Abstract
Significance: Burn assessments, including extent and severity, are some of the most critical diagnoses in burn care, and many recently developed imaging techniques may have the potential to improve the accuracy of these evaluations.
Recent Advances: Optical devices, telemedicine, and high-frequency ultrasound are among the highlights in recent burn imaging advancements. We present another promising technology, multispectral imaging (MSI), which also has the potential to impact current medical practice in burn care, among a variety of other specialties.
Critical Issues: At this time, it is still a matter of debate as to why there is no consensus on the use of technology to assist burn assessments in the United States. Fortunately, the availability of techniques does not appear to be a limitation. However, the selection of appropriate imaging technology to augment the provision of burn care can be difficult for clinicians to navigate. There are many technologies available, but a comprehensive review summarizing the tissue characteristics measured by each technology in light of aiding clinicians in selecting the proper device is missing. This would be especially valuable for the nonburn specialists who encounter burn injuries.
Future Directions: The questions of when burn assessment devices are useful to the burn team, how the various imaging devices work, and where the various burn imaging technologies fit into the spectrum of burn care will continue to be addressed. Technologies that can image a large surface area quickly, such as thermography or laser speckle imaging, may be suitable for initial burn assessment and triage. In the setting of presurgical planning, ultrasound or optical microscopy techniques, including optical coherence tomography, may prove useful. MSI, which actually has origins in burn care, may ultimately meet a high number of requirements for burn assessment in routine clinical use.

Jeffrey E. Thatcher, PhD
Scope and Significance
Clinical judgment by burn experts is the standard of care in burn depth assessment, with reported accuracy between 70–80%.1 Before a burn victim is transferred to a specialized burn center, in most cases, a nonexpert initially assesses burn size and depth, but burn depth and area estimations by non-specialists are reported to be only 60% and 51% accurate, respectively.1,2 To improve upon these burn assessments, many tools are currently available or in development to assist the burn care provider in better understanding burn injuries. Imaging devices, in particular, have been investigated in numerous preclinical and clinical burn studies, with notable success in their evaluation of burns. It is surprising, given the number of techniques that could readily assist providers, that none of the commercially available devices are used routinely in burn centers throughout the United States. We provide this review to address recent advancements in this rapidly progressing field with a focus on new progresses in multispectral imaging (MSI) technology for burn assessment. The scope of this review is to present basic concepts in burn assessment technology, including a background in tissue optical properties to facilitate readers' understanding of the many optical imaging devices for burn assessment. Included in this review are the results of a Monte Carlo simulation we customized to explain the extent to which light travels in the skin. Next, we review imaging techniques for burn assessment using recent highlights from published literature. The final section details MSI, including the information this technique can provide to clinicians and the clinical aspects of developing computer-aided diagnosis algorithms for MSI devices.
Translational Relevance
Most imaging methods discussed in this review, including MSI, are currently well positioned for translational studies. One advantage this group of technologies has as they undergo translational scientific assessment is the noninvasive mechanisms by which they operate. With a majority of these techniques functioning in the optical range of the electromagnetic spectrum and few requiring patient contact, study designs that use human subjects are usually of minimal risk. In this review, we provide information on the path for bringing MSI burn diagnosis to clinical application by developing a conceptual model of a machine learning algorithm to provide an example of the steps required for the clinical development of an imaging technology in the burn care arena.
Clinical Relevance
In this review, we also present modern commercially available burn imaging devices, including color photography, laser Doppler, indocyanine green (ICG) video angiography, thermography, and ultrasound. These devices are common in other areas of medical practice but, although effective in burn assessment, have drawbacks that keep burn surgeons searching for better alternatives. As an aid to this search, we compare and contrast recent advancements from both clinically available burn imaging technologies and emerging imaging techniques.
Background
Burn triage and care environments
When considering the translation of imaging techniques to the clinic, emphasis must be placed on both the clinical environment and clinical information relevant to each step in the spectrum of burn care. The slow adoption of technology has demonstrated that the ability to provide accurate assessment is only one facet of adoption.3 Besides providing an accurate analysis of the burn injury, imaging devices must meet a minimum level of user and environmental requirements to be adopted for routine use. These requirements include the ability to be handled by a range of users, including nurses and surgeons, in challenging use environments such as a busy emergency room, crowded operating room, or hot and humid tank rooms where nonsurgical debridement takes place. In many cases, a burn victim is initially taken to a hospital that does not provide specialized burn care. This care team documents the total body surface area (%TBSA) of the burn injury using a quick estimation technique, such as the Wallace Rule-of-9s, and estimates the burn depth. In many cases, the healthcare providers performing these initial assessments have relatively little experience in burns. Inexperienced personnel overestimate %TBSA in half of their assessments and make inaccurate burn depth estimations 40% of the time.1,2 Therefore, devices must be simple to use by a range of personnel with varying education and experience levels.
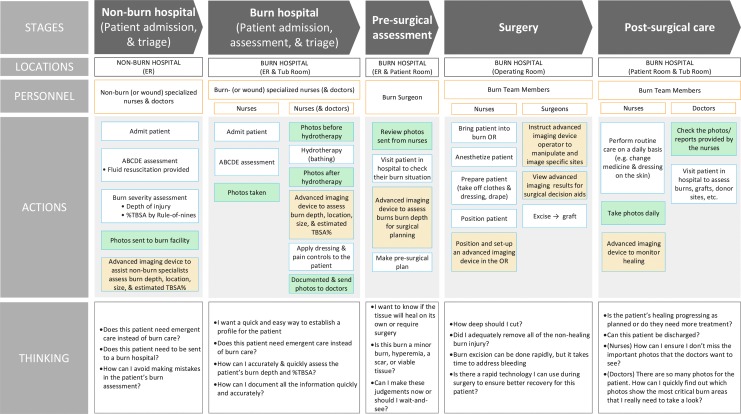
We have compiled information about the spectrum of burn care from the standpoint of imaging into a Burn Care Workflow to establish what information the burn treatment team needs and when they need that information (Fig. 1). This workflow recognizes multiple burn assessment needs that various imaging technologies can address. These needs include the following: identifying burn depth and TBSA; monitoring wound infections and wound healing progress; pre- and intraoperative assessment that instructs where and to what extent tissue should be debrided; and, with circumferential burns, the continuous monitoring of perfusion to the injury and extremities.
Figure 1.
Summary of the workflow of a typical burn patient across the spectrum of care. Stages indicate a setting of care for which the location, personnel, actions, and thoughts are presented. Because color photography is so common, we noted in green boxes where photographs are typically taken and reviewed. In orange, we present possible utilization of advanced imaging devices that assist in determining burn depth or %TBSA. TBSA, total body surface area. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Pathophysiology of a burn
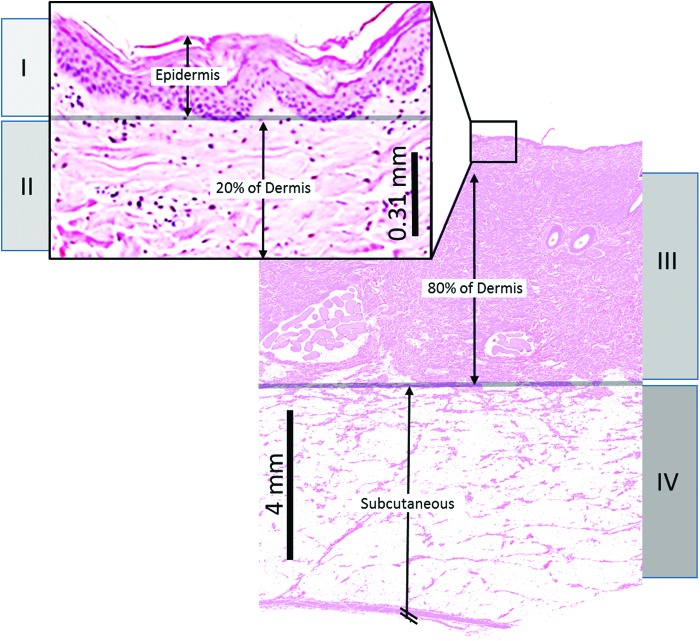
Burn imaging is accomplished through the identification of one or more specific pathological features of the burn injury and surrounding tissue. Therefore, a review of the burn pathology is valuable. Burn pathophysiology is described by two important characteristics: the depth of the injury and the physiologic response in the tissue zones surrounding the injury. The depth of cell destruction according to burn degree is shown in Fig. 2.
Figure 2.
Structure of skin depths of destroyed cells according to burn degree are shown. Burn injuries depicted are as follows: (I) superficial; (II) shallow partial thickness; (III) deep partial thickness; and (IV) full thickness. Histology is a representative dorsal skin segment from our porcine burn experiments where skin thickness is slightly greater than that in humans. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Burn severity
Superficial (first degree) burns are the shallowest burns, occurring 0.07–0.12 mm in depth, and result in vasodilation of dermal vessels due to an inflammatory response causing the clinical sign of erythema.4 All layers of the epidermis and dermis remain intact. Slightly deeper burns are classified as partial-thickness (second degree) burns, which are between 0.12 and 2.0 mm in depth, and are divided into two categories: shallow and deep.5 Shallow partial-thickness burns result in significant epidermal damage, but minimal dermal damage. These injuries are characterized by capillary damage and cell swelling that result in blisters and edema.6 Basal cells of the epidermis remain intact and allow for spontaneous healing without grafting. Some melanocytes may be damaged, possibly causing some depigmentation at the burn site. Deep partial-thickness burns extend through the epidermis and dermis, causing significant vascular damage that leads to vascular occlusion, thrombus, and edema. The original fibrous dermal collagen structure is lost, so skin grafting becomes necessary for healing.7 There is a permanent loss of melanocytes and depigmentation at the site of deep partial-thickness burns. These burns appear yellow or white, are dry, and demonstrate less blanching than shallow partial-thickness burns. The deepest burns are full-thickness (third degree) burns, injuries >2.0 mm deep that completely destroy the epidermis and dermis, with damage extending into the subcutaneous fat and/or muscle. These burns involve vascular occlusion and a loss of basal cells and melanocytes. The collagen present in the subdermal layer is denatured and coiled into aggregates rather than the normal basket-weave pattern, a pathological response characterized by some optical imaging techniques that can assess collagen structure.7 Full-thickness burns appear white/brown with no blanching, have a leathery texture, and are dry.
Burn zones
Burn zones describe the cellular response at the boundaries between the most severely injured areas and the surrounding healthy skin. These areas are important because some imaging devices determine the severity of an injury by the body's response in this boundary. Burn zones occur in a concentric pattern around the direct site of the burn insult (zone of coagulation), which becomes necrotic. The next surrounding layer (zone of stasis) is viable tissue with some ischemic effects and is therefore vulnerable to eventual thrombosis and necrosis. The outermost layer (zone of hyperemia) demonstrates inflammation and vasodilation, but ultimately completely recovers.8 Within the first 24–48 h, ischemic effects begin to lead to cell damage and death in the zone of stasis. This is known as “burn progression” or “burn conversion” and is typically used to discuss the pathophysiology of superficial partial-thickness burns evolving into deep partial-thickness burns.9 Burn injury progression continues over 2–4 days, peaking at day 3.10 The zone of coagulation, however, does not progress.11 A summary of the structural and functional changes that occur during burn progression is shown in Table 1. For readers interested in more detail on this subject, other reviews are available.12
Table 1.
Structural and functional changes in burn zones during acute and late stages
| Burn Zone | 0–24 h | Acute Stage 24–48 h | 48–72 h | Late Stage 4–7 Days |
|---|---|---|---|---|
| Structural changes | ||||
| Zone of coagulation | Collagen denaturation; cellular swelling and necrosis; thrombosis of blood vessels; area constant | Collagen coagulation; stable; area constant | Collagen coagulation; stable; area constant | Coagulative necrosis; area constant |
| Zone of stasis | Vascular stasis and ischemia; apoptotic cell death | Apoptotic cell death; vascular thrombosis; neutrophil accumulation; free radical injury | Apoptotic cell death; vascular necrosis | Apoptotic cell death; vascular necrosis; coagulative necrosis |
| Zone of hyperemia | Cytokines released; vasodilation; some damaged collagen recovers | Neutrophil accumulation; free radical injury | Inflammation | Inflammation resolves and tissue begins healing |
| Functional changes | ||||
| Zone of coagulation | Ischemia; loss of function | Loss of function of region | Loss of function of region | Loss of function of region |
| Zone of stasis | Stenosis of blood flow | Little change in depth; thrombosis of blood flow | Progressive tissue loss; ischemia | Ischemia; loss of function |
| Zone of hyperemia | Increased blood flow to region; edema | Edema decreases; inflammation | Inflammation | Tissue healing |
Light–tissue interaction
Optical techniques comprise a majority of recent advances in burn imaging, and the optical properties that define the interaction between photons and tissue as light travels through tissue are key principles for translation of these techniques to clinical use. The properties that define light transport relevant for burn assessment are measured at lengths in the order of mm-cm. These include the refractive index (n), which defines the speed of light in the medium, as well as the reduced scattering coefficient (μs/) and the absorption coefficient (μa), which define the distance that light tends to travel between either scattering or absorption events. Light scattering influences the directionality of light transport as photons cross tiny index-of-refraction mismatches within the tissue. Absorption defines the transfer of energy of a photon to an absorbing molecule. In general, scattering describes the many paths that light can take within tissue, while absorption describes the attenuation of light along those paths.
Optical imaging of a tissue surface, such as the skin, includes collecting not only photons that scatter throughout the tissue and then remit but also photons that directly reflect from the tissue surface without entering the tissue. This direct reflection, termed “specular reflection,” does not contain information about the internal tissue composition, so accurate optical imaging of tissue must reject specular reflections so that they do not overwhelm the collected signal. This can be achieved by polarization or careful selection of the angles between the light source and camera.
The optical properties of skin tissue are defined by its chemical composition and anatomical structure. Skin tissue is a highly layered (and therefore heterogeneous) medium, with different layers composed of different amounts of constituents. A specific set of compounds in the skin tissue has major influences on the optical properties and these include hemoglobin, melanin, water, and collagen. All of these molecules, except collagen, are major contributors to tissue absorption. Disease processes, including burns, that alter the configuration or concentration of these molecules in the skin are therefore discernable by optical techniques.
Depth of optical measurement
Reflectance spectra collected by diffuse wide-field imaging approaches, such as MSI, return a measured light intensity at each sampled wavelength. The variations in remitted intensity across the wavelengths are caused by the wavelength-dependent optical properties, which attenuate light uniquely at the different wavelengths in the visible and near-infrared spectrum.13 The dominant absorbers in tissue are oxygenated and deoxygenated hemoglobin, as shown in Fig. 3, with very strong absorption in the blue (near 400 nm), moderate absorption in the green (near 500 nm), and weak absorption in the far red (near 700 nm). The absorption-based attenuation of light within tissue is proportional to these absorption bands, which means that blue light penetrates less deeply into tissue than red light, as blue light is more likely to be absorbed. This phenomenon impacts the depth into tissue that is sampled by spectral imaging approaches.
Figure 3.
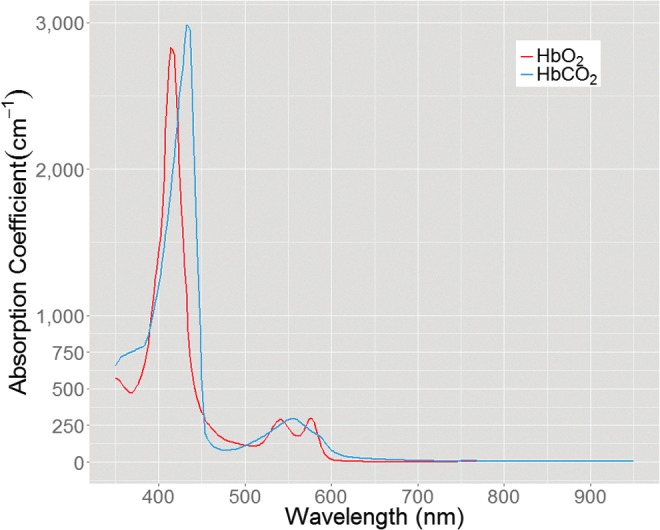
Absorption spectrum of HbO2 and HbCO2. HbO2, oxyhemoglobin; HbCO2, carboxyhemoglobin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Other molecules that interact with light in the skin include melanin, water, and collagen. Melanin comprises a very thin layer in the epidermis where it strongly absorbs light across the UV and visible spectrum (200–700 nm). Water is optically transparent in the UV and visible spectrum, but strongly absorbs IR light beginning around 1,500 nm. Although melanin and water are light absorbers, collagen accounts for much of the scattering of light in skin. Burn injuries alter the location and concentration of melanin, hemoglobin, and water, impacting light absorption patterns in the skin. Furthermore, burns also denature collagen's organized macromolecular structure, resulting in variations of light scattering as well.
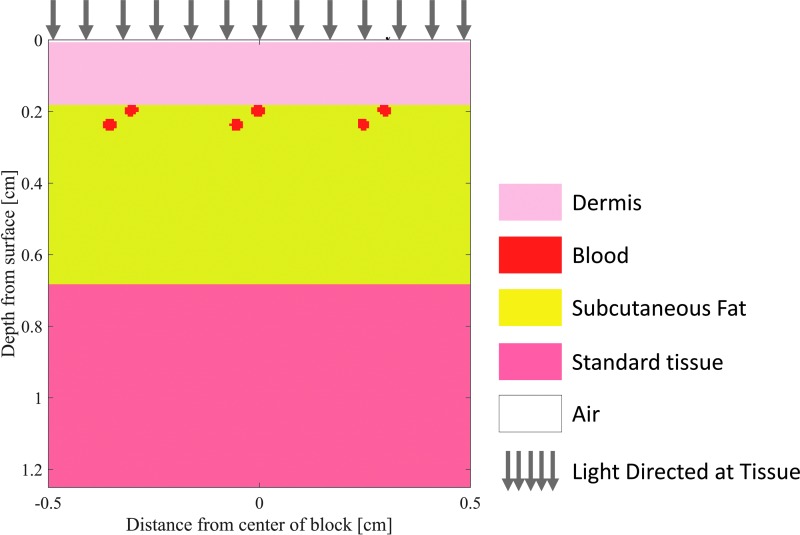
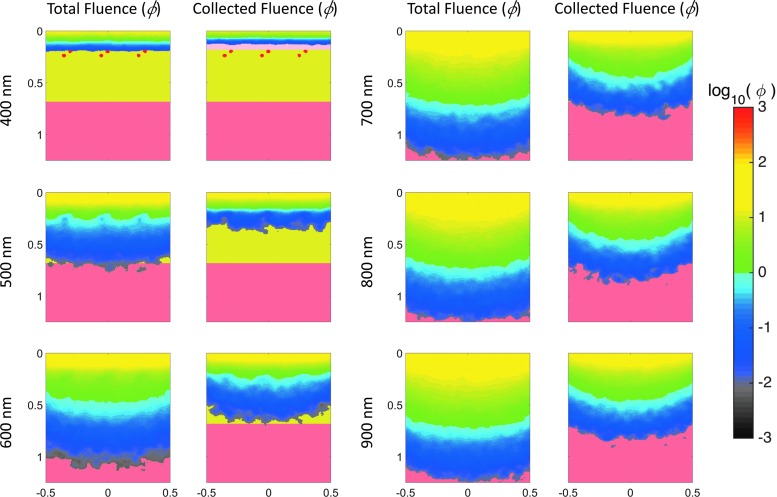
When collecting light reflected from skin to study burn tissue physiology, the depth at which the tissue can be assessed is essential. To provide insight into the depth of tissue probed by visible and near-IR light, we simulated light transport in tissue to characterize the relationship between the volume of tissue sampled and the spectral optical properties in skin. Specifically, we have customized a publically available Monte Carlo model that simulates light propagation in a three-dimensional (3D) heterogeneous tissue volume (Fig. 4; mcxyz.c) to return the photon paths of photons collected during spectral imaging of the skin surface. Figure 5 shows sensitivity “clouds” that describe the paths of photons collected during simulated measurement. The data clearly show that red light penetrates more deeply before collection than blue light, with the wavelength dependence of the average sampling depth shown in Fig. 6.
Figure 4.
Layered skin tissue model designed for Monte Carlo simulations of diffuse light. Modified from the “mcxyz.c” simulation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 5.
Monte Carlo simulations of diffuse light directed at the layered tissue of figure geometry of Fig. 4 representative of the skin. Left columns: Total fluence (ф W/cm2/W delivered) delivered to the tissue at three select wavelengths. Right columns: Pathway of backscattered light that could account for the collected fluence by a sensor positioned facing the same side of the tissue as the input light. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 6.
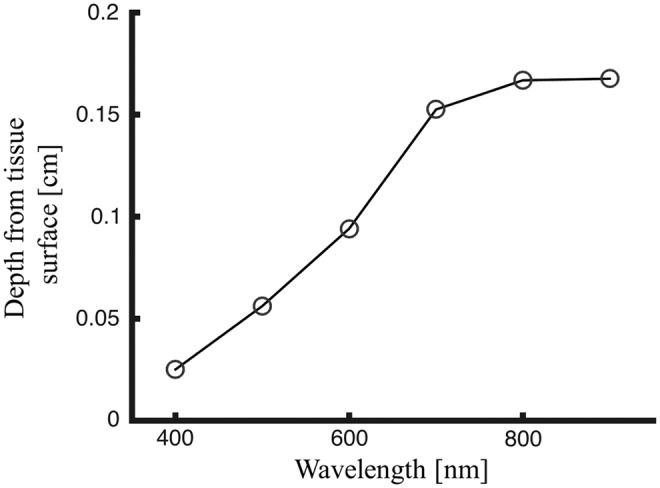
Average sampled depth of collected fluence (i.e., average depth of light backscattered after entering the skin) by wavelength based on results from the Monte Carlo simulation.
Imaging Technologies for Assessing Burn Depth
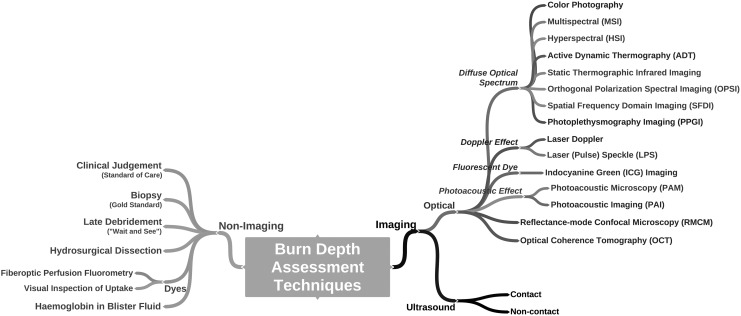
Technologies developed to assist in burn wound assessment provide a quantitative analysis of burn metrics such as severity/depth, healing potential, and infectious status. For simplicity, burn assessment tools can be separated into two categories: imaging and nonimaging (Fig. 7). While many nonimaging methods provide improved burn assessment, we focus on imaging tools herein. We review a variety of the conventional imaging technologies for burn depth assessment and then present emerging techniques. The basic physical principles of modern modalities are explained along with the burn care applications recently addressed by each technology in the scientific literature. Key aspects of these techniques are summarized as a reference for clinical personnel (Table 2). We conclude with an in-depth review of MSI, including the type of information made available to the clinician and the key steps in developing this technique for burn assessment.
Figure 7.
Conceptual diagram of burn assessment broken into imaging and nonimaging techniques.
Table 2.
Characteristics of imaging modalities used in burn care and burn research
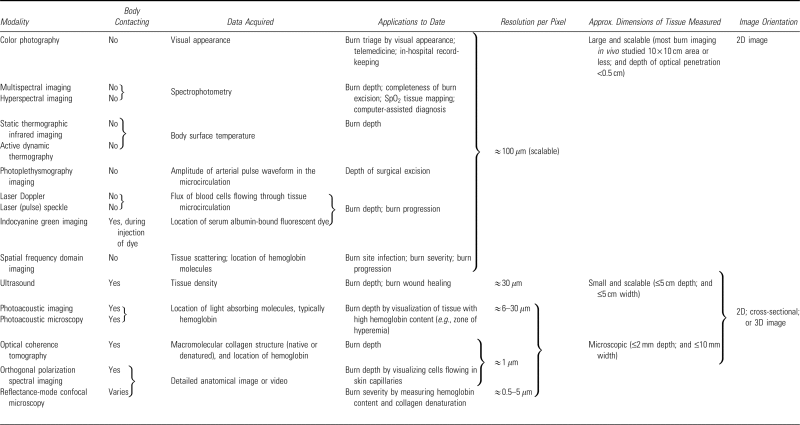 |
Color photography
As the number of burn centers and burn surgeons have been decreasing in recent years,14 telemedicine has become more valuable to fill gaps in the treatment of burn patients. Color photography is frequently offered as a low-cost rapid solution to increase access to specialized clinical judgment for burn victims.15–21 Photographs are simple to collect and share with clinicians who can use digital burn images to prepare in advance for a patient's arrival to a burn center or for remote consultation on treatment of distant patients without access to expert burn care. Color photography, however, does not provide any information beyond the visual appearance of the burn to the clinician, and the accurate assessment of burns using digital images by burn experts is doubted.22 Digital images provide instantaneousness and portability as advantages, but differences in coloration and ambient light may have a significant impact on the accuracy of interpretation. Furthermore, acute burn diagnosis is complex, and expert burn surgeons have been shown to more accurately assess both burn size23,24 and depth25,26 than general clinicians.
Most recently, Boissin et al. developed a survey with photographs of 21 burn cases of varying complexity.27 Respondents, a mix of expert burn surgeons and emergency medicine physicians, estimated burn depth size from the photos. Survey participants scored a 67% average accuracy in estimating both burn depth and burn size with no significant differences between the burn surgeons and emergency medicine physicians. The results of this trial were deemed consistent with previous studies showing that expert clinical judgment is ∼70% accurate. Therefore, we may currently conclude that color photography does expand the limited resources of burn experts, but cannot improve burn assessment independent of their expertise.
Thermography
The first major attempt at noncontact burn imaging emerged in the 1960s after thermography machines assessed skin temperature down to gradients as small as 0.1°C. As a result, thermography could be applied to measure relative perfusion in the skin.28 Theoretically, the infrared radiation emitted from a burn wound should decrease with increasing burn depth due to higher degrees of microvascular coagulation, and therefore, thermography was also applied to estimate burn depth.29,30 A 1974 survey by Hackett et al. found that static thermography was capable of assessing burn depth with a high degree of accuracy.31 Static thermography, however, is subject to a number of factors that negatively impact its intratemporal and intrauser reliability, including evaporative heating and cooling effects, wound granulation that modifies perfusion levels, and variations in the depth of vasculature across cutaneous tissue in different body locations.32,33
In response to the limitations of static thermography, a new technology called active dynamic thermography (ADT) was developed. ADT measures tissue conductance of heat transfer relative to its surroundings.34 While static thermography provides only a snapshot image of wound temperature, ADT assesses radiation trends over time to differentiate between burn depths. To provide this analysis, ADT introduces thermal energy into the skin to raise its physiologic temperature to an elevated level. Over time, regional variations in heat energy transfer from the wound surface can be detected to identify burn depth.35 Although initial data have been promising, ADT is still in the research stage of development.
ICG imaging
Another approach to augmenting burn wound assessment has been the introduction of ICG fluorescent dyes in combination with videoangiography. In this method, intravenously injected ICG binds to albumin in the blood where it remains for several minutes before degradation by the liver.36 ICG fluoresces in the near-infrared range, which conveniently penetrates deeper into dermal vasculature than other wavelengths of light.37 The fluorescent light is captured and translated into a perfusion map that demonstrates vascular degeneration in wounded areas, which is a marker to assess the severity of burned tissue.38 ICG imaging accurately differentiates burn depth, as shown in several studies.39–41
Because vascular degradation occurs quickly after injury, ICG videoangiography can be used very early following initial burn injury.37 The major drawback of ICG imaging, however, is its invasiveness. Although the safety of ICG dye has been confirmed over decades of use, rare side effects, including headache, itching, rash, and anaphylactic reactions, have been reported.41 The disadvantageous requirement to constantly purchase dye increases the expense of ICG imaging. Furthermore, as the anatomy of dermal vasculature is highly variable, it has proven very challenging to establish a threshold for wound severity without significantly more clinical investigation.
Laser Doppler and laser speckle imaging
A recently published survey highlighted laser Doppler imaging (LDI) as the technology with the most promise for future routine clinical use to determine burn depth,3 and several devices implementing this technology are already commercially available in Europe and North America. A red or near-IR wavelength laser directed at the tissue is backscatterd by red blood cells moving through the microcirculation. The backscattered light slightly varies in its color because the moving red blood cells impart a “Doppler shift” on the light. The extent of this Doppler shift determines the speed and volume (“flux”) of blood flow through burn tissue at depths from 0.1 to 2.0 mm below the skin surface.42–44 Commercial LDI devices typically translate flux measurements into color maps that estimate a range of time required for tissue to heal.
The correlation of LDI to both histological assessment as well the clinical requirement for surgical excision and grafting is quite strong—as high as 95% in multiple studies.45,46 Despite the excellent sensitivity and specificity of LDI for burn depth assessment and classification, in addition to a strong safety profile (noncontact, nonradiation), the adaptation of this technology has been slower than expected due to several key drawbacks inherent to the technology.36 Commercial LDI machines are expensive, bulky, with poor levels of user-friendliness, and require patients to lay motionless for extended periods.47 Newer laser Doppler line scanner devices have solved the long data collection times. Nonetheless, LDI assessment also varies unpredictably due to anemia, cellulitis, peripheral vascular disease, challenging tissue topography, surface moisture, dressings, and wound ointments.48 A tremendously high volume of research regarding LDI technology is available for review elsewhere.43
Laser speckle imaging (LSI) technology, using the same basic principles as LDI, addresses several of the limitations of LDI, including its expense, bulkiness, and requirement for patient motionlessness.49 LSI is the result of iterative improvements to LDI systems that give rise to a low-cost setup with increased acquisition speeds compared to LDI.50,51 LSI has undergone experimentation in animal burn models,11,49,52 and we expect the clinical utility of LSI to be further investigated in human trials in the near future.53,54
Reflectance-mode confocal microscopy
Reflectance-mode confocal microscopy (RMCM) has been described as a method to obtain “optical biopsies”.36 RMCM applies near-infrared light to the area of interest using a laser beam and collects reflected light through a specially designed aperture to investigate multiple focal planes, or depths into the tissue to a maximum depth of ∼350 μm.55 Tissue structures are contrasted by their differences in refraction index. At the junction between two different refraction indices, a portion of the incident light inherently reflects. RMCM can therefore assess burns by compiling these refraction differences in multiple focal planes to create a 3D map of the injury area.56 The high level of detail provided by RMCM theoretically allows clinicians to assess microscopic structural damage to determine the depth and extent of a burn injury. These structures include blood vessels in the dermis, cells flowing through the lumen of these vessels, the epidermal-dermal junction, and the presence of white blood cells in the tissue.57 However, human burn injuries have only undergone preliminary investigations with RMCM and the clinical accuracy, sensitivity, and specificity of this technology are unknown.58 Moreover, this considerably expensive technology requires contact with patient skin, a lengthy examination time of greater than 10 min (of note, the technology is tolerant of some degree of patient motion during this time), images a small area of tissue, and considerable expertise is necessary to interpret the resulting images.36
Ultrasound
The application of ultrasound technology for burn assessment has undergone a process of iterative improvements. Initially, pulse-echo ultrasound was used to measure burn depth.59 Ultrasound waves were found to reflect from epidermal, dermal, and subcutis boundaries and could be used to analyze the thickness of each tissue layer by measuring the time between recorded echoes from each tissue layer boundary. With B-mode ultrasonography, there was a more intuitive cross-sectional image to assess burns.60 The clinical utility of these techniques, however, was marginal, and neither technique was demonstrated to improve accuracy of clinical assessment alone, despite good correlation with histological sectioning.61,62 More recently, high-frequency ultrasonography is available as a means to assess dermal depth as well as burn wound healing.63 Although ultrasound requires skin contact, the improved resolution provided by high-frequency ultrasonography may demonstrate a worthwhile trade off, should the additional resolution improve burn diagnostics.64 Despite the initial promise of this technology to impact current practice,65 the clinical utility of high-frequency ultrasound remains to be determined.
Traditional ultrasound technology requires contact with the burn wound, a fundamental limitation that initially stymied attempts to improve the accuracy of pulse-echo and/or B-mode ultrasonography. However, novel, noncontact ultrasonography is being investigated.66 Further development to improve this device's detection of skin features, such as sweat ducts and hair follicles, is needed to bring this technique to the clinical setting.67
Orthogonal polarization spectral imaging
This optical technique takes advantage of orthogonally aligned polarized lenses in front of the light source and light detector to cancel most of the tissue surface's specular reflection. By cancelling specular reflection, the system captures only photons that traverse into the tissue. While orthogonal polarization is useful for a variety of optical imaging techniques, orthogonal polarization spectral imaging (OPSI) refers to a specific system for microscopic imaging of blood perfusion in vivo. In this system, polarized light at a wavelength of hemoglobin's absorption peak, 548 nm is directed at the tissue. Hemoglobin absorbs this light, while the rest of the backscattered light is collected by the sensor. Milner et al. studied burns with this technique in a system that collects and displays this backscattered light in the form of a video. These videos depict, in microscopic detail, cells traversing capillaries and arterioles of the skin. These dynamic measurements reveal that OPSI detects healthy dermal capillaries with individual circulating red blood cells in superficial burns.68 In deep burns, where these capillaries are damaged, the larger vessels of the deep plexus become visible with OPSI, and they are filled with stagnant clot rather than circulating blood. Much like RMCM, the practical uses of this technique are limited by its small, about 1 × 1 mm, field of view.
Optical coherence tomography
Optical coherence tomography (OCT) is a technology widely used in ophthalmology and has been described as the optical equivalent of ultrasound imaging. The image produced by OCT can be either cross-sectional (2D) or 3D images of extraordinary detail. Similar to how ultrasound machines employ sound waves, OCT generates images by measuring the arrival times of light (usually infrared) that is incident on the tissue. The challenge is in the timing of light collection because light is nearly one million times faster than sound. To do this, the system is designed so that the light backscattered from the tissue is compared to a reference beam using Michelson Interferometry (For a more in-depth explanation the reader is referred to Zysk et al.69; and for a mathematical background Wang and Wu70). The result is an image depicting the location of structures within the tissue. For instance, the epidermis, dermis, and structures such as sweat glands can be identified in detail. Image resolution ranges from 1 to 10 μm, and the imaging depth is similar to RMCM and OPSI at 1–2 mm. However, the small field of view and requirement for interpretation of the detailed image could be a challenge for application of this technique in the burn care environment.
Another iteration of OCT is polarization-sensitive OCT, which, in addition to detecting the depth of the backscattered light, captures the polarization state of that light. Polarization measurements are useful because certain molecules and structures in the tissue, including glucose, collagen, myosin, and elastin, impart specific polarization signatures onto light in a process called birefringence.71 Polarization-sensitive OCT noninvasively assessing the severity of burn wounds identifies the thermal denaturation of collagen by the reduction in skin tissue birefringence.72,73 The reduced birefringence correlates to burn severity and could be a simpler metric to apply to routine burn severity assessments.
Recent efforts to extend the sampling depth of OCT have been made, and one of which is not directly OCT, but still a scatter-sensitive approach, is spectroscopic analysis of multiply scattered light. Matthews et al. designed a system that was capable of sampling up to 9 mm depth with resolution in the millimeter scale.74 Their images are cross-sections of the tissue about 13 mm wide and 9 mm deep. In addition to detecting tissue anatomy at deeper depths compared to OCT, this system also scans across various wavelengths of light allowing spectroscopic analysis of dermal anatomy. These authors report five minute image acquisition times for high-resolution images, currently a drawback for clinical use.
Photoacoustic imaging and photoacoustic microscopy
The photoacoustic effect describes the generation of ultrasonic waves by molecular interactions with light. Photoacoustic imaging (PAI) is a hybrid imaging technology, whereby an ultrasound probe is used to detect ultrasonic waves induced by laser light. A variation in the optical absorption coefficient of different tissues allows PAI to contrast different layers and constituents of the skin. Like OCT and OPSI, photoacoustic microscopy (PAM) has good resolution with the ability to see details within a living tissue. The advantage of PAM over OCT and OPSI is that it images at deeper depths, ∼1–5 mm. In vivo imaging of tissue using this technique involves a system, whereby a laser emits light into the tissue, usually at hemoglobin's peak absorption of 584 nm, and a high-speed ultrasound transducer collects the reflected ultrasonic waves. Images appear similar to ultrasound, but the areas of high signal contrast are from structures in the tissue that are highly absorptive of the emitted light. This makes PAI and PAM useful for burn imaging because they are highly sensitive to the location of hemostasis, hemorrhage, and hyperemia.75
In a pig model of 2.0 mm deep burns, PAM clearly demonstrated the zone of hyperemia surrounding the burn in a “bowl-like” shape and, therefore, is useful for determining the depth of tissue affected by thermal damage.75 Later, PAI was used to identify the severity of burns and track their healing. We can see from the images produced by this system, a high level of detail in the skin vasculature up to 3.0 mm below the surface.76 Much like OCT and confocal microscopy, PAM image analysis will require knowledge of skin microanatomy to identify vascular changes, such as hyperemia, representative of burn severity. Because the initial hyperemic response following burn injury begins beyond 72 h, future studies with PAI that focus on the time resolved hyperemic response of a burn injury would be beneficial. Alternatively, a scientific study assessing burn edema in mice highlights the potential for this technique to identify tissue components other than hemoglobin. By taking advantage of PAI's sensitivity to highly absorbing molecules, albumin in the serum can become the image contrast element in the tissue when labeled with Evans blue (EB) and the PAI system's stimulation wavelength tuned to EB's absorbance peak. Results with this method show the location and times where albumin accumulates in the burn and the potential for PAI to monitor burn-related edema.77
Photoplethysmography imaging
Photoplethysmography imaging (PPGI) derives from the optical measurement of the skin's blood volume over time. The PPG signal is generated by measuring light interaction with dynamic changes in the vascularized tissues. Vascularized tissue expands and contracts in volume by ∼1–2% with each incoming systolic blood pressure wave at the frequency of the cardiac cycle.78 This influx of blood increases the volume of the tissue and brings additional hemoglobin proteins that strongly absorb light. Therefore, the total absorbance of light within the tissue oscillates with each heartbeat. PPGI employs a digital camera to capture over a million unique PPG signals across a large area of tissue.79 This information translates into an arterial blood flow image by mapping the amplitude of each pixel's unique PPG waveform.80,81 PPGI systems detect the PPG signal at distances up to one meter, and their field of view and resolution are similar to a digital camera.
In a pig model, PPGI was able to identify the proper point of debridement, as confirmed with histological assessment, by identifying differences in PPG signal strength between viable and nonviable tissue.82 The progression of data collection began with a measurement of PPG signals in the region of interest before introduction of a burn wound. As expected, the PPG signal across the uninjured skin uniformly indicated healthy tissue. Creating a deep partial-thickness burn in the same location stymied blood flow and reduced the PPGI signal intensity in its location, while the surrounding healthy tissue still exhibited a signal consistent with uninjured tissue. PPG images ultimately revealed significant differences between the signal intensities of burned tissue and viable wound bed such that it may be valuable as a tool to indicate a properly prepared burn wound bed.
MSI and hyperspectral imaging
Wide-field spectral imaging of tissue uses the spectral remission of reflected light in the visible and near-infrared wavelengths (400–1,100 nm) from a tissue's surface. Both hyperspectral imaging (HSI) and MSI are spectrophotometry approaches where the data are collected at multiple points simultaneously from an area. HSI returns robustly sampled spectra (e.g., with reflectance sampled at 1 nm wavelength intervals) that can be advantageous to separate optically similar compounds or to identify unknown absorbers, but it is possible to use a reduced number of selected wavelengths to characterize a selected set of physiologically relevant components (e.g., hemoglobin saturation, blood volume, water, and lipid). MSI systems can be used after a fewer number of appropriate wavelengths are selected to discriminate absorption spectra of the desired tissue components. Compared to HSI systems, an MSI system typically entails higher image resolution, a greater range of equipable wavelengths spanning the entire visible and near-IR spectrum, faster image acquisition, and reduced cost.83,84
Spectral imaging was first studied in burns in 1977 using a film camera equipped with red, green, blue, and near-IR filters.85 Initial attempts to diagnose burn depth with MSI used four wavelengths of light and relied on human interpretation of the images.85 Eventually, data interpretation was relegated to computational analysis. Afromowitz et al. concluded that red, green, and near-IR light were successful in predicting burn wound healing, and by simply using the ratios of red:near-IR and green:near-IR, they could predict burns that would heal in less than 21 days with 86% accuracy.86,87 Enabled by modern computing, a more sophisticated machine learning algorithm was later employed by Eisenbeiss et al. to diagnose burn severity with the same wavelengths of light.88 In more recent studies, an HSI imager with wavelengths in the near-IR range (650–1,050 nm at 10-nm increments) showed feasibility in differentiating superficial from partial and deep burns in a small pig model. As computers have become more powerful, increasingly complex predictive models have been employed alongside better imaging technology to gain more accurate analysis of the diffuse reflectance spectrum.89–91
MSI has also been applied to identify the proper depth of burn surgery excision. In one of our recent studies using a pig model of burn excision, we demonstrated that burn-injured tissue was detectible with ∼85% accuracy and the viable wound bed to which a skin graft could be applied is detectible by MSI with 87% accuracy.82 Analysis of the spectral difference between burn-injured tissue and wound bed tissue revealed that reflectance spectra vary most significantly at wavelengths of 515, 669, 750, and 972 nm, suggesting that burn tissues' blood, extracellular matrix, and water components makeup are quite different from healthy skin.92
Spatial frequency domain imaging
This recent technique is an extension of spectral imaging, but imparts a structural pattern to the tissue illumination on the basis that the different illumination patterns provide different tissue depth sensitivities. The imaged structured light patterns result in strips of varying light intensities that look like a like a corrugated sheet.93 By shining patterns of light with different spatial frequencies on the tissue, recording images of the backscattered light, and demodulating the image sequence, this instrument can quantify the tissue's optical properties, including hemoglobin concentration, microvascular oxygen saturation (SpO2), and reduced scattering coefficient. Importantly, interpretation of the demodulated reflectance images of different spatial frequencies allows decoupling of the scattering and absorption properties, an advantage over MSI and HSI, which cannot decouple those effects. Spatial frequency domain imaging (SFDI) has some advantages over wide-field spectral imaging, including independent quantification of absorption and scattering, quantitation of hemoglobin saturation and blood volume, and analysis of multiple spatial frequencies can inform depth-dependent tomography, which has resolution limits, but is information that cannot be gained without multiple sampled length scales (e.g., spatial frequencies). However, this information currently is not available in “real time” and requires some time for acquisition of images and processing.
The information collected by SFDI is useful for identifying burn severity and tissue changes associated with burn progression during the first 3 days following the initial burn.11,94 In addition, SFDI can identify infection in burn wounds. Nguyen et al. studied SFDI to detect the presence of infection in full-thickness burns in a pig model.95 Their results revealed that the scattering coefficient and absorption coefficient of infected burns began to deviate from noninfected burns 3 days after introduction of methicillin-resistant Staphylococcus aureus (MRSA) to the burn. Benefits of this technology for burn assessment include the independent quantification of absorption and scattering and its wide scanning area that can be greater than 10 × 10 cm. Ongoing efforts are focused on acquiring and processing image sequences at or near real time.
Combination systems
Many of these systems utilize the same types of electronics and hardware for illumination and sensing. Thus, two or more techniques can be combined to produce images composed of multiple tissue optical properties. Ganapathy et al. developed a combined OCT and PSI imaging system for burn assessment.54 This combination of optical imaging techniques could be considered analogous to Doppler ultrasound, where anatomical information (analogous to OCT) is combined with blood perfusion data (analogous to PSI). In acute pig burns, the technique provided an average of 0.85 AUC (i.e., area under the receiver operator characteristic curve) in identifying superficial, partial-thickness, and full-thickness burns.
Further Understanding of Msi
All of the techniques described previously have advantages and disadvantages. However, our recent work in MSI has lead us to believe that it possesses a high likelihood of fulfilling the demanding needs of burn diagnosis from a technical, environmental, and user standpoint. MSI captures multiple independent measurements from the tissue in rapid succession and is flexible to diagnosing not just the severity of the burn but identifying many other tissues, including the viable wound bed and hyperemia. Other advantages include the following: a large and scalable field of view, rapid data collection time, highly accurate determination of burn physiology, and adaptability to multiple diagnoses across the spectrum of burn care.
MSI data analysis
Primarily, analysis of MSI data can achieve either mapping of blood oxygen concentration in the microcirculation or classification of tissue types in the image. Both methods have key clinical applications. However, each analysis is accomplished with different processing methods.
Images of tissue oxygenation
MSI can quantify the volume fraction of hemoglobin and the relative percentage of oxygenated hemoglobin.96,97 Mapping the blood oxygen concentration from MSI image data typically draws on the same optical contrast used to inform standard contact pulse oximeter electronics. Pulse oximetry relies on a variation in the absorption of light by oxyhemoglobin (HbO2) and deoxy- or carboxyhemoglobin (HbCO2). There is change in the molecular configuration of hemoglobin when bound to oxygen. This configurational change causes the absorption of light by HbCO2 to be higher in the red wavelengths (∼650 nm) and slightly lower in the near-IR wavelengths (∼950 nm) compared to HbO2. Thus, it is possible to use an MSI system to measure the ratio of absorption at the red wavelengths of light compared to the near-IR wavelengths and determine the relative percentage of hemoglobin molecules that are bound to oxygen or carbon dioxide. Unlike PPGI calculations of the intensity of the pulse waveform that take multiple seconds to acquire, calculation of SpO2 by the ratio of red:near-IR light is performed by instantaneously collecting the red and near-IR absorbance measurements together, thereby eliminating the variable of tissue blood volume that varies with each heartbeat.
Tissue classification by diffuse spectrum analysis
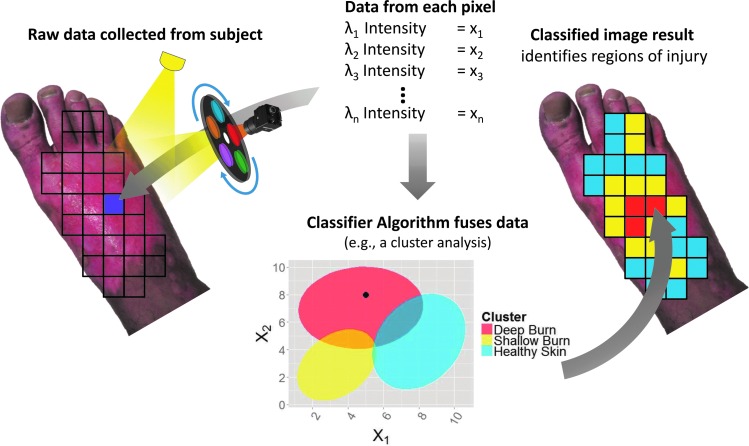
A second method to produce images from multispectral data is by comparing the tissue's diffuse reflectance spectrum to a known reference library of reflectance spectra. This technique classifies the unknown spectra as a tissue type or pathology represented in the reference library in a stochastic manner (Fig. 8). This technique is also known as computer-aided diagnosis, machine learning, or pattern recognition.98 Computer-aided diagnosis is the process by which a computer algorithm is trained to recognize patterns in new data based on similar patterns in a previously analyzed database.
Figure 8.
Application of a classification algorithm to MSI collected from one pixel. First, the multispectral data are collected for the entire area of interest. From one pixel, we obtain a low-resolution reflectance spectrum. Applying the values found at this pixel to the classifier identifies the type of tissue at that particular pixel. Once performed for each pixel of the MSI data cube, the results combine into an image. MSI, multispectral imaging. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
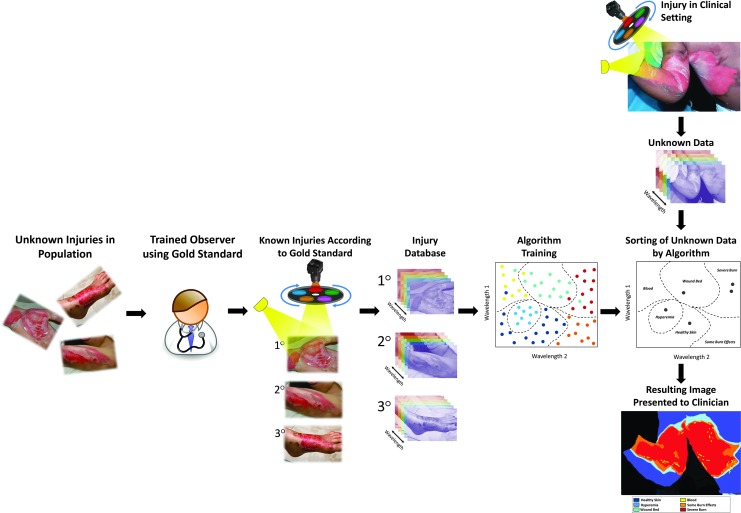
Considering the diagnosis of burn depth, developing a spectral imaging system for this purpose includes the following key steps (Fig. 9): initially, the algorithm requires “training” to recognize important spectral patterns from different burn thicknesses. An adequate sample of subjects representing the range of burn severities of interest (e.g., superficial, partial thickness, and full thickness) obtained with the MSI system is required to build a database before creating the classification algorithm. In classification by supervised learning, spectral data are organized into its proper burn category by a person using a reference called the ground truth, such as histological assessment. The ground truth reference is needed to organize and train a classification algorithm generated as well as for validating the accuracy of the algorithm. Various mathematical techniques accomplish the same classification problem, and selecting the proper technique is a matter of computational assessment. Once this is complete, the thresholds for classifying the patient data are ready and the system can begin validation. Classifier generation and validation are typically a feedback loop in the development of the algorithm, whereby the developer modifies features of the algorithm until the established accuracy reached.
Figure 9.
The development and application of an MSI-based machine learning model for burn wound classification. Horizontal arm: Steps used to build an accurate training database or library for the algorithm. Initially, a sample of the tissue classes is selected and sorted into their appropriate class using expert-guided criteria. Images of these tissues are gathered and used to populate a tissue reference training database. From these data, machine learning algorithms assign quantitative thresholds to the tissue classes of interest. Vertical arm: Once the classification algorithm is complete, the technique generates classified outputs in seconds to minutes of patients in the clinical setting. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
We recently developed an MSI device to assist burn surgeons in determining the adequacy of surgical excision. As discussed earlier, MSI captures multiple and independent measurements from the tissue in ∼2 s. Our MSI system classified six different tissue types in one burn image of a pig model of burn debridement. We found that a variety of clinically relevant burn tissues have a unique reflectance spectrum, including shallow to deep partial-thickness burns as well as hyperemia, and we also demonstrated that that the length of time since the initial injury has a significant effect on burn reflectance spectra.92 We continued to develop this technique by applying machine learning to the tissue spectral measurements. The result has been favorable in validation testing where we found an average of 87% accuracy in the classification of tissues relevant to diagnose the preparedness of a wound bed in a burn surgery animal model.82
Summary
All of the above technologies demonstrated success in burn assessment, but given the number and complexity of each system, the selection of imaging technology to augment the provision of burn care can be difficult for clinicians to navigate. Regarding the tissue area imaged, about half of these devices are able to scan large areas (>10 × 10 cm) of the body's surface, while the rest produce progressively smaller images, down to microscopic levels. Owing to the wavelength of energy used, ultrasound has the greatest depth of imaging at ∼5 cm, whereas optical methods can only penetrate up to 0.35 cm, depending on wavelength.99 Technologies that can image a large surface area quickly, like thermography, multispectral, or LSI, would be suitable for initial burn assessment and triage. While in the setting of presurgical planning, during which the surgeon has more time available to review the burn and might only need to spot check specific areas, technologies like OCT, OPSI, PAM, or ultrasound may find more usefulness.
Clearly, there are better alternatives to the most commonly used burn assessment techniques, clinical judgment and photography, but none are currently as user friendly. Burn care providers have spoken clearly that improved human factors oriented designs and user-friendliness are necessary features of any commercial burn care device.3 To this end, noncontact methods are safer and therefore more likely to be sustainable in a clinical setting. Portability, accessibility, accuracy, and cost will also play key roles in acceptance of any assistive technology.
As burn care continues to improve in the US over the last century, burn victims are surviving with more severe burns. In this day, misjudgment of the extent and depth of the burn is not a leading cause of morbidity or mortality, and only one facet of care.100 While judging burn depth is critical, and more accurate assessment should result in better outcomes and reduced cost, considering other applications where imaging devices could benefit the treatment team and patient is essential. A few of these applications include the following: earlier identification of infection during treatment and healing; determining the depth of excision to reduce the skin graft area, improve functional outcomes, or reduce scarring for better cosmetic outcomes; the ability to track burn and devices that improve the effectiveness of nonburn-specialized care providers. In particular, a few techniques not discussed in this review, fluorescence imaging for detection of wound infection is emerging onto the wound care market.101 In addition, research in the Terahertz regime of the electromagnetic spectrum demonstrates the ability to image a burn without removing wound dressings.102
One important future development for burn depth imaging is predicting the conversion of healthy tissue to necrosis or apoptosis owing to burn progression. Techniques that may be useful to predict the extent of burn conversion would be able to detect features in the zone of stasis, including inflammation, edema, and ischemia. Correlating imaging measurements to these pathological processes at different times after burn injury will be a major effort in future imaging studies to help physicians make faster decisions to operate and increase tissue sparing.
Take-Home Messages.
• Before the patient is transferred to a burn center, a physician or nurse with relatively little experience in burn wounds initially assesses burn size and depth with only 60% and 51% accuracy, respectively.
• Clinical judgment alone is the standard of care in burn depth assessment with a reported accuracy by experts between 70–80%.
• Imaging technologies report greater accuracy in burn depth assessment (>80%) compared to clinical judgment alone.
• The clinical setting requires much from burn diagnostics to gain adoption including the following: ease-of-use; ability to assess large burn areas quickly; and high accuracy.
• Optical methods comprise the majority of recent advancements in burn imaging, and light can penetrate deeply enough into the skin to make assessments on burn severity and healing.
• Imaging techniques measure anatomic or physiologic parameters of the tissue, but seldom both. Therefore, knowledge of the appropriate imaging technology, when it is best applied, and how to interpret results is necessary for imaging to provide assistance to the clinician.
• Imaging technologies for burn depth assessments, infection monitoring, and diagnosing the preparedness of wound bed for grafting are supported by preclinical studies, clinical studies, or both.
• Technologies that can image a large surface area quickly, like thermography or LSI, would be suitable for initial burn assessment and triage.
• In the setting of presurgical planning, ultrasound or optical microscopy techniques may be more useful.
• An emerging technique in medicine, MSI, is capable of providing clinicians with images that depict the presence and severity of burns in a wide field of view. MSI has demonstrated promising accuracy in preclinical studies.
• Computer-assisted diagnosis of burns with MSI technology will require clinical trials to build reliable reference libraries before these devices can be utilized in routine care.
Abbreviations and Acronyms
- μa
absorption coefficient
- μs/
scattering coefficient
- 2D
two dimensional
- 3D
three dimensional
- ADT
active dynamic thermography
- AUC
area under the receiver-operator characteristic curve
- EB
Evans blue
- HbCO2
carboxyhemoglobin
- HbO2
oxyhemoglobin
- HSI
hyperspectral imaging
- ICG
indocyanine green
- LDI
laser Doppler imaging
- LSI
laser speckle imaging
- MRSA
methicillin-resistant Staphylococcus aureus
- MSI
multispectral imaging
- n
refractive index
- OCT
optical coherence tomography
- OPSI
orthogonal polarization spectral imaging
- PAI
photoacoustic imaging
- PAM
photoacoustic microscopy
- PPGI
photoplethysmography imaging
- RMCM
reflectance-mode confocal microscopy
- SFDI
spatial frequency domain imaging
- SpO2
oxygen saturation
- TBSA
total body surface area
- W
watts
Acknowledgments and Funding Sources
We sincerely thank Professor James H. Holmes, MD of Wake Forest, and Professor Steven E. Wolf, MD of UT Southwestern Medical Center for their detailed discussions about the burn care workflow. This work was supported, in part, by the Biomedical Advanced Research and Development Authority (BARDA).
Author Disclosure and Ghostwriting
J.E. Thatcher and J.M. DiMaio receive salary from Spectral MD, Inc. and have ownership in Spectral MD, Inc. through stock. J.J. Squiers, D.R. King, Y. Lu, and E.W. Selke receive salary from Spectral MD, Inc.
About the Authors
Jeffrey E. Thatcher, PhD, is the Chief Scientist at Spectral MD, Inc. where he oversees technology and applications research for medical imaging systems. His interests are in translational research of noninvasive imaging for wound care. His work in burn imaging was recognized by the 2015 Burke/Yannas award for original research in biomedical engineering from the American Burn Association. John J. Squiers, BSE, focuses on clinical applications and clinical trial design as Clinical Specialist at Spectral MD, Inc. Stephen C. Kanick, PhD, is an Assistant Professor of Engineering at Dartmouth where his research focuses on developing biophotonics for better methods to diagnose and treat cancer. Darlene R. King is a biomedical engineer at Spectral MD, Inc. Yang Lu, PhD, is an algorithm development engineer at Spectral MD, Inc. Yulin Wang, PhD, is a human factors engineer at Spectral MD, Inc. Rachit Mohan is a student at Columbia University and recently completed an internship with Spectral MD, Inc. Eric W. Sellke, BS, is a biomedical engineer at Spectral MD, Inc. J. Michael DiMaio, MD, is the founder and CEO of Spectral MD, Inc. He received his MD from the University of Miami and completed his thoracic surgery residency at Duke. He serves as the Director of Postgraduate Education and Publications at Baylor Research Institute.
References
- 1.Pape S, Skouras C, Byrne P. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 2000;27:233–239 [DOI] [PubMed] [Google Scholar]
- 2.Nichter L, Williams J, Bryant C, Edlich R. Improving the accuracy of burn-surface estimation. Plast Reconstr Surg 1985;76:428–433 [DOI] [PubMed] [Google Scholar]
- 3.Resch T, Drake R, Helmer S, Jost G, Osland J. Estimation of burn depth at burn centers in the United States: a survey. J Burn Care Res 2014;35:491–497 [DOI] [PubMed] [Google Scholar]
- 4.Kaczmarek M, Nowakowski A, Renkielska A. Rating burn wounds by dynamic thermography. Quant Infrared Thermogr J 2000;5:376–381 [Google Scholar]
- 5.Wang L. Optical Science and Engineering: Photoacoustic Imaging and Spectroscopy, Boca Raton, FL: CRC Press, 2009 [Google Scholar]
- 6.Shupp J, Nasabzadeh T, Rosenthal D, Jordan M, Fidler P, Jeng J. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–873 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka R, Fukushima S, Sasaki K, et al. . In vivo visualization of dermal collagen fiber in skin burn by collagen-sensitive second-harmonic-generation microscopy. J Biomed Opt 2013;18:61231. [DOI] [PubMed] [Google Scholar]
- 8.Papp A, Kiraly K, Härmä M, Lahtinen T, Uusaro A, Alhava E. The progression of burn depth in experimental burns: a histological and methodological study. Burns 2004;30:684–690 [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw J. Progressive changes in the depth of burns. JAMA 1963;186:169. [DOI] [PubMed] [Google Scholar]
- 10.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol 2010;19:777–783 [DOI] [PubMed] [Google Scholar]
- 11.Burmeister D, Ponticorvo A, Yang B, et al. . Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model. Burns 2015;41:1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari V. Burn wound: How it differs from other wounds? Indian J Plast Surg 2012;45:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques S. Optical properties of biological tissues: a review. Phys Med Biol 2013;58:R37–R61 [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Pujols S, Thompson K, Sheldon G, Fraher E, Ricketts T, Cairns BA. Burn care: are there sufficient providers and facilities? Bull Am Coll Surg 2011;96:33–37 [PubMed] [Google Scholar]
- 15.Boccara D, Chaouat M, Uzan C, Lachere A, Mimoun M. Retrospective analysis of photographic evaluation of burn depth. Burns 2011;37:69–73 [DOI] [PubMed] [Google Scholar]
- 16.Jones O. Measurements of the clinical competence of doctors and nurses to process telemedicine referrals for burns patients. J Telemed Telecare 2005;11:89–90 [DOI] [PubMed] [Google Scholar]
- 17.Jones O, Wilson D, Andrews S. The reliability of digital images when used to assess burn wounds. J Telemed Telecare 2003;9:S22–S24 [DOI] [PubMed] [Google Scholar]
- 18.Kiser M, Beijer G, Mjuweni S, Muyco A, Cairns B, Charles A. Photographic assessment of burn wounds: a simple strategy in a resource-poor setting. Burns 2013;39:155–161 [DOI] [PubMed] [Google Scholar]
- 19.Roa L, Gomez-Cia T, Acha B, Serrano C. Digital imaging in remote diagnosis of burns. Burns 1999;25:617–623 [DOI] [PubMed] [Google Scholar]
- 20.Wallace D, Jones S, Milroy C, Pickford M. Telemedicine for acute plastic surgical trauma and burns. J Plast Reconstr Aesthet Surg 2008;61:31–36 [DOI] [PubMed] [Google Scholar]
- 21.Shokrollahi K, Sayed M, Dickson W, Potokar T. Mobile phones for the assessment of burns: we have the technology. Emerg Med J 2007;24:753–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hop M, Moues C, Bogomolova K, et al. . Photographic assessment of burn size and depth: reliability and validity. J Wound Care 2014;23:144–152 [DOI] [PubMed] [Google Scholar]
- 23.Berkebile B, Goldfarb I, Slater H. Comparison of burn size estimates between prehospital reports and burn center evaluations. J Burn Care Rehabil 1986;7:411–412 [DOI] [PubMed] [Google Scholar]
- 24.Wachtel T, Berry C, Wachtel E, Frank H. The interrater reliability of estimating the size of burns from various burn area chart drawings. Burns 2000;26:156–170 [DOI] [PubMed] [Google Scholar]
- 25.Hlava P, Moserova J, Konigova R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast 1983;25:202–208 [PubMed] [Google Scholar]
- 26.Jaskille A, Shupp J, Jordan M, Jeng J. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res 2009;30:937–947 [DOI] [PubMed] [Google Scholar]
- 27.Boissin C, Laflamme L, Wallis L, Fleming J, Hasselberg M. Photograph-based diagnosis of burns in patients with dark-skin types: the importance of case and assessor characteristics. Burns 2015;41:1253–1260 [DOI] [PubMed] [Google Scholar]
- 28.Lawson R, Gaston J. Temperature measurements of localized pathologicl processes. Ann N Y Acad Sci 1964;121:90–98 [DOI] [PubMed] [Google Scholar]
- 29.Mladick R, Goergiade N, Thorne F. A clinical evaluation of the use of thermography in determining degree of burn injury. Plast Reconstr Surg 1966;38:512–518 [DOI] [PubMed] [Google Scholar]
- 30.Watson A, Vasilescu C. Thermography in plastic surgery. J R Coll Surg Edinb 1972;17:247–252 [PubMed] [Google Scholar]
- 31.Hackett M. The use of thermography in the assessment of depth of burn and blood bupply of flaps, with preliminary reports on its use in Dupuytren's contracture and treatment of varicose ulcers. Br J Plast Surg 1974;27:311–317 [DOI] [PubMed] [Google Scholar]
- 32.Liddington M, Shakespeare P. Timing of the thermographic assessment of burns. Burns 1996;22:26–28 [DOI] [PubMed] [Google Scholar]
- 33.Anselmo V, Zawacki B. Infra-red photography as a diagnostic tool for the burn ward. Proc Soc Photo Opt Instr Eng 1973;8:181 [Google Scholar]
- 34.Renkielska A, Nowakowski A, Kaczmarek M, Ruminksi J. Burn depth evaluation based on active dynamic IR thermal imaging-a preliminary study. Burns 2006;32:867–875 [DOI] [PubMed] [Google Scholar]
- 35.Prindeze N, Fathi P, Mino M, et al. . Examination of the early diagnostic applicability of active dynamic thermography for burn wound depth assessment and concept analysis. J Burn Care Res 2015;36:626–635 [DOI] [PubMed] [Google Scholar]
- 36.Kaiser M, Yafi A, Cinat M, Choi B, Durkin A. Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities. Burns 2011;37:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerath M, Schomacker K, Sheridan R, Nishioka N. Burn wound assessment in porcine skin using indocyanine green fluorescence. J Trauma 1999;46:1085–1088 [DOI] [PubMed] [Google Scholar]
- 38.Kamolz L, Andel H, Haslik W, et al. . Indocynanine green video angiographies help to identify burns requiring operation. Burns 2003;29:785–791 [DOI] [PubMed] [Google Scholar]
- 39.Braue E, Graham J, Doxzon B, et al. . Noninvasive methods for determining lesion depth from vesicant exposure. J Burn Care Res 2007;28:275–285 [DOI] [PubMed] [Google Scholar]
- 40.Still J, Law E, Klavuhn K, et al. . Diagnosis of burn depth using laser induced indocyanine green fluorescence: a preliminary clinical trial. Burns 2011;27:364–371 [DOI] [PubMed] [Google Scholar]
- 41.Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and review of the literature. Cathet Cardiovasc Diagn 1989;17:231–233 [DOI] [PubMed] [Google Scholar]
- 42.Stern M. In vivo evaluation of microcirculation by coherent light scattering. Nature 1975;245:56–58 [DOI] [PubMed] [Google Scholar]
- 43.Jaskille A, Ramella-Roman J, Shupp J, Jordan M, Jeng J. Critical review of burn depth assessment techniques: part II. Review of laser Doppler technology. J Burn Care Res 2010;31:151–157 [DOI] [PubMed] [Google Scholar]
- 44.Briers J. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas 2001;22:R35–R66 [DOI] [PubMed] [Google Scholar]
- 45.Niazi Z, Essex T, Papini R, Scott D, McLean N, Black M. New laser Doppler scanner, a valuable adjunct in burn depth assessment. Burns 1993;19:485–489 [DOI] [PubMed] [Google Scholar]
- 46.Jeng J, Bridgeman A, Shivnan L, et al. . Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial. Burns 2003;29:665–670 [DOI] [PubMed] [Google Scholar]
- 47.McGill D, Sorensen K, MacKay I, Taggart I, Watson S. Assessment of burn depth; a prospective. Blinded comparisons of laser Doppler imaging and videomicroscopy. Burns 2007;33:833–842 [DOI] [PubMed] [Google Scholar]
- 48.Holland A, Ward D, Farrell B. The influence of burn wound dressings on laser Doppler imaging assessment of a standardized cutaneous injury model. J Burn Care Res 2007;28:871–878 [DOI] [PubMed] [Google Scholar]
- 49.Crouzet C, Nguyen J, Ponticorvo A, Bernal N, Durkin A, Choi B. Acute discrimination between superficial-partial and deep-partial thickness burns in a preclinical model with laser speckle imaging. Burns 2015;41:1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roustit M, Cracowski J. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 2012;19:47–64 [DOI] [PubMed] [Google Scholar]
- 51.Boas D, Dunn A. Laser speckle contrast imaging in biomedical optics. J Biomed Opt 2010;15:011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steward C, Gallant-Behm C, Forrester K, Tulip J, Hard D, Bray R. Kinetics of blood flow during healing of excisional full-thickness skin wounds in pigs as monitored by laser speckle perfusion imaging. Skin Res Technol 2006;12:247–253 [DOI] [PubMed] [Google Scholar]
- 53.Lindahl F, Tesselaar E, Sjoberg F. Assessing paediatric injuries using laser speckle contrast imaging. Burns 2013;39:662–666 [DOI] [PubMed] [Google Scholar]
- 54.Ganapathy P, Tamminedi T, Qin Y, et al. . Dual-imaging system for burn depth diagnosis. Burns 2014;40:67–81 [DOI] [PubMed] [Google Scholar]
- 55.Calzavara-Pinton P, Longo C, Venturini M, Sala R, Pellacani G. Reflectance confocal microscopy for in vivo skin imaging. Photochem Photobiol 2008;84:1421–1430 [DOI] [PubMed] [Google Scholar]
- 56.Rajadhyaksha M, Gonzalez S, Zavislan J, Anderson R, Webb R. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol 1999;113:293–303 [DOI] [PubMed] [Google Scholar]
- 57.Kaiser M, Yafi A, Cinat M, et al. . Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities. Burns 2011;37:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altintas A, Altintas M, Ipaktchi K, et al. . Assessment of microcirculatory influence on cellular morpohology in human burn wound healing using reflectance-mode-confocal microscopy. Wound Repair Regen 2009;17:498–504 [DOI] [PubMed] [Google Scholar]
- 59.Goans R, Cantrell J, Jr., Meyers F. Ultrasonic pulse-echo determination of thermal injury in deep dermal burns. Med Phys 1977;4:259–263 [DOI] [PubMed] [Google Scholar]
- 60.Kalus A, Aindow J, Cauldfield M. Application of ultrasound in assessing burn depth. Lancet 1979;27:188–189 [DOI] [PubMed] [Google Scholar]
- 61.Wachtel T, Leopold G, Frank H, Frank D. B-mode ultrasonic echo determination of depth of thermal injury. Burns Inc Therm Inj 1986;12:432–437 [DOI] [PubMed] [Google Scholar]
- 62.Brink J, Sheets P, Dines K, Etchison M, Hanke C, Sadove A. Quantitative assessment of burn injury in porcine skin with high-frequency ultrasonic imaging. Invest Radiol 1986;21:645–651 [DOI] [PubMed] [Google Scholar]
- 63.Gnyawali S, Barki K, Mathew-Steiner S, et al. . High-resolution harmonics ultrasound imaging for non-invasive characterization of wound healing in a pre-clinical swine model. PLoS One 2015;10:e0122327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster F, Zhang M, Zhou Y, et al. . A new ultrasound instrument for in vivo microimaging of mice. Ultrasound Med Biol 2002;28:1165–1172 [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Mill J, Kravchuk O, Kimble R. Ultrasound assessed thickness of burn scars in association with laser Doppler imaging determined depth of burns in paediatric patients. Burns 2010;36:1254–1262 [DOI] [PubMed] [Google Scholar]
- 66.Iraniha S, Cinat M, VanderKam V, et al. . Determination of burn depth with noncontact ultrasonography. J Burn Care Rehabil 2000;21:333–338 [DOI] [PubMed] [Google Scholar]
- 67.Monstrey S, Hoeksema H, Verbelen J, Pirayesh Blondeel P. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761–769 [DOI] [PubMed] [Google Scholar]
- 68.Milner S, Bhat S, Gulati S, Gherardini G, Smith C, Bick R. Observations on the microcirculation of the human burn wound using orthogonal polarization spectral imaging. Burns 2005;31:316–319 [DOI] [PubMed] [Google Scholar]
- 69.Zysk A, Nguyen F, Oldenburg A, Marks D, Boppart S. Optical coherence tomography: a review of clinical development from bench to bedside. J Biomed Opt 2007;12:051403. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Wu H. Biomedical Optics: Principles and Imaging. Hoboken, NJ: John Wiley & Sons, Inc., 2007 [Google Scholar]
- 71.Ghosh N, Vitkin I. Tissue polarimetry: concepts, challenges, applications, and outlook. J Biomed Opt 2011;16:110801. [DOI] [PubMed] [Google Scholar]
- 72.Pierce M, Sheridan R, Park B, Cense B, de Boer J. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography. Burns 2004;30:511–517 [DOI] [PubMed] [Google Scholar]
- 73.Kim K, Pierce M, Maguluri G, et al. . In vivo imaging of human burn injuries with polarization-sensitive optical coherence tomography. J Biomed Opt 2012;17:0660102. [DOI] [PubMed] [Google Scholar]
- 74.Matthews T, Medina M, Maher J, Levinson H, Brown W, Wax A. Deep tissue imaging using spectroscopic analysis of multiply scattered light. Optica 2014;1:105–111 [Google Scholar]
- 75.Zhang H, Stoica G, Wang L. Imaging acute thermal burns by photoacoustic microscopy. J Biomed Opt 2006;11:054033. [DOI] [PubMed] [Google Scholar]
- 76.Ida T, Kawaguchi Y, Kawauchi S, et al. . Real-time photoacoustic imaging system for burn diagnosis. J Biomed Opt 2014;19:086013. [DOI] [PubMed] [Google Scholar]
- 77.Tsunoi Y, Sato S, Kawauchi S, Ashida H, Saitoh D, Terakawa M. In vivo photoacoustic molecular imaging of the distribution of serum albumin in rat burned skin. Burns 2013;39:1403–1408 [DOI] [PubMed] [Google Scholar]
- 78.Webster J. Design of Pulse Oximeters, Medical Science Series. New York: Institute of Physics Publishing, 1997 [Google Scholar]
- 79.Severinghaus J, Honda Y. History of blood gas analysis. VII. Pulse oximetry. J Clin Monit 1987;2:3. [DOI] [PubMed] [Google Scholar]
- 80.Thatcher J, Plant K, King D, Block K, Fan W, DiMaio J. Dynamic tissue phantoms and their use in assessment of a non-invasive optical plethysmography imaging device. Proceedings of SPIE 9107, Smart Biomedical and Physiological Sensor Technology XI 2014:910718 [Google Scholar]
- 81.Mo W, Mohan R, Li W, et al. . The importance of illumination in a non-contact photoplethysmography imaging system for burn wound assessment. In Proceedings of SPIE 9303, Photonic Therapeutics and Diagnostics XI San Francisco, CA, 2015:93030M [Google Scholar]
- 82.Thatcher J, Li W, Rodriguez-Vaqueiro Y, et al. . Multispectral and photoplethysmography optical imaging techniques identify important tissue characteristics in an anima. J Burn Care Res 2016;37:38–52 [DOI] [PubMed] [Google Scholar]
- 83.Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt 2014;19:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, He X, Wang Y, Liu H, Xu D, Guo F. Review of spectral imaging technology in biomedical engineering: achievements and challenges. J Biomed Opt 2013;18:100901. [DOI] [PubMed] [Google Scholar]
- 85.Anselmo V, Zawacki B. Multispectral photographic analysis. A new quantitative tool to assist in the early diagnosis of thermal burn depth. Ann Biomed Eng 1977;5:179–193 [DOI] [PubMed] [Google Scholar]
- 86.Afromowitz M, Van Liew G, Heimbach D. Clinical evaluation of burn injuries using an optical reflectance technique. IEEE Transactions on Biomedical Engineering Vols, BME-34:1987 [DOI] [PubMed]
- 87.Afromowitz M, Callis J, Heimbach D, DeSoto L. Norton M. Multispectral imaging of burn wounds: a new clinical instrument for evaluating burn depth. IEEE Trans Biomed Eng 1988;35:842–850 [DOI] [PubMed] [Google Scholar]
- 88.Eisenbeiss W, Marotz J, Schrade J. Reflection-optical multispectral imaging method for objective determination of burn depth. Burns 1999;25:697–704 [DOI] [PubMed] [Google Scholar]
- 89.Calin M, Parasca S, Savastru R, Manea D. Characterization of burns using hyperspectral imaging technique—a preliminary study. Burns 2015;41:118–124 [DOI] [PubMed] [Google Scholar]
- 90.Yeong E, Hsiao T, Chiang H, Lin C. Prediction of burn healing time using artificial neural networks and reflectance spectrometer. Burns 2005;31:415–420 [DOI] [PubMed] [Google Scholar]
- 91.Li W, Mo W, Zhang X, et al. . Outlier detection and removal improves accuracy of machine learning approach to multispectral burn diagnostic imaging. J Biomed Opt 2015;20:121305. [DOI] [PubMed] [Google Scholar]
- 92.King D, Li W, Squiers J, et al. . Surgical wound debridement sequentially characterized in a porcine burn model with multispectral imaging. Burns 2015;41:1478–1487 [DOI] [PubMed] [Google Scholar]
- 93.Cuccia D, Bevilacqua F, Durkin A, Ayers F, Tromberg B. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt 2009;14:024012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen J, Crouzet C, Mai T, et al. . Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt 2013;18:66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen T, Ramella-Roman J, Moffatt L, Ortiz R, Jordan M, Shupp J. Novel application of a spatial frequency domain imaging system to determine signature spectral differences between infected and noninfected. J Burn Care Res 2013;34:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jolivot R, Benezeth Y, Marzani F. Skin parameter map retrieval from a dedicated multispectral imaging system applied to dermatology/cosmetology. Int J Biomed Imaging 2013;2013:Article ID 978289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zonios G, Bykowski J, Kollias N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Dermatol 2001;117:1452–1457 [DOI] [PubMed] [Google Scholar]
- 98.Liu N, Salinas J. Machine learning in burn care and research: a systematic review of the literature. Burns 2015;41:1636–1641 [DOI] [PubMed] [Google Scholar]
- 99.Bashkatov A, Genina E, Kochubey V, Tuchin V. Optical properties of human skin subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J Phys D Appl Phys 2005;38:2543–2555 [Google Scholar]
- 100.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DaCosta R, Kulbatski I, Lindvere-Teene L, et al. . Point-of-care autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: first-in-human results. PLoS One 2015;10:e0116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor Z, Singh R, Culjat M, et al. . Reflective terahertz imaging of porcine skin burns. Opt Lett 2008;33:1258–1260 [DOI] [PubMed] [Google Scholar]