establishing repositories of fibroblasts and other somatic cells that can be efficiently reprogrammed to iPSCs opens the possibility of maintaining a renewable resource of pluripotent lines that can be used as a source of terminally differentiated, disease-relevant cells.
Herein, we present data regarding a human Friedreich's ataxia primary fibroblast repository we have been developing over the past 4 years. In addition to FRDA-specific genetic and functional characterization, we describe the initial fibroblast derivation process and general characterization steps that can be applied to other disease-oriented cell line repositories. Finally, our experience regarding the costs of establishment, maintenance, characterization, and dissemination of lines from the FRDA fibroblast repository is presented.
Results
Establishing primary fibroblast lines from human biopsies
In all cases, appropriate institutional requirements were fulfilled before collection of patient samples. FRDA patients signed adequate consent forms allowing for derivation of cell lines, including immortalized and self-renewing lines, as well as distribution of lines for research purposes. All patients signed informed consent forms, and the study was approved by the Committees for the Protection of Human Subjects of The Children's Hospital of Philadelphia (IRB 10-007864) and the Institutional Review Board (IRB) for Human Use at the University of Alabama at Birmingham (IRB N131204003).
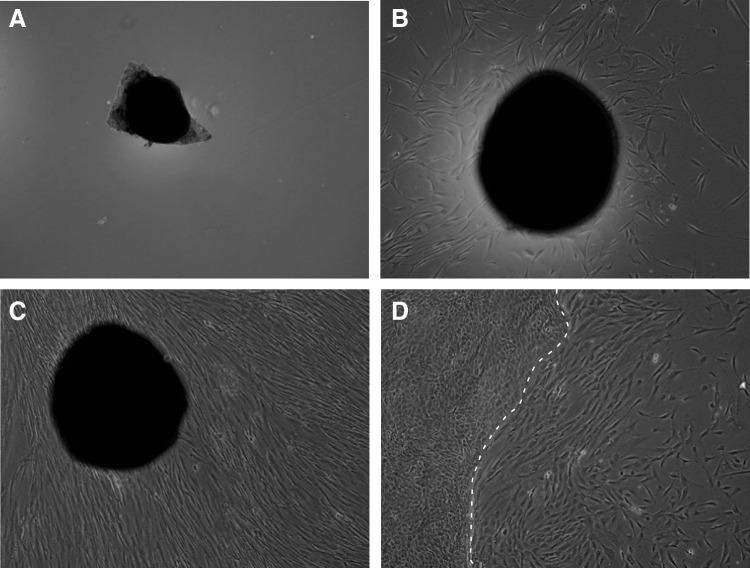
A 3-mm skin biopsy was collected from the forearm of an FRDA patient, transferred to media lacking fetal bovine serum (FBS), and shipped overnight in a cooled, biological material-approved container. Biopsy material is regarded as a biohazard and handled using aseptic technique in a laminar hood using personal protective equipment. First, biopsy material is washed with Hank's Balanced Salt Solution containing penicillin and streptomycin and subsequently suspended in an enzyme mixture containing collagenase IV, dispase, and trypsin. The material is then cut into small pieces and incubated at 37°C with gentle shaking. Digested tissue fragments are transferred to conical tubes with Dulbecco's Modified Eagle's medium (DMEM)/F12 media containing 20% FBS and antibiotics. Following centrifugation, the skin pieces are plated onto gelatin-treated plates. Typically, outgrowths of fibroblasts are observed 7–14 days after the initial plating, and media are added as necessary during this incubation period (Fig. 1A–D). Details of this procedure can be found in the studies by Li et al.11,15
FIG. 1.
Derivation of human fibroblasts from a skin biopsy. Small pieces of a 3-mm forearm skin biopsy were plated on gelatin-coated tissue culture dishes (A). Typically, initial fibroblast outgrowths are visible after 7–14 days (B). Fourteen to 28 days after initial plating, intense outgrowth of fibroblasts from the skin fragments can be observed (C). Frequently, keratinocytes are also observed during derivation of fibroblast lines. The white line indicates the border between keratinocytes (shown on the left side of the image) and fibroblasts (visible on the right side) (D). Fibroblast culturing conditions favor proliferation of fibroblast cells, resulting in loss of keratinocytes after the first passage of cells.
Characterization of fibroblast lines
Currently, the repository holds 50 FRDA patient fibroblast lines. Forty-seven of these represent the most common homozygous GAA expansion mutation. The remaining three lines are from patients carrying the compound heterozygous mutation of an expanded GAA allele along with the G130V point mutation in the second copy of the FXN gene.16,17 The repository also includes six FRDA carrier lines with one expanded GAA allele and the second GAA tract within the unaffected range. The size of the repeats varies from 150 to 1500 GAAs, with the average length for allele 1 (shorter GAA tract) being 600 GAAs and for allele 2 being 980 GAAs. The repository currently contains 25 samples each from male and female FRDA patients. The average age of onset of the entire cohort is 25 years, with a range from 6 to 47 years.
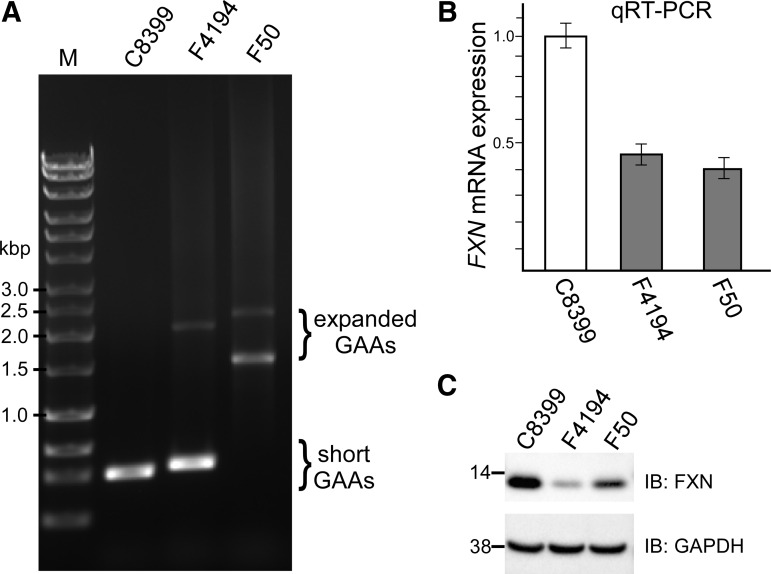
Basic molecular characterization of the established fibroblast lines includes (i) determination of GAA repeat length, (ii) analysis of frataxin mRNA and protein expression, and (iii) testing for Mycoplasma. As a standard operating procedure (SOP), we use two sets of FXN primers to determine the number of GAA repeats in the expanded alleles. The short set amplifies the GAA tract plus 498 bp of flanking sequence, and the long set amplifies 1370 bp of DNA flanking the repeats (Fig. 2A). The detailed procedure was recently described by Li et al.15 For FXN mRNA quantitation, we rely on real-time quantitative RT-PCR using SYBR Green reagents and primers spanning exons 3 and 4 (Fig. 2B). To quantitate frataxin by western blot, we use an anti-frataxin H-155 polyclonal antibody and normalize to GAPDH expression levels11,15 (Fig. 2C). The cell lines are also tested for Mycoplasma contamination using the MycoAlert Detection Kit (Lonza).
FIG. 2.
Characterization of FRDA fibroblast lines. Initial characterization of the FRDA fibroblasts is conducted using approximately1.5 × 106 cells cultured on three wells of a standard six-well plate. Determination of the size of GAA repeats using PCR (A). Short GAA alleles are amplified in unaffected controls (C8399, Coriell Cell Repositories), heterozygous mutation carriers, or compound heterozygotes carrying a point mutation on one FXN allele and a GAA expansion on the second allele (F4194). The two expanded alleles can be distinguished in an FRDA cell line homozygous for GAA expansions (F50). Determination of frataxin mRNA and protein expression using qRT-PCR (B) and western blot (C). FRDA, Friedreich's ataxia; qRT-PCR, real-time quantitative reverse transcription polymerase chain reaction.
Cryopreservation, recovery, and banking of lines
Our SOP is to culture eight to twelve 10-cm dishes of confluent FRDA fibroblasts for cryopreservation. Lines are frozen in complete DMEM containing 20% FBS and 10% dimethyl sulfoxide at a density corresponding approximately 1–2 × 106 cells/mL in 1 mL aliquots. Initial freezing is conducted in freezing containers at a rate of approximately 1°C/min in a −80°C freezer. After 24 hours, vials are transferred to the vapor phase of a liquid nitrogen dewar. Spare liquid nitrogen storage systems are in place in case of equipment failure. An electronic record of all cell lines that includes the number of vials, passage number, and freezing date is kept in a password-protected database with an appropriate backup system along with printed inventory records (Table 1). Recovery of lines is conducted using a standard quick-thaw procedure, and typically, more than 90% of cells attach and begin proliferating within 24 hours.
Table 1.
Friedreich's Ataxia Fibroblast Cell Line Repository
| FXN level vs. average controls | Phenotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | Gender | Age of sampling | Age of onset | No. GAA repeats allele 1, allele 2 | SYBR | Frataxin by western blot | C | D | H | Date of freezing | No. of vials | Passage |
| 68 | F | 21 | 7 | 570, 1200 | 0.13 | 0.13 | + | − | + | 05/21/2012 | 17 | P2, 3, 7, 12 |
| 4230 | F | 28 | 6 | 870, 1470 | 0.15 | 0.17 | + | + | + | 08/05/2014 | 20 | P7, 9, 12 |
| 4497 | F | 44 | 30 | 526, 826 | 0.22 | 0.20 | + | − | + | 11/29/2012 | 14 | P2, 3, 7 |
| 4259 | M | 37 | 15 | 404, 920 | 0.24 | 0.16 | + | − | + | 12/06/2012 | 4 | P2, 7 |
| 4194 | F | 23 | 13 | 540, G130V | 0.51 | 0.16 | − | − | − | 07/26/2014 | 8 | P3, 5 |
| 50 | M | 33 | 13 | 353, 616 | 0.40 | 0.48 | + | − | − | 05/18/2015 | 5 | P2 |
Example of information for six FRDA lines included in the electronic database of the FRDA fibroblast repository.
C, clinically diagnosed cardiomyopathy; D, diabetes; FRDA, Friedreich's ataxia; H, hearing loss/deficit.
Costs of establishment and maintenance of repository
Establishing cell line repositories representative of rare diseases is extremely important from the perspective of discovering mechanisms of pathogenesis, identification of new therapeutic targets, and preclinical testing of new drug candidates. The major obstacle in creating such repositories is access to patient material; hence, collaboration between physicians and research scientists is necessary. In addition, completion of all regulatory requirements (patient consent, IRB protocols, and proper material transfer agreements) is necessary before initiating sample collection. Cell line repositories are typically limited to renewable and easily accessible cell types, such as laboratory immortalized lymphocytes, primary fibroblasts, or keratinocytes. More recently, owing to the development of somatic cell reprogramming approaches,18,19 such repositories can be supplemented with iPSC lines as well as those cells differentiated from the iPSCs (e.g., cardiomyocytes, neurons, and pancreatic cells). Moreover, multiple specimens collected from a single patient over time are an important addition to a repository for the prospect of longitudinal studies. It is important to note that fibroblasts are not directly involved in the pathogenesis of neurological or neuromuscular diseases, such as Friedreich's ataxia, and therefore might not robustly represent all the underlying molecular features of the disease. Additionally, the metabolism of primary fibroblasts depends somewhat on passage number and age of the culture. These points should be weighed when considering fibroblasts as a model for drug screening and/or mechanistic studies. However, the accessibility and reprogramming capacity of fibroblasts make them a valuable primary resource.
The number of samples accommodated in a rare disease cell line repository is a function of the patient population size and typically will not exceed a few hundred lines. Thus, costs associated with establishment and maintenance of fibroblast lines are relatively low. Preparation of initial biopsy material is less than $100 per sample, including disposable materials and time for qualified personnel. An additional $100–200 per sample is necessary to propagate and cryopreserve the fibroblast lines. Liquid nitrogen systems, including backup dewars, that can accommodate up to 7,000 vials are an initial investment of approximately $20,000, with yearly costs of liquid nitrogen supplies not exceeding $2,500. Periodic defrosting and expansion of individual fibroblast lines are necessary to replenish stocks that are disseminated to requesting laboratories, thus increasing maintenance costs.
It is important to note that although primary human fibroblasts are especially valuable cell types due to their competence for somatic cell reprogramming, their replicative capacity is limited. Hence, for projects requiring greater cell numbers, we generate immortalized lines by overexpression of hTERT.20,21 Obtaining these lines by retroviral transduction of the hTERT gene bears a cost of $500.
A limited number of our fibroblast cell lines have been reprogrammed from fibroblasts to iPSCs (Table 2) using retroviral transduction of Oct4, Sox2, Klf4, and c-Myc (OSKM) transcription factors as described by Ku et al.22 and Polak et al.23 or by Sendai virus delivery of these factors (CytoTune-iPS 2.0 Sendai Reprogramming Kit; Life Technologies). These lines are also available upon request. The cost of obtaining and maintenance of these lines is much higher (approximately $5,000 per line) than the parental fibroblast cells due to initial expenses associated with reprogramming (viral vectors), significant costs of media, small molecule supplements, feeder cells, or growth matrix, as well as personnel time necessary to establish an iPSC line (approximately 3 months). However, these pluripotent stem cells represent an unlimited supply of FRDA patient material that can be further differentiated into disease-relevant cell types for downstream applications.
Table 2.
FRDA iPSC Lines Reprogrammed from Patient Fibroblasts
| Cell line | Mutation GAA1/GAA2 | Reprogramming | No. of clones |
|---|---|---|---|
| 68 | 570/1200 | OSKM Retro | 5 |
| 4491 | 559/G130V | OSKM Retro | 3 |
| GM03665 | 816/1400 | OSKM Retro | 3 |
| GM04078 | 341/480 | OSKM Retro | 4 |
| 68_E | 570/– and 1200/– | OSKM Retro | 2 |
| 68 | 570/1200 | OSKM Sendai | 3 |
| 4259 | 404/920 | OSKM Sendai | 2 |
| 281 | 630/806 | OSKM Sendai | 3 |
Lines designated GM were derived using fibroblasts obtained from Coriell Cell Repositories. Line 68_E is a zinc finger-edited line with one GAA allele corrected by excision of the expanded sequence.15 Two different methods, retroviral and Sendai virus transduction, were used to establish these lines. All lines underwent rigorous testing for pluripotency, lineage differentiation, and karyotype analyses. As GAA repeats continuously expand in FRDA iPSCs, the repeat number is based on determination in fibroblasts prior to reprogramming.
Importantly, the data regarding the costs of generation and maintenance of the FRDA patient cell line repository are for informational purposes only. The fibroblast and iPSC lines are available on a collaborative basis at no cost to the requesting laboratory.
Conclusions
Clinicians and scientists studying orphan diseases face the specific challenge of limited access to biospecimens due to the small number of patients suffering from the particular condition. Coriell Cell Repositories and other large multidisease biorepositories that bank and distribute cell lines and specimens obtained from patients suffering from different rare diseases are extremely valuable resources to the scientific community. However, by serving a broad spectrum of diseases, they are limited in their capacity to accumulate significant numbers of cell lines from a given rare condition. For comparison, currently only two primary fibroblast lines from FRDA patients are available from Coriell Cell Repositories. In addition, derivation of more challenging cell lines, such as primary fibroblasts or iPS cells, in large quantities from a broad spectrum of disease samples would require substantial personnel commitment. Importantly, specific characterization of each derived line involves an intimate knowledge of the respective disease. Thus, individual laboratories and collaborative consortia of a few research groups dedicated to a single disease or a group of similar conditions are well positioned to establish such disease-focused, well-characterized repositories.
One of the most important aspects in creating research resources is the support of patient advocacy groups, such as the Friedreich's Ataxia Research Alliance (FARA), in spreading awareness regarding the critical role of cell repositories for development of potential therapies. The initial setup of a relatively small repository containing less than 100 cell lines, as described above, requires a substantial time and financial investment. However, the long-term maintenance, derivation, and characterization of new lines can be conducted with a modest budget. Moreover, the financial cost is certainly outweighed by the benefits of conducting rare disease research using multiple cell lines or samples that more accurately represent the diversity of the patient population.
Sustainability of biorepositories can be achieved through several means. First, the role of foundations and advocacy groups, such as the FARA or National Ataxia Foundation, is essential for long-term support of cell line derivation and biobanking. Federal grant funding for creating new cell line resources and small, disease-focused repositories is dedicated to the current needs of the principal research project or may come from special supplements that can be linked with such an award. Finally, partnerships between academic laboratories and biotechnology and pharmaceutical companies create avenues for offsetting the costs of cell line derivation and repository maintenance. Creating biorepositories of well-characterized patient samples and cell lines available for academic as well as commercial institutions is critical for developing successful therapeutic strategies to combat rare diseases.
A list of available fibroblast cell lines in the Friedreich's ataxia repository described herein can be found at www.uab.edu/medicine/biochem/91-napeierala-lab-focus.
Requests should be submitted via the “Request Form” link found on the Web site or e-mailed directly to Drs. Lynch or Napierala (lynchd@mail.med.upenn.edu or mnapiera@uab.edu).
Acknowledgments
The authors would like to thank all FRDA patients for skin biopsy samples. The authors do not declare any conflicts of interest. All authors read the article, contributed comments and suggestions, and approved the final version of the article.
These studies were supported by NIH 7R01NS081366 from NINDS to M.N. and grants from the Muscular Dystrophy Association (MDA0789 to M.N.), Friedreich's Ataxia Research Alliance (to D.L.), and Friedreich's Ataxia Research Alliance and FARA Ireland (to M.N. and J.S.B.).
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Thoene J. Why should we care about Rare Disease Day? Neurol Rev 2015;23:S4 [Google Scholar]
- 2.Polak U, McIvor E, Dent SY, et al. . Expanded complexity of unstable repeat diseases. BioFactors 2013;39:164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marmolino D. Friedreich's ataxia: Past, present and future. Brain Res Rev 2011;67:311–330 [DOI] [PubMed] [Google Scholar]
- 4.Campuzano V, Montermini L, Molto MD, et al. . Friedreich's ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996;271:1423–1427 [DOI] [PubMed] [Google Scholar]
- 5.Pandolfo M. The molecular basis of Friedreich ataxia. Adv Exp Med Biol 2002;516:99–118 [DOI] [PubMed] [Google Scholar]
- 6.Pastore A, Puccio H. Frataxin. A protein in search for a function. J Neurochem 2013;126 Suppl 1:43–52 [DOI] [PubMed] [Google Scholar]
- 7.Martelli A, Wattenhofer-Donze M, Schmucker S, et al. . Frataxin is essential for extramitochondrial Fe-S cluster proteins in mammalian tissues. Hum Mol Genet 2007;16:2651–2658 [DOI] [PubMed] [Google Scholar]
- 8.Pandolfo M. Friedreich's ataxia. In: Wells RD, and Ashizawa T. (ed) Genetic Instabilities and Neurological Diseases. 2nd edn. San Diego, CA: Elsevier-Academic Press; 2006:277–296 [Google Scholar]
- 9.Lynch DR, Regner SR, Schadt KA, et al. . Management and therapy for cardiomyopathy in Friedreich's ataxia. Expert Rev Cardiovasc Ther 2012;10:767–777 [DOI] [PubMed] [Google Scholar]
- 10.Lynch DR, Pandolfo M, Schulz JB, et al. . Common data elements for clinical research in Friedreich's ataxia. Mov Disord 2013;28:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Lu Y, Polak U, et al. . Expanded GAA repeats impede transcription elongation through the FXN gene and induce transcriptional silencing that is restricted to the FXN locus. Hum Mol Genet 2015;24:6932–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro L, De Michele G, Perretti A, et al. . Relation between trinucleotide GAA repeat length and sensory neuropathy in Friedreich's ataxia. J Neurol Neurosurg Psychiatry 1999;66:93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Biase I, Rasmussen A, Monticelli A, et al. . Somatic instability of the expanded GAA triplet-repeat sequence in Friedreich ataxia progresses throughout life. Genomics 2007;90:1–5 [DOI] [PubMed] [Google Scholar]
- 14.Perdomini M, Hick A, Puccio H, et al. . Animal and cellular models of Friedreich ataxia. J Neurochem 2013;126 Suppl 1:65–79 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Polak U, Bhalla AD, et al. . Excision of expanded GAA repeats alleviates the molecular phenotype of Friedreich's ataxia. Mol Ther 2015;23:1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cossee M, Durr A, Schmitt M, et al. . Friedreich's ataxia: Point mutations and clinical presentation of compound heterozygotes. Ann Neurol 1999;45:200–206 [DOI] [PubMed] [Google Scholar]
- 17.Delatycki MB, Knight M, Koenig M, et al. . G130V, a common FRDA point mutation, appears to have arisen from a common founder. Hum Genet 1999;105:343–346 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M, et al. . Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 19.Inoue H, Nagata N, Kurokawa H, et al. . iPS cells: A game changer for future medicine. EMBO J 2014;33:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales CP, Holt SE, Ouellette M, et al. . Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet 1999;21:115–118 [DOI] [PubMed] [Google Scholar]
- 21.Jiang XR, Jimenez G, Chang E, et al. . Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet 1999;21:111–114 [DOI] [PubMed] [Google Scholar]
- 22.Ku S, Soragni E, Campau E, et al. . Friedreich's ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell 2010;7:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polak U, Hirsch C, Ku S, et al. . Selecting and isolating colonies of human induced pluripotent stem cells reprogrammed from adult fibroblasts. J Vis Exp 2012;60:e3416. [DOI] [PMC free article] [PubMed] [Google Scholar]