Abstract
Pluripotent stem cell-derived cardiomyocytes (CMs) have great potential in the development of new therapies for cardiovascular disease. In particular, human induced pluripotent stem cells (iPSCs) may prove especially advantageous due to their pluripotency, their self-renewal potential, and their ability to create patient-specific cell lines. Unfortunately, pluripotent stem cell-derived CMs are immature, with characteristics more closely resembling fetal CMs than adult CMs, and this immaturity has limited their use in drug screening and cell-based therapies. Extracellular matrix (ECM) influences cellular behavior and maturation, as does the geometry of the environment—two-dimensional (2D) versus three-dimensional (3D). We therefore tested the hypothesis that native cardiac ECM and 3D cultures might enhance the maturation of iPSC-derived CMs in vitro. We demonstrate that maturation of iPSC-derived CMs was enhanced when cells were seeded into a 3D cardiac ECM scaffold, compared with 2D culture. 3D cardiac ECM promoted increased expression of calcium-handling genes, Junctin, CaV1.2, NCX1, HCN4, SERCA2a, Triadin, and CASQ2. Consistent with this, we find that iPSC-derived CMs in 3D adult cardiac ECM show increased calcium signaling (amplitude) and kinetics (maximum upstroke and downstroke) compared with cells in 2D. Cells in 3D culture were also more responsive to caffeine, likely reflecting an increased availability of calcium in the sarcoplasmic reticulum. Taken together, these studies provide novel strategies for maturing iPSC-derived CMs that may have applications in drug screening and transplantation therapies to treat heart disease.
Introduction
Cardiovascular disease is the number one cause of death in the United States and most of the developed world. It most commonly manifests as a myocardial infarction, an ischemic event in the heart that results in the death of millions to billions of cardiomyocytes (CMs). The loss of CMs can impair heart function, and repair is limited due to the extremely low proliferation rate of endogenous CMs. Compounding the insult, damaged tissue undergoes fibrosis, which increases the risk of arrhythmia and subsequent heart failure. There is no available drug treatment that can regenerate the lost myocardium, resulting in a critical need to find new treatment strategies.
Recent studies have focused on the potential regenerative capacity of exogenous CMs as a novel treatment following myocardial infarction and, in particular, on the derivation of these from induced pluripotent stem cells (iPSCs) generated from the patient's own cells. Several studies report transplantation of human embryonic stem cell (hESC) and iPSC-derived CMs into ischemic hearts, largely in mouse and rat, where improvements in heart function have been observed.1–8
A limitation to this approach is that pluripotent stem cell-derived CMs generated through current methods are immature, displaying characteristics of fetal CMs. Specifically, the cells show disorganized sarcomere structures, weak force contraction, automaticity (spontaneous beating), improper calcium handling and electrophysiological signaling, low expression of critical cardiac proteins, and altered responses to drugs when compared with mature CMs.9 Not surprisingly then, there is growing evidence that mature CMs outperform immature CMs for tissue replacement strategies and screening of novel pharmacologic compounds—they produce stronger force contraction and show appropriate calcium handling and electrophysiology.10–12 In addition, the loss of automaticity seen in mature CMs (which require pacemaker cells to trigger beating) will greatly improve safety by eliminating potential arrhythmic events. Last, the use of mature CMs for drug screening has clear advantages over the use of immature cells for predicting efficacy and potential side effects.13 It is clear therefore that strategies are needed that promote more efficient generation of mature CMs from iPSCs.
Several methods have been developed to induce maturation of pluripotent stem cell derived-CMs, including exposure to triiodothyronine (T3 thyroid hormone),11 exposure to non-CM cells,14 mechanical stress,15 or microRNA,16 as well as overexpression of potassium channels17 and long-term culture.10 In addition, there is also evidence that extracellular matrix (ECM) can affect both cell and organ maturation.18–25 Importantly, two-dimensional (2D) culturing on porcine cardiac ECM affects rat and human CM development and maturation,26–28 and cardiac ECM can have therapeutic effects when injected into the heart after a myocardial infarction.29 Studies have shown that fetal and adult cardiac ECMs have different effects on rat neonatal CM proliferation30; however, whether this is true for human iPSC-derived CMs is not known. It is also unclear how a 2D versus a three-dimensional (3D) growth environment might influence CM maturation or whether matrix from fetal versus adult heart might make a difference. In this study, we compare the effects of 2D versus 3D and fetal versus adult matrix on the maturation of iPSC-derived CMs, and find that both source and geometry of the matrix are important.
Materials and Methods
iPSC maintenance and cardiac differentiation
All experiments were performed with approval from UC Irvine's Human Stem Cell Research Oversight Committee and used the human WTC-11 GCaMP iPSC line, which was a gift from Dr. Bruce Conklin (UCSF).31 The WTC-11 iPSC line was derived from a healthy male volunteer with a normal electrocardiogram and no known family history of cardiac disease. The GCaMP iPSC line was generated using nuclease-mediated (TALEN) introduction of GCaMP6f,32 and the resulting cell line reports calcium fluxes through GFP fluorescence. The iPSCs were maintained on Growth Factor-Reduced Matrigel (Corning, Salt Lake City, UT) and fed daily with mTeSR1 medium (StemCell Technologies, Vancouver, Canada). To induce CM differentiation, iPSCs were seeded as single cells into 12-well plates coated with 1 mg Matrigel Growth Factor Reduced (#354230; BD) in the presence of 10 μM Y-27632 (Ascent, Cambridge, MA). Cardiac induction was initiated 2–4 days later (designated as Day 0), when cells were 75–90% confluent by the addition of glycogen synthase kinase 3 inhibitor (CHIR99021, 6 μM; Tocris, Inc., Bristol, United Kingdom) in RPMI/B27 (without insulin; Life Technologies, Carlsbad, CA).33 Twenty-four hours later (Day 1), the medium was changed to fresh RPMI/B27 (without insulin). On Day 3, Wnt inhibitor (IWP2, 5 μM; Tocris) was added in RPMI/B27 (without insulin), and at Day 5, cells were fed fresh RPMI/B27 (without insulin). The cells were subsequently fed RPMI/B27 (with insulin) (Life Technologies) every 3 days starting at Day 7. Cells began to spontaneously beat by Day 12.
Cardiac tissue decellularization and ECM generation
Bovine adult and fetal hearts were purchased from Sierra Medical. Before decellularization, the tissue (100–200 g) was processed by removing all excess fat and stored at −80°C for at least 16 h. Next, the tissue was cut into 3 mm3-sized pieces and subjected to continuous stirring at 330 RPM during incubation in the following solutions at room temperature: ddH20 for 30 min, 2× phosphate-buffered saline (PBS) for 15 min, sodium dodecyl sulfate (SDS) (1% for adult tissue and 0.5% for fetal tissue; Bio-Rad) for 72 h, and 1% Triton for 90 min. This was followed by seven washes in ddH20 for 30 min each, and 18 h in PBS with antibiotic–antimycotic (Life Technologies) at 4°C.
Decellularized tissues were lyophilized and then milled using a cheese grater (Amazon.com, Seattle, WA) to create a fine powder. The ECM powder was digested with 2 mg/mL pepsin (Sigma-Aldrich, St. Louis, MO) diluted in 0.01 M cell culture grade hydrochloric acid (HCl). Approximately 200 mg of cardiac ECM was added to 20 mg of pepsin and continuously stirred at 330 RPM at room temperature for 48–72 h or until fully digested. The digested ECM was divided into 1 mL aliquots and stored at −80°C until needed.
Multiphoton imaging and fiber analysis
Fibrillar collagen can be identified by second-harmonic generation (SHG) signals when incident light interacts with its noncentrosymmetric structure, and SHG microscopy has been widely used to study collagen fibers in vivo and in vitro.34–36 In addition, elastin fibers are autofluorescent due to elastin's tricarboxilic, triamino pyridinium derivatives, and are therefore visualized using multiphoton microscopy.37
Imaging was performed in the Laboratory for Fluorescence Dynamics (LFD) at UCI.38 A Ti:Sapphire laser (Mai Tai; Spectra Physics, Irvine, CA) was used for two-photon fluorescence excitation with a wavelength of 740 nm (SHG) and 860 nm (autofluorescence) with an incident power of 20 mW. The signal was collected using a water objective with a long working distance (LUMPlanFl 40 × /0.80 W; Olympus, Tokyo, Japan). The SHG signal was obtained using a 320–390 nm bandpass filter. The autofluorescent signal was collected using a 500 nm long-pass filter. Images were captured every 5 μm through the 3D scaffolds with a field of view of 8464 μm2. Data acquisition was performed in SimFCS (software developed by the LFD). The images were analyzed using ctFIRE MATLAB (http://loci.wisc.edu/software/ctfire) by extracting information corresponding to the fiber width and number. The data were collected from three separate z-stacks for each sample.
Statistical analysis
Split-Unit ANOVA was used to analyze quantitative reverse transcription polymerase chain reaction (qRT-PCR) data using R analysis (δp > 0.05, δδp > 0.01). Figures show the standard error of the mean with n = 3 unless otherwise stated. Student's t-test was performed on GCaMP calcium transients and standard error of the mean is shown (***p < 0.001, **p < 0.01, *p < 0.05).
Additional information and explanation of methods can be found in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/tea).
Results
Characterization of ECM from decellularized adult and fetal bovine cardiac tissue
To investigate the potential differences between fetal and adult cardiac ECMs and their respective contributions to human iPSC-derived CM maturation, we isolated cardiac ECM through SDS- and Triton-X100 detergent-mediated decellularization of fetal and adult bovine heart muscle. We found that similar polymerization characteristics could be obtained using 1% SDS for the adult tissue and 0.5% SDS for the fetal tissue (Supplementary Table S1). Importantly, the fetal cardiac ECM decellularized with either 0.5% or 1% SDS induced a similar gene expression profile compared with adult cardiac ECM when used in 2D assays (Supplementary Fig. S1). H&E staining confirmed the removal of cells during the decellularization process (Fig. 1A–D).
FIG. 1.
Characterization of cardiac ECM from decellularized bovine fetal and adult heart tissue. Decellularized adult bovine cardiac tissue has a more prominent fibrillar structure than fetal cardiac tissue. (A, C, E, and G) Fetal heart tissue. (B, D, F, and H) Adult heart tissue. (A, B) Control tissue. (C–H) Decellularized tissue. (E, F) Elastin fibers visualized by autofluorescence at 860 nm. (G, H) Collagen fibers visualized by second-harmonic generation at 740 nm. (I) Quantification of the number of collagen fibers comparing fetal and adult decellularized cardiac tissue. (J) Collagen fiber width comparing fetal and adult decellularized cardiac tissue. Results are expressed as mean ± standard error (n = 3; *p < 0.05, **p < 0.01, Student's t-test). “#” refers to number of collagen fibers per field of view (FOV). ECM, extracellular matrix. Color images available online at www.liebertpub.com/tea
The protein compositions of fetal and adult cardiac ECMs were characterized by nano-LC MS/MS. At the score thresholds employed (Supplementary Table S2),55–57 the prominent proteins were fibrinogen, collagen, periostin, fibrillin, fibulin, fibronectin, and other matrix proteins (Supplementary Table S2). The fetal cardiac ECM samples contained two unique proteins not found in the adult samples, mimecan and versican (Supplementary Table S2). To examine the structure and distribution of collagen and elastin within the ECM, we used SHG to detect collagen fibers and autofluorescence to detect elastin fibers. The elastin fibers of the fetal ECM, before pepsin digestion, were more evenly distributed, while there were more defined organized bundles in the adult ECM (Fig. 1E, F). In contrast to fetal ECM, the decellularized adult cardiac ECM displayed a higher collagen fiber signal intensity and larger bundles evident by increased fiber width and number (Fig. 1G–J). Hydrogels were formed with the adult and fetal cardiac ECMs, and storage modulus (G′) and loss modulus (G″) were measured by parallel plate rheology. The adult cardiac ECM (67.5 ± 12.6 Pa) was ∼10-fold stiffer than fetal cardiac ECM (7.2 ± 3.2 Pa) (Table 1 and Supplementary Fig. S2).
Table 1.
Mechanical Properties of Fetal and Adult Cardiac ECM Hydrogels
| Source (3D hydrogel) | Storage modulus (G′, Pa) | Loss modulus (G″, Pa) |
|---|---|---|
| Fetal (n = 3) | 7.2 ± 3.2 | 1.3 ± 0.58 |
| Adult (n = 3) | 67.5 ± 12.6 | 26.2 ± 14.1 |
Parallel plate rheology was used to determine storage and loss moduli at an oscillation rate of 1 Hz with a 1% strain amplitude. Results are expressed as mean ± standard error.
ECM, extracellular matrix.
3D adult cardiac ECM promotes expression of CM maturation genes
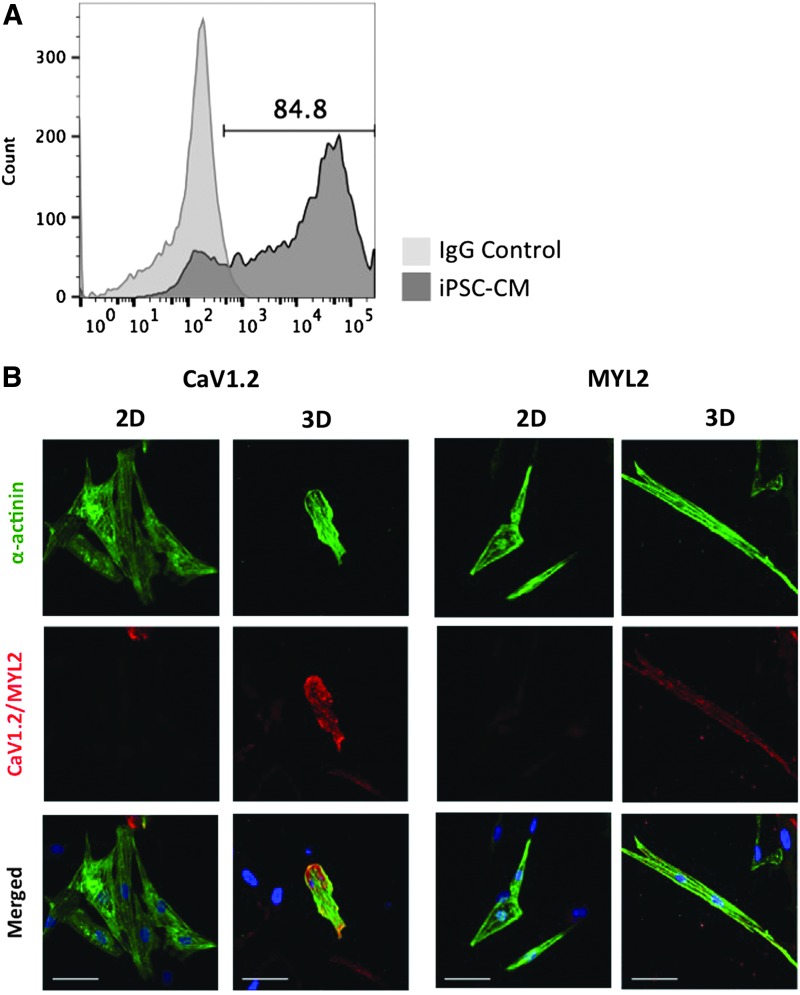
To determine if cardiac ECM affects iPSC-derived CM maturation, we examined gene expression (by qRT-PCR) in cells cultured in 2D and 3D fetal and adult cardiac ECMs (Supplementary Table S4). After 7 days in culture, iPSC-derived CMs seeded into 3D cardiac ECM (from fetal or adult tissue) expressed higher levels of numerous maturation-related genes compared with cells in 2D gels. These included genes related to calcium handling, such as Junctin (JCN), L-type voltage-dependent calcium channel (CACNA1C/CaV1.2), calsequestrin 2 (CASQ2), and sodium–calcium exchanger 1 (NCX1), which were augmented between 2- and 120-fold (Fig. 2A, B and Supplementary Table S3). We also saw similar increases in expression of several other genes related to cardiac maturation, including inward rectifier potassium ion channel (KCNJ2/Kir2.1), Triadin, sarcoplasmic reticulum (SR) Ca2+ ATPase (SERCA2a), and potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4) in 3D versus 2D culture (Supplementary Fig. S3). In addition, we also noted strong induction of these same genes, such as JCN, when comparing adult with fetal matrix, with the augmentation being particularly marked in 3D cultures (Fig. 2C, D). We next examined the expression of key structural and calcium-handling genes at the protein level. After 21 days of cardiac induction, 50–90% of differentiated cells expressed cardiac troponin T (cTNT), which is a marker of CM differentiation (Fig. 3A). Confirming our qRT-PCR data, we found a strong induction of CaV1.2, MYL2, and connexin 43 (Cx43) protein in iPSC-derived CMs cultured in 3D adult cardiac ECM, compared with 2D (Fig. 3B and Supplementary Fig. S4). In addition, the iPSC-derived CMs cultured on adult cardiac ECM exhibited increased expression of MYL2 compared with cells on fetal ECM (Supplementary Fig. S5). Thus, both the source (fetal vs. adult) and the geometry (2D vs. 3D) of ECM strongly influence maturation of iPSC-derived CMs.
FIG. 2.
3D adult cardiac ECM increases iPSC-derived CM expression of structural and functional cardiac genes. Gene expression was measured using qRT-PCR and expression normalized to 2D fetal cardiac ECM. Cardiac genes measured were JCN, CACNA1C/CaV1.2, Cx43, CASQ2, NCX1, and MYL2. (A) Gene expression in iPSC-derived CMs cultured in (3D) or on (2D) fetal cardiac ECM (*p > 0.01, **p > 0.001, Student's t-test). (B) Gene expression in iPSC-derived CMs cultured in (3D) or on (2D) adult cardiac ECM. (C, D) Data from (A, B) replotted to aid direct comparison of gene expression in iPSC-derived CMs cultured on adult and fetal 2D cardiac ECMs (C) and 3D cardiac ECM (D). Unless otherwise indicated, results are expressed as mean ± standard error. (n = 3; δp > 0.05, δδp > 0.01, Split-Unit ANOVA). 2D, two-dimensional; 3D, three-dimensional; CACNA1C/CaV1.2, L-type voltage-dependent calcium channel; CASQ2, calsequestrin 2; Cx43/GJA1, connexin-43; iPSC, induced pluripotent stem cell; JCN, junctin; MYL2, myosin light chain 2; NCX1, sodium–calcium exchanger 1; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
FIG. 3.
3D adult cardiac ECM increases iPSC-derived CM protein expression of structural and calcium-handling proteins. iPSC-derived CMs express sarcomere and calcium-handling proteins. (A) Flow cytometry analysis for cTNT(+) cells after 21 days of differentiation. (B) Immunofluorescent staining for MYL2 and CaV1.2 in iPSC-derived CMs cultured in 2D and 3D cardiac ECMs. (Right panels) MYL2 staining. (Left panels) CaV1.2 staining. Scale bar is 50 μm. Color images available online at www.liebertpub.com/tea
Cardiac ECM geometry affects iPSC-derived CM calcium signaling
The genetically encoded fluorescent calcium flux reporter GCaMP631 was utilized to assess the functional effects of ECM geometry on iPSC-derived CM calcium handling. We first compared single cells in 2D culture with single cells embedded in 3D gels. When cultured for 7 days in 3D cardiac ECM, the iPSC-derived CMs showed a significant decrease in beat rate compared with 2D culture (Fig. 4A). Interestingly, we also saw a decrease in calcium transient amplitude, maximum upslope, and maximum downslope and a concomitant increase in time to 50% decay (Fig. 4B–E). These data demonstrate a clear effect of matrix geometry on CM calcium handling. In vivo CMs are in close association and the formation of syncytia promotes electrical coupling. To mimic this, we cultured aggregates of iPSC-derived CMs in 3D gels and also allowed these to settle onto 2D gels. Perhaps, not surprisingly, we found the calcium-handling capabilities of the cells under these conditions to be quite different from those seen when the cells are not in close association. Representative traces of calcium influx are shown in Figure 4F. Calcium transient amplitude, maximum upslope, and maximum downslope were all increased (they were decreased with single cells) and the time to peak, time to 50% decay, and time to 75% decay were correspondingly decreased when cells were in 3D versus 2D (Fig. 4G–J and Supplementary Fig. S6A–E). Beat rate was not significantly altered by matrix geometry (data not shown). We also examined iPSC-derived CM behavior in a third model—cardiac spheroids embedded in 3D hydrogels comprising adult porcine cardiac ECM, fibrin, or collagen I. Compared with either fibrin or collagen I, cells in native cardiac matrix showed a decreased beat rate (Supplementary Fig. S7). These data are consistent with the promotion of CM maturation by native heart matrix.
FIG. 4.
3D adult cardiac ECM increases calcium signaling in iPSC-derived CMs. A GCaMP reporter was used to visualize calcium transients in iPSC-derived CMs after 7 days in 2D and 3D adult cardiac ECMs. (A–E) iPSC-derived CMs cultured in 3D as single cells displayed decreased calcium signaling and kinetics. (F–J) iPSC-derived CMs cultured in 3D as aggregates displayed an increase in calcium signaling and kinetics compared with 2D. (A) Beat rate of iPSC-derived CMs cultured in 2D and 3D adult/fetal cardiac ECMs. (B, G) Amplitude. (C, H) Max Upslope. (D, I) Max Downslope. (E, J) Time to 50% decay. (F) Representative calcium wave transient of iPSC-derived CM culture in 2D and 3D adult cardiac ECMs. Results are expressed as mean ± standard error (n = 4–12; *p < 0.05, **p < 0.01, ***p < 0.001, Student's t-test).
3D adult cardiac ECM increases iPSC-derived CM response to drugs
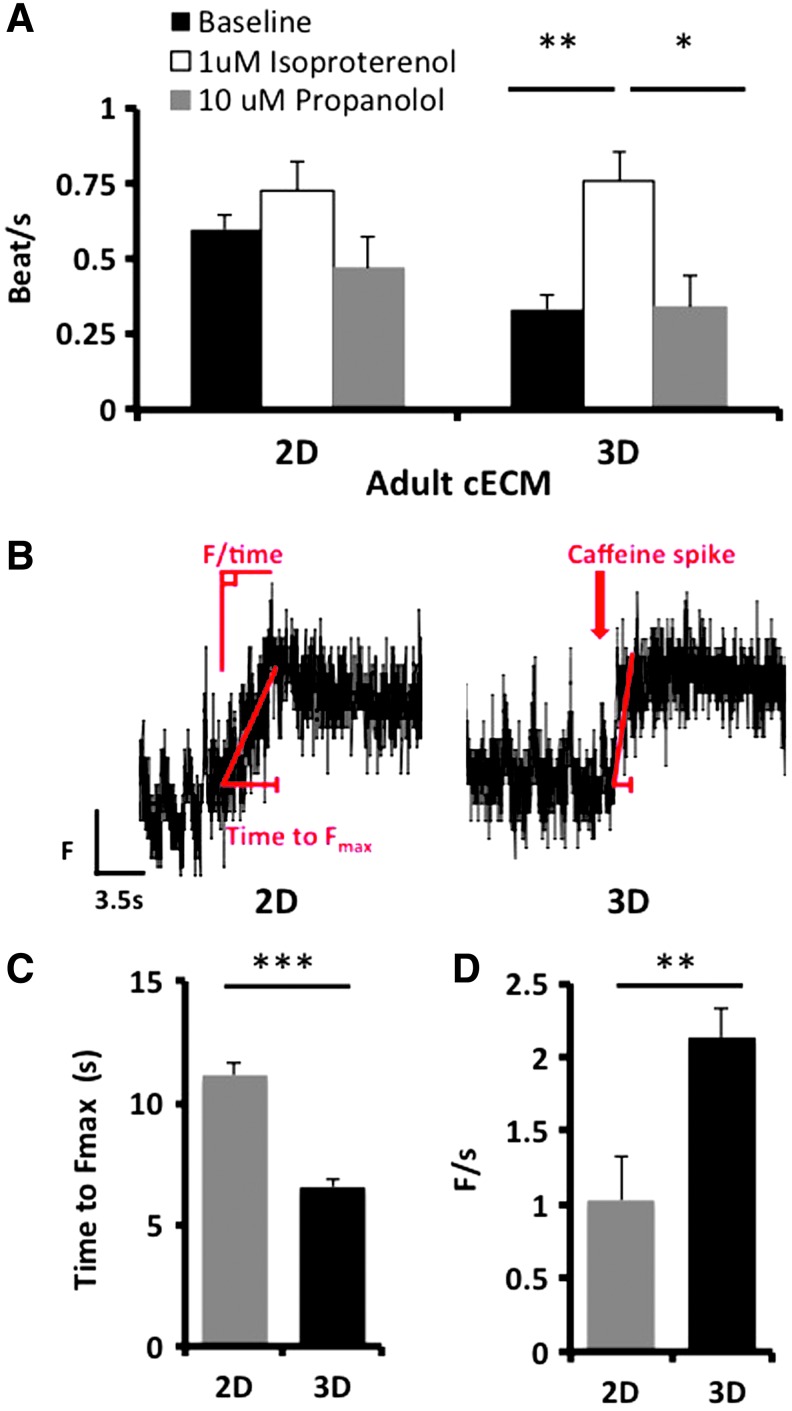
To further assess the functionality of iPSC-derived CMs in 3D cardiac ECM, cells were exposed to isoproterenol and propranolol, drugs known to modulate β-adrenergic signaling and consequently calcium handling. Consistent with the known behavior of adult CMs, the iPSC-derived CMs increased their beat rate upon the addition of 1 μM isoproterenol (Fig. 5A) and this was decreased to baseline levels following the addition of 10 μM propranolol (Fig. 5A). Caffeine targets the ryanodine receptor (RyR), resulting in an increase in available calcium. Exposure of iPSC-derived CMs to caffeine resulted in a more rapid release of calcium in 3D culture than in 2D (Fig. 5B). The upslope was steeper and the time to maximum calcium concentration was quicker (Fig. 5C, D). Thus, iPSC-derived CMs are responsive to drugs with known cardiac effects and these effects are more pronounced in 3D versus 2D culture.
FIG. 5.
3D adult cardiac ECM increases iPSC-derived CM response to drugs. iPSC-derived CMs cultured in 3D were more responsive to β-adrenergic stimuli and caffeine than cells in 2D cultures. (A) The effect of isoproterenol and propranolol on the beat rate of iPSC-derived CMs. (B) A representative fluorescent signal of the iPSC-derived CM calcium transient in response to 20 mM caffeine cultured in 2D and 3D adult cardiac ECMs. (C) The time to maximum fluorescent output (Fmax) was measured after the addition of caffeine. (D) The velocity of calcium release F/time. Results are expressed as mean ± standard error (n = 13–17; **p < 0.01 ***p < 0.001, Student's t-test). Color images available online at www.liebertpub.com/tea
Discussion
Myocardial infarction results in the death of millions to billions of CMs, and the lack of endogenous CM regeneration within the heart means that these cells are not replaced. A potential strategy to help these patients is to transplant CMs grown outside of the body, which will require establishment of procedures for the generation of large quantities of mature, or close to mature, CMs. iPSC-derived CMs provide an unlimited, autologous cell source that could be utilized for therapeutic transplantation as well as drug screening, disease modeling, and cardiotoxicity studies. A drawback, however, is that current protocols generate iPSC-derived CMs that are immature and display characteristics that resemble fetal CMs, as demonstrated by a weak force contraction, improper electrophysiology, and reduced or absent expression of several critical cardiac proteins.10,39 Consequently, there is considerable interest in finding strategies that promote maturation of iPSC-derived CMs. In the current study, we investigated the properties and composition of native cardiac ECM from adult and fetal bovine hearts and tested their ability to promote iPSC-derived CM maturation in both 2D and 3D conformations. We found several notable differences that call into question the continued use of 2D cultures for the generation of iPSC-derived CMs, notably that the composition and stiffness of the 3D matrix convey critical maturation signals to the developing CMs.
Multiple groups have investigated strategies to induce the maturation of pluripotent stem cell-derived CMs10–12,14,40–42; however, a majority of these studies investigated maturation of CMs in 2D. While 2D studies have certainly advanced our knowledge of CM maturation, the 3D environment greatly impacts cell behavior and maturation,23,43 and so a full understanding of this process will require the use of a more physiologic (3D) environment.44 Previous studies have shown that growing pluripotent stem cell-derived CMs in 3D benefits maturation induction40–42,45; however, these studies used only single-protein ECM scaffolds, which do not recapitulate the complexity of the in vivo ECM. DeQuach et al. showed that porcine cardiac ECM affects CM maturation26; however, only modest changes were observed, likely due to the use of a 2D geometry.
In the current study, we found that iPSC-derived CMs grown in a complex 3D scaffold behave differently to those in single protein gels (collagen I or fibrin) and that cells in 3D differ considerably from those cultured in 2D. This was especially notable when we studied calcium handling and expression of maturation markers. Although a complete mechanistic understanding of how the geometry of the cultures impacts cell phenotype is not yet available, we hypothesize that biomechanical cues, likely mediated through integrins,44,46–48 are critical.
The idea of biomechanical cues driving cell maturation is supported by our finding that the fetal and adult cardiac ECMs derived from decellularized heart tissue have a similar composition, but differ in their mechanical properties and fiber architecture. The adult ECM has a higher density of collagen compared with the fetal ECM, which is similar to what has been shown in rat adult cardiac ECM.30 In addition, the stiffness of the adult ECM hydrogel is 10-fold greater than the fetal hydrogel. These data are in agreement with findings that adult hearts are much stiffer than fetal hearts (10–50 KPa vs. 6 KPa),44 and previous studies have shown that substrate mechanics, particularly stiffness, influence CM growth and maturation.44,49 We think it is likely that the induction of maturation genes, such as JCN, by 3D culture and by the presence of adult versus fetal matrix, is largely the result of gel stiffness, which is a consequence of gel composition. It is also possible that specific matrix proteins engage with specific integrins to mediate maturation signals; however, we do not have any data to directly support this hypothesis.
Our investigation into iPSC-derived CM maturation concentrated on examining calcium handling. We found that iPSC-derived CMs cultured in 3D cardiac ECM showed increased expression of calcium-handling genes compared with 2D cultures, which is consistent with previous studies that primarily focused on the expression of ion channels and contractile machinery.10,11,14 Our data showing an increase in calcium-handling genes supports the hypothesis that a 3D geometry significantly influences CM maturation. In vivo, adult CMs express higher levels of calcium-handling proteins than do fetal cells,39 which again is consistent with the idea that expression of calcium-handling genes can be used as a measure of CM maturity. Interestingly, CMs of the adult heart express NCX1 at a lower level than CMs of the fetal heart39; however, we see increased NCX1 expression in our 3D cultured CMs, which is similar to results described by Rog-Zielinska et al.50 We speculate that the 3D cardiac ECM is only one component involved in promoting CM maturation and that additional maturation stimuli (hormones, growth factors, mechanical stretching, signals from non-CM) may also affect NCX1 expression. Additionally, CMs cultured in 3D, compared with 2D, had increased expression of Kir2.1, which encodes an inwardly rectifying K+ channel. This was recently shown to be the key ion channel that prevents the arrhythmias associated with immature CMs.51 Indeed, expression of Kir2.1 in immature CMs made them electrophysiological indistinguishable from mature CMs. Conversely, the increase in HCN4 expression in CMs grown in 3D may indicate that our CMs have not yet undergone ventricular CM specification.52 Furthermore, only a subset of maturation genes we studied reached elevated levels indicative of maturation when cells were cultured in 3D gels, which is consistent with our CM maturation model being incomplete.
Consistent with changes in gene expression, we found significant differences in the calcium handling in CMs, as measured by the calcium flux reporter GCaMP6. Recent publications show that mature CMs display increased calcium signaling and kinetics.10,11 In line with this, we found that in aggregates of iPSC-derived CMs, calcium transient amplitude, maximum upslope, and maximum downslope were all increased and the time to peak and time to 50% and 75% decay were correspondingly decreased when cells were in 3D versus 2D. These data are entirely consistent with our findings of increased expression of calcium handing genes/proteins such as JCN, CASQ2, CaV1.2, Triadin, and SERCA2a in 3D, which would act to increase the amount of calcium within the SR and create CMs with a greater capacity to handle calcium during calcium-induced calcium release. Furthermore, numerous publications have demonstrated that CMs with increased calcium signaling also exhibit functional changes such as increased contractile forces and enhanced contractile kinetics.10,11,51,53
Interestingly, in contrast to this, when we seeded CMs as single cells into 3D gels (to allow a more direct comparison of matrix effects in 2D vs. 3D) we found that calcium-handling kinetics were different—calcium transient amplitude, maximum upslope, and maximum downslope were all decreased and the time to 50% decay was correspondingly increased when cells were in 3D versus 2D. This strongly implies that gene expression is not enough to determine calcium-handling characteristics. Rather, it is the association of cells and the coupling of these calcium-handling systems that determines the kinetics of calcium handling. Clearly, future studies in 3D should focus on aggregated, rather than single, cells.
In the current study, CMs are cultured for 7 days before measuring calcium signaling, similar to Yang et al.11; however, others have cultured CMs for over 100 days before assaying for maturation.10 Interestingly, our unpublished data demonstrate that iPSC-derived CMs cultured long term (comparing a 5-month with a 2-month culture) exhibit increased calcium signaling and kinetics, suggesting that maturation continues over an extended period of time. Additionally, the CM T-tubules were examined and there was no apparent difference between the T-tubules of iPSC-derived CMs grown in 2D and 3D adult cardiac ECMs (data not shown). We speculate that development of mature T-tubules may occur over a longer time period than we studied in these experiments. Since endogenous CMs take several years before maturation is complete, it is not entirely surprising that maturation of CMs in culture may take considerable time.
An important characteristic of CMs, and one that needs to be recapitulated by iPSC-derived CMs, is their response to drugs. We found that iPSC-derived CMs cultured in 3D were more responsive to various stimuli compared with cells cultured in 2D. The addition of the β-adrenergic agonist, isoproterenol, to the iPSC-derived CMs cultured in 3D induced an increased beat rate compared with cells in 2D culture. Furthermore, exposure to caffeine induced a steeper slope and a shorter time to maximum calcium concentration in the CMs cultured in the 3D cardiac ECM compared with 2D. Caffeine is known to increase RyR sensitivity by lowering the luminal calcium threshold for calcium release from the SR.54 We hypothesize that the 3D culturing of iPSC-derived CMs increases the slope and decreases the time to maximum calcium signaling due to an increase in absolute calcium and/or faster release of calcium from the SR caused by the increased expression of JCN, CASQ2, and Triadin, which are all proteins that handle calcium within the SR and modulate its release through the RyR.
In summary, we have used 3D adult cardiac ECM as a strategy to promote iPSC-derived CM maturation. We determined that the age (fetal vs. adult) of the cardiac ECM modulates its mechanical properties and fiber architecture and these aspects affect CM gene and protein expression. After 7 days of culture within the 3D adult cardiac ECM hydrogels, the iPSC-derived CMs showed increased expression of many calcium-handling genes, consistent with the increase in these genes seen in adult versus fetal CMs. We also report that calcium signaling in the iPSC-derived CMs is enhanced in 3D compared with 2D, consistent with the changes in gene and protein expression. The utilization of 3D adult cardiac ECM to culture pluripotent stem cell-derived CMs better recapitulates the in vivo environment than traditional 2D cultures or the use of non-native matrix, thereby promoting CM maturation. Our studies help advance strategies for maturing pluripotent stem cell-derived CMs so that they can be safely and effectively used for therapeutic applications to treat heart disease.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Bruce Conklin for contributing the WTC-11 iPSC GCaMP cell line, Dr. Karen Christman for the porcine cardiac ECM, and UC Irvine's facilities, including Laboratory for Fluorescence Dynamics (P41-GM103540; P50-GM076516) and Optical Biology Center (CA-62203). This work was supported by NIH UH3 TR00048 (S.C.G., CCWH); NSF DGE-0549479 (A.H.F.); CIRM TG2-01152 (A.H.F.); and NIH SIG 1S10OD016328 (P.D.G.).
Disclosure Statement
No competing financial interests exist.
References
- 1.van Laake L.W., Passier R., den Ouden K., Schreurs C., Monshouwer-Kloots J., Ward-van Oostwaard D., van Echteld C.J., Doevendans P.A., and Mummery C.L. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res 3, 106, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Shiba Y., Fernandes S., Zhu W.Z., Filice D., Muskheli V., Kim J., Palpant N.J., Gantz J., Moyes K.W., Reinecke H., Van Biber B., Dardas T., Mignone J.L., Izawa A., Hanna R., Viswanathan M., Gold J.D., Kotlikoff M.I., Sarvazyan N., Kay M.W., Murry C.E., and Laflamme M.A. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon S.H., Kang S.W., Park S.J., Bae D., Kim S.J., Lee H.A., Kim K.S., Hong K.S., Kim J.S., Do J.T., Byun K.H., and Chung H.M. The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 34, 4013, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Caspi O., Lesman A., Basevitch Y., Gepstein A., Arbel G., Habib I.H., Gepstein L., and Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 100, 263, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., Gantz J.A., Fugate J.A., Muskheli V., Gough G.M., Vogel K.W., Astley C.A., Hotchkiss C.E., Baldessari A., Pabon L., Reinecke H., Gill E.A., Nelson V., Kiem H.P., Laflamme M.A., and Murry C.E. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong S.G., Huber B.C., Hee Lee W., Kodo K., Ebert A.D., Ma Y., Nguyen P.K., Diecke S., Chen W.Y., and Wu J.C. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation 132, 762, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi T., Miyagawa S., Pearson J.T., Fukushima S., Saito A., Tsuchimochi H., Sonobe T., Fujii Y., Yagi N., Astolfo A., Shirai M., and Sawa Y. Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in a myocardial infarction rat heart. Cell Transplant 24, 2479, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Masumoto H., Ikuno T., Takeda M., Fukushima H., Marui A., Katayama S., Shimizu T., Ikeda T., Okano T., Sakata R., and Yamashita J.K. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep 4, 6716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Pabon L., and Murry C.E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 114, 511, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundy S.D., Zhu W.Z., Regnier M., and Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 22, 1991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Rodriguez M., Pabon L., Fischer K.A., Reinecke H., Regnier M., Sniadecki N.J., Ruohola-Baker H., and Murry C.E. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol 72, 296, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D., Shadrin I.Y., Lam J., Xian H.Q., Snodgrass H.R., and Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuji T.G., Minami I., Kurose Y., Yamauchi K., Tada M., and Nakatsuji N. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: qualitative effects on electrophysiological responses to drugs. Stem Cell Res 4, 201, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kim C., Majdi M., Xia P., Wei K.A., Talantova M., Spiering S., Nelson B., Mercola M., and Chen H.S. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev 19, 783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tulloch N.L., Muskheli V., Razumova M.V., Korte F.S., Regnier M., Hauch K.D., Pabon L., Reinecke H., and Murry C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 109, 47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuppusamy K.T., Jones D.C., Sperber H., Madan A., Fischer K.A., Rodriguez M.L., Pabon L., Zhu W.Z., Tulloch N.L., Yang X., Sniadecki N.J., Laflamme M.A., Ruzzo W.L., Murry C.E., and Ruohola-Baker H. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A 112, E2785, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieu D.K., Fu J., Chiamvimonvat N., Tung K.C., McNerney G.P., Huser T., Keller G., Kong C., and Li R.A. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation 6, 191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkholtz C.B., Shea L.D., and Woodruff T.K. Extracellular matrix functions in follicle maturation. Semin Reprod Med 24, 262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solozobova V., Wyvekens N., and Pruszak J. Lessons from the embryonic neural stem cell niche for neural lineage differentiation of pluripotent stem cells. Stem Cell Rev 8, 813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y., Liu S., Huang J., Guo W., Chen J., Zhang L., Zhao B., Peng J., Wang A., Wang Y., Xu W., Lu S., Yuan M., and Guo Q. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. BioMed Res Int 2014, 648459, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukumoto S., and Yamada Y. Review: extracellular matrix regulates tooth morphogenesis. Connect Tissue Res 46, 220, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann D.R., and Dours-Zimmermann M.T. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol 130, 635, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gieseck R.L., III, Hannan N.R., Bort R., Hanley N.A., Drake R.A., Cameron G.W., Wynn T.A., and Vallier L. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One 9, e86372, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otonkoski T., Banerjee M., Korsgren O., Thornell L.E., and Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab 10 Suppl 4, 119, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Przybyt E., van Luyn M.J., and Harmsen M.C. Extracellular matrix components of adipose derived stromal cells promote alignment, organization, and maturation of cardiomyocytes in vitro. J Biomed Mater Res A 103, 1840, 2015 [DOI] [PubMed] [Google Scholar]
- 26.DeQuach J.A., Mezzano V., Miglani A., Lange S., Keller G.M., Sheikh F., and Christman K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One 5, e13039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y., Liu Z., O'Neill J., Wan L.Q., Freytes D.O., and Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res 4, 605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French K.M., Boopathy A.V., DeQuach J.A., Chingozha L., Lu H., Christman K.L., and Davis M.E. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater 8, 4357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., and Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 30, 5409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams C., Quinn K.P., Georgakoudi I., and Black L.D., III Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater 10, 194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddah M., Heidmann J.D., Mandegar M.A., Walker C.D., Bolouki S., Conklin B.R., and Loewke K.E. A non-invasive platform for functional characterization of stem-cell-derived cardiomyocytes with applications in cardiotoxicity testing. Stem Cell Reports 4, 621, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., Looger L.L., Svoboda K., and Kim D.S. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., and Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc 8, 162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theodossiou T.A., Thrasivoulou C., Ekwobi C., and Becker D.L. Second harmonic generation confocal microscopy of collagen type I from rat tendon cryosections. Biophys J 91, 4665, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams R.M., Zipfel W.R., and Webb W.W. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 88, 1377, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosignani V., Jahid S., Dvornikov A.S., and Gratton E. A deep tissue fluorescence imaging system with enhanced SHG detection capabilities. Microsc Res Tech 77, 368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards-Kortum R., and Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem 47, 555, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Crosignani V., Dvornikov A., Aguilar J.S., Stringari C., Edwards R., Mantulin W.W., and Gratton E. Deep tissue fluorescence imaging andin vivobiological applications. J Biomed Opt 17, 116023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J., Fu J.D., Siu C.W., and Li R.A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells 25, 3038, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Mihic A., Li J., Miyagi Y., Gagliardi M., Li S.H., Zu J., Weisel R.D., Keller G., and Li R.K. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials 35, 2798, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Nunes S.S., Miklas J.W., Liu J., Aschar-Sobbi R., Xiao Y., Zhang B., Jiang J., Masse S., Gagliardi M., Hsieh A., Thavandiran N., Laflamme M.A., Nanthakumar K., Gross G.J., Backx P.H., Keller G., and Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10, 781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M., Schulte J.S., Heinick A., Piccini I., Rao J., Quaranta R., Zeuschner D., Malan D., Kim K.P., Ropke A., Sasse P., Arauzo-Bravo M., Seebohm G., Scholer H., Fabritz L., Kirchhof P., Muller F.U., and Greber B. Universal cardiac induction of human pluripotent stem cells in two and three-dimensional formats: implications for in vitro maturation. Stem Cells 33, 1456, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann W.H. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 90, 223, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Tallawi M., Rai R., Boccaccini A.R., and Aifantis K.E. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng Part B Rev 21, 157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendel J.S., Ye L., Zhang P., Tranquillo R.T., and Zhang J.J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A 20, 1325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayomy A.F., Bauer M., Qiu Y., and Liao R. Regeneration in heart disease—is ECM the key? Life Sci 91, 823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross R.S., and Borg T.K. Integrins and the myocardium. Circ Res 88, 1112, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Wang P.Y., Yu J., Lin J.H., and Tsai W.B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater 7, 3285, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Jacot J.G., McCulloch A.D., and Omens J.H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95, 3479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rog-Zielinska E.A., Craig M.A., Manning J.R., Richardson R.V., Gowans G.J., Dunbar D.R., Gharbi K., Kenyon C.J., Holmes M.C., Hardie D.G., Smith G.L., and Chapman K.E. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ 22, 1106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieu D.K., Fu J.D., Chiamvimonvat N., Tung K.C., McNerney G.P., Huser T., Keller G., Kong C.W., and Li R.A. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol 6, 191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sartiani L., Bettiol E., Stillitano F., Mugelli A., Cerbai E., and Jaconi M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells 25, 1136, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Sheehy S.P., Pasqualini F., Grosberg A., Park S.J., Aratyn-Schaus Y., and Parker K.K. Quality metrics for stem cell-derived cardiac myocytes. Stem Cell Reports 2, 282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong H., Jones P.P., Koop A., Zhang L., Duff H.J., and Chen S.R. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J 414, 441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wisniewski J.R., Zougman A., Nagaraj N., and Mann M. Universal sample preparation method for proteome analysis. Nat Methods 6, 359, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Chou W., Ngo T., and Gershon P.D. An overview of the vaccinia virus infectome: a survey of the proteins of the poxvirus-infected cell. J Virol 86, 1487, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishihama Y., Rappsilber J., and Mann M. Modular stop and go extraction tips with stacked disks for parallel and multidimensional Peptide fractionation in proteomics. J Proteome Res 5, 988, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.