Abstract
To study mucosal immunity and conduct HIV vaccine trials, it is important to be able to cryopreserve mucosal specimens and recover them in functional viable form. Obtaining a good recovery depends, in part, on cooling the cells at the appropriate rate, which is determined by the rate of water transport across the cell membrane during the cooling process. In this study, the cell membrane permeabilities to water at subzero temperatures of human vaginal mucosal T cells and macrophages were measured using the differential scanning calorimetry method proposed by Devireddy et al. in 1998. Thermal histograms were measured before and after cell lysis using a Slow-Fast-Fast-Slow cooling program. The difference between the thermal histograms of the live intact cells and the dead lysed cells was used to calculate the temperature-dependent cell membrane permeability at subzero temperatures, which was assumed to follow the Arrhenius relationship, 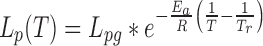 , where Lpg is the permeability to water at the reference temperature (273.15 K). The results showed that Lpg = 0.0209 ± 0.0108 μm/atm/min and Ea = 41.5 ± 11.4 kcal/mol for T cells and Lpg = 0.0198 ± 0.0102 μm/atm/min and Ea = 38.2 ± 10.4 kcal/mol for macrophages, respectively, in the range 0°C to −40°C (mean ± standard deviation). Theoretical simulations predicted that the optimal cooling rate for both T cells and macrophages was about −3°C/min, which was proven by preliminary immune cell cryopreservation experiments.
, where Lpg is the permeability to water at the reference temperature (273.15 K). The results showed that Lpg = 0.0209 ± 0.0108 μm/atm/min and Ea = 41.5 ± 11.4 kcal/mol for T cells and Lpg = 0.0198 ± 0.0102 μm/atm/min and Ea = 38.2 ± 10.4 kcal/mol for macrophages, respectively, in the range 0°C to −40°C (mean ± standard deviation). Theoretical simulations predicted that the optimal cooling rate for both T cells and macrophages was about −3°C/min, which was proven by preliminary immune cell cryopreservation experiments.
Introduction
Most HIV infections occur through the cervicovaginal mucosa, so understanding the effects of medical interventions such as vaccines and microbicides on the female genital tract is important.1,2 This has created a strong need to be able to cryopreserve, store, and ship immune cells from the female genital mucosa, in particular in the context of clinical trials conducted at many sites around the world. However, no universally accepted cryopreservation procedure exists for mucosal leukocytes.
The fate of a cell after freezing and thawing is determined by heat and mass transfer and the biophysical response of the cell to environmental changes, which depends on the fundamental cryobiological characteristics of the cell, including the osmotically inactive cell volume (Vb), cell membrane permeability to water (Lp) and to cryoprotective agent (CPA) (Ps), intracellular ice formation temperature (TIIF), osmotic tolerance limit of the cell, cytotoxicity of CPA to the cell, and others.3–7 To develop an optimal procedure, we studied the fundamental cryobiological characteristics of human vaginal T cells and macrophages. In this article, we describe a set of those studies: the measurement of membrane permeabilities to water (Lp) at subzero temperatures. The rate at which water crosses the cell membrane at subzero temperatures determines the rate at which cells can be cooled with the greatest recovery. Based on Mazur's two-factor hypothesis, if cells are cooled too slowly, cellular injury can occur due to severe cell dehydration and the solution effect; by contrast, if they are cooled too quickly, a large amount of water will crystallize inside the cell, which can disrupt the membrane and be lethal to the cell (ice injury)5,7 The optimal cooling rate will fall between those two extremes and depend on the membrane permeability to water.
There have been many methods proposed and applied by researchers to measure the cell membrane permeability to water, primarily at temperatures above the freezing point.8–18 However, the membrane permeability at subzero temperatures, for example, in the range of 0°C to −35°C, plays an important role because cell death by solution injury or ice injury occurs in this range. One method to determine the permeability in this range is to measure the Lp values at a few suprazero temperatures (e.g., 22°C, 10°C, and 4°C), and then predict the Lp at subzero temperatures with the Arrhenius relationship. However, this may lead to inaccuracy in Lp prediction possibly due to the liquid–gel (solid) phase change of lipid and membrane protein conformation.17,19,20 Therefore, direct measurement of the Lp values at subzero temperatures is vital for the optimization of the cooling process.
A method of direct measurement of Lp at subzero temperatures using differential scanning calorimetry (DSC) was proposed by Devireddy et al.21 Later, this method was applied for the measurements of cryobiological properties of both cells and tissues.22–28 In this study, we used this method to investigate the temperature-dependent cell membrane permeability to water for human vaginal mucosal T cells and macrophages. Based on the results, we predicted the theoretically optimal cooling rates for the immune cells and tested those rates in preliminary cryopreservation experiments.
Materials and Methods
Theory of the DSC measurements
The theory of measuring cell membrane properties at subzero temperatures with DSC was first developed by Devireddy et al.21 In our study, a similar Slow-Fast-Fast-Slow (SFFS) cooling protocol was applied to cell suspensions of pure, sorted vaginal T cells or macrophages. Details of the method and theory derivation are as presented in Devireddy et al.21 In brief, heat transfer is measured for a suspension of live cells during the first slow freezing (4°C/min), and then measured again for the same cell suspension during the second slow freezing after lysis by repeated fast freezing (200°C/min). Based on the difference between the two thermograms, the volume response of cells during freezing can be calculated as follows:
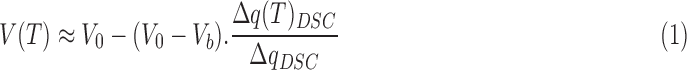 |
where V(T) is the cell volume at temperature T, V0 is the initial volume in isotonic environment, Vb is the osmotically inactive volume, ΔqDSC is the total difference of heat release between the first and the last slow cooling steps, and ΔqDSC(T) is the partial difference of heat release between the two slow cooling steps down to temperature T. The osmotically inactive volume, Vb, of human vaginal T cells and macrophages was determined to be 51.6% V0 and 45.7% V0, respectively, by the Boyle–Van't Hoff relationship (Shu et al., submitted).29
Water transport across cell membranes during cooling
After V(T) of cells is calculated based on the DSC measurements, the cell membrane permeability to water at any temperature below 0°C, Lp(T), can be simulated according to Mazur and Toner's model of water transport across cell membranes and the following Arrhenius equation6,30,31:
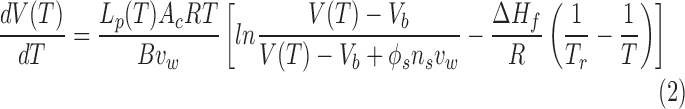 |
 |
where Ac = 4πr2 is the cell surface area and assumed constant during cooling, R is the universal gas constant, B is the cooling rate, T0 is the initial temperature, Vb is the osmotically inactive cell volume, ns is the number of moles of salts, vw is the specific molar volume of water, øs is the dissociation constant ( = 2 for NaCl), Lpg is the Lp at the reference temperature Tr (generally 273.15 K), and Ea is the activation energy of the dependence of Lp on temperature.
Equations (2) and (3) are applied to calculate the cell membrane permeability to water at subzero temperatures based on the DSC results. They are also used to predict the optimal cooling rate for a cell type based on the Lp(T) results.
DSC calibration and experiments
Before each set of experiments, the DSC 8500 (PerkinElmer, Waltham, MA) was carefully calibrated following the user manual, including the calibrations of temperature, heat flow, and furnace control. Besides indium as the standard reference material for the calibration, two more reference materials, n-octane and n-dodecane, were also used to calibrate the temperature and heat flow because their subzero freezing/melting temperatures are close to or within the DSC scanning temperature range in the experiment (0°C to −40°C). After calibration, the phase transition temperature error was <0.2°C and latent heat error <1%. The DSC furnace was calibrated in the temperature range of −80°C to 200°C. After calibration, the differences between the real sample temperature and programmed temperature were <0.05°C in the isothermal equilibration step and <0.5°C in the scanning step with cooling rate lower than 10°C/min.
In the DSC experiments, 5–10 μL of each cell suspension (weighed with microbalance) was added to the aluminum pan provided by the PerkinElmer Company; 0.1–0.2 mg freeze-dried Pseudomonas syringae (ATCC, Manassas, VA) was added to each sample as an ice nucleator to reduce supercooling. Then, the pan was sealed with the crimper. The mass of each sample was precisely measured. The Slow-Fast-Fast-Slow DSC scanning protocol in our experiments was very similar to that used in Devireddy et al.,21 except the modification that repeated fast cooling steps were applied to lyse the cells.
Human vaginal mucosal specimens
Human vaginal tissue specimens were obtained from vaginal repair surgeries at the University of Washington Medical Center under a waiver of consent approved by the Institutional Review Board of the University of Washington.
Sorting of vaginal T cells and macrophages
T cells and macrophages were purified from vaginal tissues by dissection, enzymatic digestion, and flow cytometric sorting. The vaginal epithelium was trimmed to small pieces, about 1 × 1 × 2 mm, and rested overnight in cell culture medium at 4°C. Cells were subsequently isolated from the epithelium by digestion with collagenase type II (Sigma-Aldrich, St. Louis, MO), as described previously.32 The pieces of tissue were digested in a solution of collagenase type II and DNase (both at 700 units/mL) at 37°C with shaking for a maximum of four rounds of 30 minutes each. In between digestions, tissues were passed through a blunt needle and syringe, and the cell suspension separated from the tissues by filtration through a 70-μm strainer. Once the cell suspension was obtained, pure populations of vaginal T cells and macrophages were isolated by flow cytometric sorting on a four-laser BD FACSAria II (408, 488, 535, and 633 nm). Cells were stained with CD45 APC, CD3 FITC, and CD14 PE-Cy7 (all mouse anti-human from BD Biosciences, San Jose, CA) and 0.1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) for viability. All antibodies were titrated before use and used at the minimum saturating dose. T cells were defined as live CD45+CD3+CD14− and macrophages defined as live CD45+CD3−CD14+ events. The purified cell populations were suspended in 1× phosphate-buffered saline at about 3 × 107 cells/mL and stored on ice before use within 8 hours.
Cryopreservation of the human vaginal immune cells with different cooling rates
Human vaginal mucosal cells were cryopreserved at different cooling rates. After digestion, cells were suspended in RPMI-1640 culture medium at 4°C with 12.5% bovine serum albumin and 200,000 cells aliquoted into each cryovial. A 2× solution of cryopreservation medium was added dropwise for a final volume ratio of 1:1. The final concentration of cryoprotective agents was 6% dimethylsulfoxide, 5% ethylene glycol, and 6% hydroxyethyl starch. The cryovials were then frozen to −80°C at either 1°C/min in a Mr. Frosty or at 2.5°C/min, 3.5°C/min, or 10°C/min in a cooling rate-controlled freezer (CryoMed; Fisher Scientific, Hanover Park, IL). The samples were then transferred to the vapor phase of a liquid nitrogen tank and stored for several days. For thawing, the frozen samples were taken out of liquid nitrogen and thawed quickly in a 37°C water bath with gentle agitation for about 30 seconds until only tiny ice crystals remained in the vial. One milliliter of 37°C cell culture medium was added to each cryovial dropwise over about 30 seconds, the cell suspension was transferred to 15-mL conical tubes containing 10 mL, 37°C, cell culture medium, and the cells were washed by centrifugation at 300 g for 10 minutes.
Cell viability assessment
After thawing, cell number and viability were determined by flow cytometry. Cells were stained with fixable live/dead aqua (Life Technologies, Grand Island, NY) at a dilution of 1:50 for 20 minutes and washed. They were subsequently stained with CD45 APC, CD3 FITC, and CD14 PE-Cy7 at the minimum saturating doses for 20 minutes (all mouse anti-human from BD Biosciences) and washed again. Finally, the samples were transferred to Trucount absolute counting tubes (BD Biosciences) and fixed with 1% paraformaldehyde. Samples were acquired on a five-laser BD LSRII (355, 408, 488, 535, and 633 nm) within 1 week. Events were gated on CD45+, divided into CD14+CD3− and CD14−CD3+, and gated for viability using FlowJo version 9 (FlowJo, LLC, Ashland, OR). The number of cells per sample was determined relative to the number of Trucount beads acquired. Recovery reflects the number of live cells recovered out of the number of live cells in the fresh control sample from the same donor. Data were analyzed in R version 3.2.033,34 using the packages, dplyr35 and ggplot2.36
Results
Cell membrane permeability to water (Lp(T))
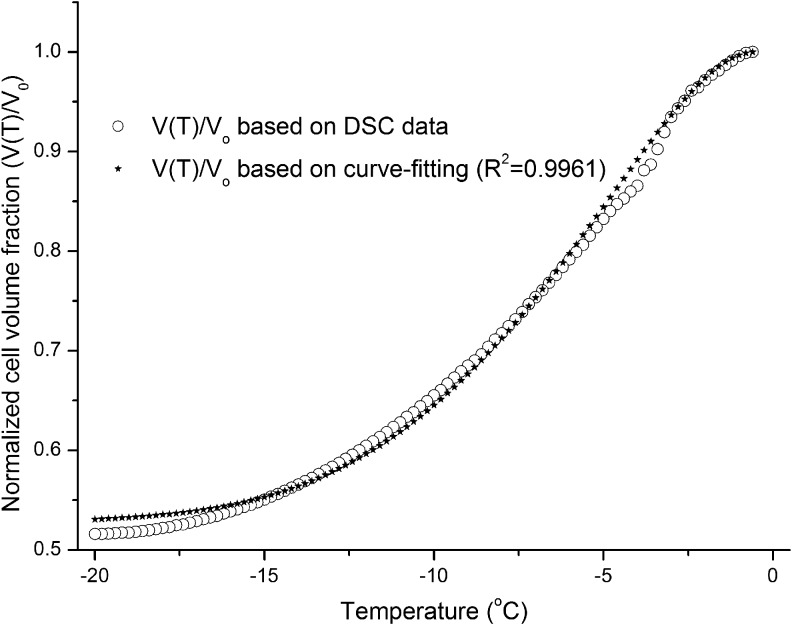
From the experimental DSC heat flow (mW)–time (minutes) data, the thermogram difference between the live and dead cell suspensions (ΔqDSC) and the partial thermogram difference at temperature T [ΔqDSC(T)] were determined with Pyris software (PerkinElmer). The cell volume change history V(T) was determined as described in Equation (1).21 The water transport model [Eqs. (2) and (3), cooling rate B = 4°C/min] and MLab software were then used to simulate Lpg (cell membrane permeability to water at the reference temperature Tr, 273.15 K) and Ea (activation energy) by nonlinear regression curve fitting of the experimental V(T) data (MLab for PC; Civilized Software, Inc., Silver Spring, MD). An example of the experimental V(T) curve based on DSC data and the curve-fitting results for the estimation of Lpg and Ea is shown in Figure 1. The estimated Lpg and Ea results for human vaginal mucosal T cells and macrophages are shown in Table 1.
FIG. 1.
Simulation of Lpg and Ea by nonlinear regression curve fitting of the normalized cell volume fraction changes during cooling [V(T)/V0].
Table 1.
Lpg and Ea for Human Vaginal Mucosal T Cells and Macrophages (Mean ± Standard Deviation, n = 12)
| Lpg(μm/atm/min) | Ea(kcal/mol) | |
|---|---|---|
| Human vaginal mucosal T cells | 0.0209 ± 0.0108 | 41.5 ± 11.4 |
| Human vaginal mucosal macrophages | 0.0198 ± 0.0102 | 38.2 ± 10.4 |
Prediction of optimal cooling rates for human vaginal mucosal T cells and monocytes
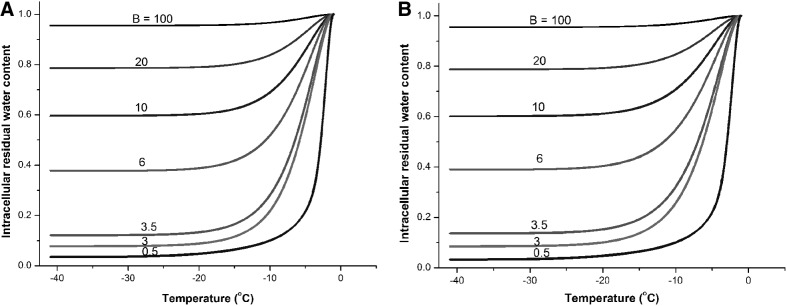
After the cell membrane permeabilities to water at subzero temperatures were obtained, the water transport process across cell membranes during cooling was simulated. The numerical simulation results are shown in Figure 2A and B for human vaginal mucosal T cells and macrophages, respectively. It shows that cell dehydration generally stalls at temperatures lower than −20°C because Lp decreases dramatically with lower temperature. For example, Lp of T cells at −20°C decreases to 0.72% of the Lp at the reference temperature (273.15 K). From the results, cells undergo severe dehydration at slow cooling rates (e.g., less than 10% water remaining in the cell at cooling rate 0.5°C/min), which may lead to solute injury to the cells. At high cooling rates, much water remains inside the cell (e.g., ∼80% water remaining in the cell at cooling rate 20°C/min). Assuming that about 10% of cellular water should remain in the cell when ice forms,4,37 the predicted optimal cooling rates for human vaginal mucosal T cells and macrophages are both about 3°C/min.
FIG. 2.
Simulation of intracellular residual water content normalized to initial water content for the prediction of the optimal cooling rates. (A) Simulation for T cells. (B) Simulation for macrophages. Cooling rate B in unit: °C/min.
Experimental verification of the optimal cooling rate
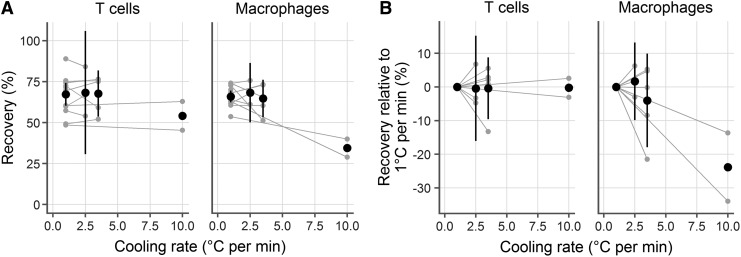
To test the prediction that ∼3°C/min is optimal for mucosal immune cells, we froze cells at various cooling rates (2.5°C/min, 3.5°C/min, and 10°C/min) and measured viability and recovery after thawing. In each case, we conducted experiments in parallel, freezing two cryovials at 1°C/min and two cryovials at the experimental cooling rate. After storing in liquid nitrogen for a week, we thawed the samples side by side and measured recovery, averaging the replicate cryovials within each experiment. In total, we conducted experiments with 10 samples, using 1°C/min (standard for peripheral blood mononuclear cells, n = 10) and 2.5 (n = 3), 3.5 (n = 5), or 10 (n = 2) °C/min. This gave a common reference point for each comparison.
We found that the predicted cooling rate of about 3°C/min provided good recoveries (Fig. 3A, B). In fact, 1°C/min, 2.5°C/min, and 3.5°C/min yielded similar recoveries for both cell types. At 2.5°C/min, 0.44% (95% confidence interval [CI]: −16.08, 15.2) fewer live T cells and 1.67% (95% CI: −9.88, 13.23) more live macrophages were recovered than at 1°C/min. Statistical testing for a difference in recovery between 1°C/min and 2.5°C/min (by paired t-test, taking a p-value of 0.05 as significant) gave p-values of 0.91 for T cells and 0.60 for macrophages. Similarly, there were no significant differences between 3.5°C/min and 1°C/min: the difference was −0.39% (95% CI: −9.58, 8.81; p = 0.91) for T cells and −4.02% (95% CI: −17.94, 9.89; p = 0.47) for macrophages.
FIG. 3.
Experimental confirmation of the predicted optimal cooling rate. Human vaginal CD3+ T cells (left) and CD14+ macrophages (right) were cryopreserved at 1°C/min and 2.5°C/min, 3.5°C/min, or 10°C/min. Each gray dot represents the average of duplicate cryovials frozen from a single donor and gray lines indicate pairing. Black symbols indicate the mean of the experiments at that temperature and the black lines represent the 95% confidence interval (omitted for the 10°C/min case where n = 2). (A) Cell recovery rates at different cooling rates. (B) Recovery rates relative to that at 1°C/min.
However, a cooling rate of 10°C/min was notably worse than 1°C/min for CD14 macrophages, with a mean difference of −23.83% (95% CI: −152.7, 105.04; p = 0.26). Surprisingly, it did not make a difference for T cells, where the difference was only −0.25% (95% CI: −36.39, 35.89; p = 0.94).
Discussion
DSC was used to measure the cell membrane permeabilities of human vaginal macrophages and T cells to water at subzero temperatures, using the approach proposed by Devireddy et al.21 The results showed that in the temperature-dependent relationship of cell membrane permeability 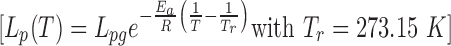 , Lpg was 0.0209 μm/atm/min and Ea was 41.5 kcal/mol for mucosal T cells and Lpg was 0.0198 μm/atm/min and Ea was 38.2 kcal/mol for mucosal macrophages. Lp decreases rapidly with temperature due to the relatively high Ea, hence water loss during cell freezing mostly happens above −10°C. Based on these measured cell membrane permeabilities, optimal cooling rates were predicted to be about 3°C/min for both the mucosal T cells and macrophages. In cryopreservation experiments, viable vaginal T cells and macrophages were recovered at similar rates whether frozen at 1°C/min, 2.5°C/min, or 3.5°C/min. Recovery dropped dramatically for vaginal macrophages at 10°C/min, although the result was not statistically significant given the small sample size.
, Lpg was 0.0209 μm/atm/min and Ea was 41.5 kcal/mol for mucosal T cells and Lpg was 0.0198 μm/atm/min and Ea was 38.2 kcal/mol for mucosal macrophages. Lp decreases rapidly with temperature due to the relatively high Ea, hence water loss during cell freezing mostly happens above −10°C. Based on these measured cell membrane permeabilities, optimal cooling rates were predicted to be about 3°C/min for both the mucosal T cells and macrophages. In cryopreservation experiments, viable vaginal T cells and macrophages were recovered at similar rates whether frozen at 1°C/min, 2.5°C/min, or 3.5°C/min. Recovery dropped dramatically for vaginal macrophages at 10°C/min, although the result was not statistically significant given the small sample size.
The error in the estimates of Lpg and Ea may be caused by many factors, such as the validity of the assumptions (equilibrium cooling for the slow cooling steps, all cells lysed in the fast cooling steps, and others) and the accuracy of the DSC measurements. Another possible factor is the accuracy of the osmotically inactive cell volume (Vb). The dependence of the cell membrane permeability on Vb was investigated. Our results showed that if Vb changed by ±10%, Lpg values for T cells and macrophages changed by ±8%–11%, while the change in Ea for both cell types was within ±2%.
Some other cell types have been tested by different methods (DSC, photomicrography, or Coulter counter) in different temperature ranges, as shown in Table 2. We can see that many factors may influence the values of Lpg and Ea, including the cell type, cell origin, temperature range, presence of CPA, osmotic environment, cooling rate, and others. For example, sperm from different species have dramatically different cell membrane characteristics, where Lpg may vary from 0.0017 to 0.02 μm/min/atm and Ea may vary from 9.8 to 85.5 kcal/mol. The most similar experiments to ours in the subzero temperature range (0 to −50°C) were conducted on Epstein-Barr virus transformed (EBVT) lymphocytes. Our Ea value is about three times higher than that for EBVT and our Lpg is about five times lower. Human lymphocytes from blood have also been studied at suprazero temperatures (10°C–37°C) by two different methods (photomicrography or Coulter counter). The activation energies differed by about fivefold in those two studies. Our activation energy in the subzero temperature range was 3–12 times higher. Human monocytes, a precursor of macrophages, have also been studied at temperatures around zero. In this case, the activation energy we measured is about three times higher than what was reported in the article. Of course, mucosal T cells and macrophages are not the same as T cells and monocytes from the blood, so it is reasonable to expect that they have unique properties.
Table 2.
Lpg and Ea of Some Cell Types
| Cell type | Lpg(Tr = 0°C) (μm/min/atm) | Ea(kcal/mol) | Temperature range (°C) | Method | Ref. |
|---|---|---|---|---|---|
| Haploid sperm | 0.0017 | 9.8 | 0 to −50 | DSC | 39 |
| Human sperm | 0.14 | 85.5 | 0 to −50 | DSC | 40 |
| Boar sperm | 0.02 | 29.3 | 0 to −50 | DSC | 41 |
| Equine sperm | 0.02 | 32.7 | 0 to −50 | DSC | 25 |
| Mouse sperm | 0.01 | 22.5 | 0 to −50 | DSC | 26 |
| Xiphophorus maculatus sperm | 0.0053–0.0093 | 9.79–29.0 | 0 to −50 | DSC | 28 |
| Morone saxatilis sperm | 0.005–0.016 | 37.8–40.2 | 0 to −50 | DSC | 42 |
| EBVT lymphocytes | 0.1 | 15.5 | 0 to −50 | DSC | 21 |
| Human oocyte | 14.7 (with DMSO) | 10 to 34 | Photomicrography | 43 | |
| Human prostatic adenocarcinoma cells | 50.5 | 0 to 23 | Photomicrography | 16 | |
| Human monocyte | 14.59 | −7 to −10 | Photomicrography | 44 | |
| Human lymphocyte from blood | 3.42 | 23 to 37 | Photomicrography | 45 | |
| Human lymphocyte from blood | 11.1–17.4 | 10 to 37 | Coulter counter | 46 |
EBVT, Epstein-Barr virus transformed; DMSO, dimethylsulfoxide; DSC, differential scanning calorimetry.
While the DSC method is useful because it allows measurement of the average membrane permeabilities of many cells with spherical or nonspherical shape at low temperatures, it also has some limitations. First, it is assumed that all cells are killed by the fast cooling step, which was performed twice to increase reliability in this work, but it is possible that some cells may remain intact. Additionally, the compromised membranes of some cell types (ghosts), such as red blood cells, can self-reseal during the thawing step before the last slow cooling step.38 To test the sufficiency of the fast cooling steps, suspensions of mucosal immune cells were immersed in liquid nitrogen (for cooling at 100–300°C/min) and stained with trypan blue after thawing. Less than 4% of total cells remained viable, suggesting that the fast cooling steps used here were adequate.
Second, it is assumed that in the slow cooling steps, equilibration cooling happens without sufficient supercooling or ice crystallization inside the cells. Theoretical simulation of cooling at −4°C/min (the slow cooling rate used in the DSC experiments; Fig. 2A, B) indicates that the residual water content remaining inside the cells during cooling was small (17.2% for T cells and 18.9% for macrophages at −40°C). At such a low cooling rate, the effects of intracellular supercooling or ice formation can be ignored. A lower cooling rate (e.g., −1°C/min) could be better because it achieves even more cell dehydration. However, the DSC scanning program would take much longer and solute injury may also be potentially induced in slower cooling.
Additionally, it is worth noting that no measurements were done in the presence of cryoprotective agents, so the predicted optimal cooling rates do not take the effect of CPAs into account. The effects of CPA may influence many properties of the cell and the solution, such as cell membrane permeability to water, IIF temperature and probability, solution viscosity, and phase transition properties. The effect of CPAs on the cell membrane properties and IIF kinetics of mucosal immune cells at subzero temperatures will be studied in future work.
Acknowledgments
This study was supported by funds received through the Mucosal Immunology Group (http://public.hivmucosalgroup.org), a group funded by a supplement to the HIV Vaccine Trials Network (HVTN) grant, an HIV/AIDS clinical trials network funded by the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (UM1AI068618); by a supplement to R33AI094412 funded by NIAID; and by the Bill and Melinda Gates Foundation (OPP1032522). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 2010;33:542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker BD, Virgin HW. Immunology and the elusive AIDS vaccine. Nature 2010;464:224–231 [DOI] [PubMed] [Google Scholar]
- 3.Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J 2000;41:187–196 [DOI] [PubMed] [Google Scholar]
- 4.Mazur P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys 1990;17:53–92 [DOI] [PubMed] [Google Scholar]
- 5.Mazur P. Freezing of living cells: Mechanisms and implications. Am J Physiol 1984;247:125–142 [DOI] [PubMed] [Google Scholar]
- 6.Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol 1963;47:347–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazur P. Cryobiology: The freezing of biological systems. Science 1970;168:939–949 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Purtteman JJP. Heimfeld S, Folch A, Gao D. Development of a microfluidic device for determination of cell osmotic behavior and membrane transport properties. Cryobiology 2007;55:200–209 [DOI] [PubMed] [Google Scholar]
- 9.Diller KR, Bradley DA. Measurement of the water permeability of single human granulocytes on a microscopic stopped-flow mixing system. J Biomech Eng 1984;106:384–393 [DOI] [PubMed] [Google Scholar]
- 10.Gao DY, Benson CT, Liu C, McGrath JJ, Critser ES, Critser JK. Development of a novel microperfusion chamber for determination of cell membrane transport properties. Biophys J 1996;71:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao DY, McGrath JJ, Tao J, Benson CT, Critser ES, Critser JK. Membrane transport properties of mammalian oocytes: A micropipette perfusion technique. J Reprod Fertil 1994;102:385–392 [DOI] [PubMed] [Google Scholar]
- 12.Hunter J, Bernard A, Fuller B, McGrath J, Shaw R. Measurements of the membrane water permeability (Lp) and its temperature-dependence (activation-energy) in human fresh and failed-to-fertilize oocytes and mouse oocyte. Cryobiology 1992;29:240–249 [DOI] [PubMed] [Google Scholar]
- 13.Leibo SP. Water permeability and its activation energy of fertilized and unfertilized mouse ova. J Membr Biol 1980;53:179–188 [DOI] [PubMed] [Google Scholar]
- 14.McGrath JJ. Quantitative measurement of cell membrane transport: Technology and applications. Cryobiology 1997;34:315–334 [DOI] [PubMed] [Google Scholar]
- 15.McGrath JJ. A microscope diffusion chamber for the determination of the equilibrium and non-equilibrium osmotic response of individual cells. J Microsc 1985;139:249–263 [DOI] [PubMed] [Google Scholar]
- 16.Takamatsu H, Komori Y, Zawlodzka S, Fujii M. Quantitative examination of a perfusion microscope for the study of osmotic response of cells. J Biomech Eng 2004;126:402–409 [DOI] [PubMed] [Google Scholar]
- 17.Tseng H, Sun S, Shu Z, Ding W, Reems J, Gao D. A microfluidic study of megakaryocytes membrane transport properties to water and dimethyl sulfoxide at suprazero and subzero temperatures. Biopreserv Biobank 2011;9:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vian AM, Higgins AZ. Membrane permeability of the human granulocyte to water, dimethyl sulfoxide, glycerol, propylene glycol and ethylene glycol. Cryobiology 2014;68:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe JH, Hoekstra FA, Crowe LM, Anchordoguy TJ, Drobnis E. Lipid phase transitions measured in intact cells with Fourier transform infrared spectroscopy. Cryobiology 1989;26:76–84 [DOI] [PubMed] [Google Scholar]
- 20.Eletr S, Inesi G. Phase changes in the lipid moieties of sarcoplasmic reticulum membranes induced by temperature and protein conformational changes. Biochim Biophys Acta 1972; 290:178–185 [DOI] [PubMed] [Google Scholar]
- 21.Devireddy RV, Raha D, Bischof JC. Measurement of water transport during freezing in cell suspensions using a differential scanning calorimeter. Cryobiology 1998;36:24–155 [DOI] [PubMed] [Google Scholar]
- 22.Devireddy RV, Barratt PR, Storey KB, Bischof JC. Liver freezing response of the freeze-tolerant wood frog, Rana sylvatica, in the presence and absence of glucose. I. Experimental measurements. Cryobiology 1999;38:310–326 [DOI] [PubMed] [Google Scholar]
- 23.Devireddy R, Bischof J. Measurement of water transport during freezing in mammalian liver tissue: Part II—The use of differential scanning calorimetry. J Biomech Eng 1998;120:559–569 [DOI] [PubMed] [Google Scholar]
- 24.Devireddy R, Coad J, Bischof J. Microscopic and calorimetric assessment of freezing processes in uterine fibroid tumor tissue. Cryobiology 2001; 42:225–243 [DOI] [PubMed] [Google Scholar]
- 25.Devireddy R, Swanlund D, Olin T, et al. . Cryopreservation of equine sperm: Optimal cooling rates in the presence and absence of cryoprotective agents determined using differential scanning calorimetry. Biol Reprod 2002;66:222–231 [DOI] [PubMed] [Google Scholar]
- 26.Devireddy R, Swanlund D, Roberts K, Bischof J. Subzero water permeability parameters of mouse spermatozoa in the presence of extracellular ice and cryoprotective agents. Biol Reprod 1999;61:764–775 [DOI] [PubMed] [Google Scholar]
- 27.Li G, Thirumala S, Leibo S, Devireddy R. Subzero water transport characteristics and optimal rates of freezing rhesus monkey (Macaca mulatta) ovarian tissue. Mol Reprod Dev 2006;73:1600–1611 [DOI] [PubMed] [Google Scholar]
- 28.Pinisetty D, Huang C, Dong Q, Tiersch T, Devireddy R. Subzero water permeability parameters and optimal freezing rates for sperm cells of the southern platyfish, Xiphophorus maculatus. Cryobiology 2005;50:250–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shu Z, Hughes SM, Fang C, et al. . A study of the osmotic characteristics, water permeability, and cryoprotectant permeability of human vaginal immune cells. Cryobiology (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toner M, Cravalho EG, Karel M. Cellular response of mouse oocytes to freezing stress: Prediction of intracellular ice formation. J Biomech Eng 1993;115:169–174 [DOI] [PubMed] [Google Scholar]
- 31.Toner M, Cravalho EG, Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J Appl Phys 1990;67:1582–1593 [Google Scholar]
- 32.Mckinnon L, Hughes S, Rosa S, et al. . Optimizing viable leukocyte sampling from the female genital tract for clinical trials: An international multi-site study. PLoS One 2014;9:e85675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat 1996;5:299–314 [Google Scholar]
- 34.R Core Team. R: A language and environment for statistical computing. 2015. Available at: www.R-project.org/ Last accessed November13, 2015
- 35.Wickham H, Francois R. dplyr: A grammar of data manipulation. R package version 0.4.1. 2015. Available at: http://CRAN.R-project.org/package=dplyr Last accessed November13, 2015
- 36.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer New York; 2009 [Google Scholar]
- 37.Mazur P. The role of intracellular freezing in death of cells cooled at supraoptimal rates. Cryobiology 1977;14:251–272 [DOI] [PubMed] [Google Scholar]
- 38.Bodemann H, Passow H. Factors controlling resealing of membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol 1972;8:1–26 [DOI] [PubMed] [Google Scholar]
- 39.He Y, Dong Q, Tiersch T, Devireddy R. Variation in the membrane transport properties and predicted optimal rates of freezing for spermatozoa of diploid and tetraploid pacific oyster, Crassostrea gigas. Biol Reprod 2004;70 1428–1437 [DOI] [PubMed] [Google Scholar]
- 40.Devireddy R, Swanlund D, Roberts K, Pryor J, Bischof J. The effect of extracellular ice and cryoprotective agents on the water permeability parameters of human sperm plasma membrane during freezing. Hum Reprod 2000; 15:1125–1135 [DOI] [PubMed] [Google Scholar]
- 41.Devireddy R, Fahrig B, Godke R, Leibo S. Subzero water transport characteristics of boar spermatozoa confirm observed optimal cooling rates. Mol Reprod Dev 2004; 67:446–457 [DOI] [PubMed] [Google Scholar]
- 42.Thirumala S, Campbell WT, Vicknair MR, Tiersch TR, Devireddy RV. Freezing response and optimal cooling rates for cryopreserving sperm cells of striped bass, Morone saxatilis. Theriogenology 2006;66:964–973 [DOI] [PubMed] [Google Scholar]
- 43.Paynter S, Cooper A, Gregory L, Fuller B, Shaw R. Permeability characteristics of human oocytes in the presence of the cryoprotectant dimethylsulphoxide. Hum Reprod 1999;14:2338–2342 [DOI] [PubMed] [Google Scholar]
- 44.McCaa C, Diller K, Aggarwal S, Takahashi T. Cryomicroscopic determination of the membrane osmotic properties of human monocytes at subfreezing temperatures. Cryobiology 1991;28:391–399 [DOI] [PubMed] [Google Scholar]
- 45.Porsche AM, Körber C, Englich S, Hartmann U, Rau G. Determination of the permeability of human lymphocytes with a microscope diffusion chamber. Cryobiology 1986;23:302–316 [DOI] [PubMed] [Google Scholar]
- 46.Hempling HG, Thompson S, Dupre A. Osmotic properties of human lymphocyte. J Cell Physiol 1977;93:293–302 [DOI] [PubMed] [Google Scholar]