Abstract
Significance: This review article puts together all the studies performed so far in realizing terahertz (THz) spectra as a probing mechanism for burn evaluation, summarizing their experimental conditions, observations, outcomes, merits, and demerits, along with a comparative discussion of other currently used technologies to present the state of art in a condensed manner. The key features of this noncontact investigation technique like its precise burn depth analysis and the approaches it follows to convert the probed data into a quantitative measure have also been discussed in this article.
Recent Advances: The current research developments in THz regime observed in device design technologies (like THz time domain spectrometer, quantum cascade THz lasers, THz single-photon detectors, etc.) and in understanding its unique properties (like nonionizing nature, penetrability through dry dielectrics, etc.) have motivated the research world to realize THz window as a potential candidate for burn detection.
Critical Issues: Application of appropriate medical measure for burn injury is primarily subjective to proper estimation of burn depth. Tool modality distinguishing between partial and full-thickness burn contributing toward correct medical care is indeed awaited.
Future Directions: The overview of THz imaging as a burn assessment tool as provided in this article will certainly help in further nurturing of this emerging diagnostic technique particularly in improving its detection and accompanied image processing methods so that the minute nuances captured by the THz beam can be correlated with the physiological–anatomical changes in skin structures, caused by burn, for better sensitivity, resolution, and quantitative analysis.

Moumita Dutta, BTech
Scope and Significance
In this review article we have started by introducing a critical overview of the current technologies used for burn assessment and subsequently have discussed the prospect of terahertz (THz) frequency regime to be leveraged as a future alternative, overcoming the limitations of the current analysis techniques. First, we have discussed how THz technology evolved over time, primarily focusing on its biological applications, then moved forward with its interaction with water that determines the THz spectral window that can be used for this purpose, followed by a discussion on the features of THz imaging systems developed and finally putting together the promising results so far achieved in this domain.
Translational Relevance
Although histological specimen analysis always have proved to be the best assessment tool, its invasive and time-consuming nature1 created the urge of developing some other noninvasive means of burn evaluation. The current noninvasive mechanisms primarily employ two different diagnostic probes, elastic waves/matter waves and electromagnetic (EM) waves like, for example, ultrasound (∼106 Hz) and Infrared (∼1013 Hz) imaging systems, respectively, exploring their different spectral windows based on the need and feasibility of the technology. However, each comes with its own limitations, which needs to be addressed to proceed toward the development of a more efficient and reliable tool. To understand and appreciate the need for venturing into the emerging field of THz technologies for finding a better solution, it is important to analyze the relative efficacies of the existing mechanisms and comprehend how THz probing can provide a potentially promising outcome.
Clinical Relevance
Burn injuries can be caused by exposure to a wide variety of sources, including heat, electricity, radiation, chemical agents, and friction. According to the American Burn Association, ∼500,000 people seek treatment for burn injuries every year.2 Of that population, about 45,000 have burn injuries requiring medical treatment with about 3,500 cases resulting in death. Thermal injuries are common to all military conflicts and historically constitute ∼5–10% of all warfare military casualties.3,4 As a result of the improvised explosive devices currently being used in combat, burns are the primary injury in ∼5% of the personnel being evacuated. Even relatively small burns can be incapacitating, disfiguring, and painful and can strain the logistical and manpower resources of military medical units and thus overwhelm medical-resource allocation requirements. Moreover, they cause serious psychological implications for both combat and medical personnel.5,6
Discussion
Background
Burn injuries are defined as first, second, or third degree according to the depth of the wound and the layers of skin affected.7 First degree: Involves the epidermis (outer layer) only. These burns do not form blisters and epidermal regeneration occurs. Second degree: Involves the epidermis and varying levels of the dermis (middle layer). Blisters may form, but the underlying basement layer remains intact and epidermal regeneration occurs. Although it is hard to diagnose the difference readily, superficial second-degree burns are treated differently than deep second-degree burns because of their ability to heal without treatment and long-term outcomes. Third degree: All layers of the skin, including the entire dermis and hypodermis (adipose layer) are involved, blisters form, and will leave a scar.7 The depth and general appearance of the burn are used to determine the optimal treatment to improve healing outcomes.8
First-degree burns heal without the need for drastic medical intervention. Third-degree burn wounds typically require sharp debridement and grafting. Second-degree burns can heal spontaneously within 14 days without the formation of a scar. Deep partial-thickness and third-degree burns are always debrided and grafted. The partial-thickness second-degree burns are more difficult to assess. While skin grafts can improve the aesthetic outcomes of large burns, concerns exist over their inability to resist contractures, excessive contraction of surrounding skin that can limit the range of movement. Other concerns associated with skin grafts include excessive scarring, the need for multiple surgical procedures and limited donor site availability. So if not sufficiently deep, second-degree burns can be kept clean and covered with antimicrobial treatment instead of debridement.7 There are not, however, many instruments available to the surgeons to easily assess burn depth and differentiate between partial-thickness and deep partial-thickness burns8 and, therefore, decide whether to debride or not. The Thz technology reviewed in this article can be a useful and portable tool helping this purpose. Superficial burns can also convert to deep-partial or full-thickness burns over 3–5 days postinjury.9 There are three distinct zones pertaining to burn injury: the zone of coagulation, zone of stasis, and zone of hyperemia. The zone of coagulation contains irreversibly damaged tissue.9 Perfusion within the zone of stasis may also be reduced due to the local upregulation of factors such as bradykinin that stimulate microthrombosis.10 Ultimately, as perfusion to the site of injury is reduced, the zone of stasis becomes susceptible to necrosis, thus resulting in burns that progress from partial-thickness to deep partial- or full-thickness.9,11 Rather than treat burn wounds after they progress to deep partial-thickness in depth, early assessment of burn depth and intervention may also be able to prevent burn conversion. Such treatments may prevent the shift of superficial second-degree burns to deep partial-thickness burns that scar and form contractures.
Existing technologies for burn assessment
Several efforts have been made so far to develop noninvasive detection tool, with resolution and sensitivity sufficing the need for proper detection of the degree of skin burn. Endeavors made to develop accurate burn depth determinant modalities are stretched over varying technological arenas, such as Laser Doppler Imaging (LDI),12 photoacoustic,13 nuclear imaging,14 ultrasound,15 thermal imaging,16 are to name a few.
Exploration of ultrasound for burn examination got initiated back in 1977, where the pulse-echo mode was exploited to correlate the depth of burn to the time differences of the echoes measured from a burnt tissue with that of the known time intervals for a healthy skin.15 The elastic wave-based imaging technique gathered reliable significance when Brink17 reported its comparative proficiency with histological sectioning. The pulse-echo system takes advantage of the variable speed of the elastic wave traversing through different tissue layers with varying density to resolve their physiological specifics. Then came the first noncontact ultrasonography, where the probe was capable enough to assess the wound even from a distance of 1 inch.18 The underlying technique being pulse-echo in nature, the resolution of the monitoring technique remains limited to a number of acoustic parameters like the frequency or rather the wavelength of the probing spectra used, the electromechanical coupling, the impedance matching etc. As wavelength is inversely proportional to frequency, with increasing frequency better resolution is achieved (due to finer probing wavelength); however, although the technique beautifully examines the burn depths caused by a burn ranging from 1 to 45 s, which penetrates to the dermis and subcutaneous layers, the correlation fails to resolve a depth of 0.57 mm occurring for a burn of 1 s surfacing in the epidermis.1 Endeavors have also been made to couple the ultrasound imaging with Doppler effects to further enhance its resolution.19
LDI, emerged as a coupled phenomenon of Doppler flowmetry and laser imaging, has become one of the mostly recognized techniques for burn assessment. Although it reportedly achieves an accuracy of 97% in burn prediction,20 as pointed out in Ref.21 it is not a direct measure of the burn depth, rather it correlates the flow of blood in a burnt area with the depth of the wound to assess its severity. The accuracy, therefore, lies on the correlation methods it adopts.
To achieve a better resolution, one has to move toward higher frequencies. The EM waves have been exploited as an extensive diagnostic probe for that matter. The infrared (IR) imaging adheres to the emissive technique; thermal imaging is based on the localized variation in temperature due to change in blood circulation in the burnt tissues. Tissues with different heat content produce different black-body radiation, and the relative change in the emitted radiation of the burnt tissues as compared with that of the healthier ones is used as the parameter for burn assessment. Human body temperature at ambient condition being around 37°C emits a black-body radiation of ∼30 THz. Following the notion that deeper wounds would be colder than the superficial ones (due to higher rate of depletion of vascular circulation), the translated temperature from the emitted radiation can be inversely correlated to the depth of burn. This was supported by the investigation reported in Ref.16, which stated that a full-thickness wound would be 2° cooler than that of healthy skin. On the other hand, another study22 claimed that an accuracy as high as 90% can be achieved if the degree change in temperature corresponding to various aspects of wound is better translated using the same thermal imaging mechanism. Despite being a fast diagnostic technique, as reportedly claimed by Refs.23,24 its limitation lies in its misinterpretation. As cooler areas are interpreted as deeper depth of burn, sometimes thermal imaging infers the reduced heat content due to natural heat loss from the burnt tissues as deeper wounds. Therefore, higher the time delay between the analysis and the injury, the probability of obtaining inaccurate assessment increases.
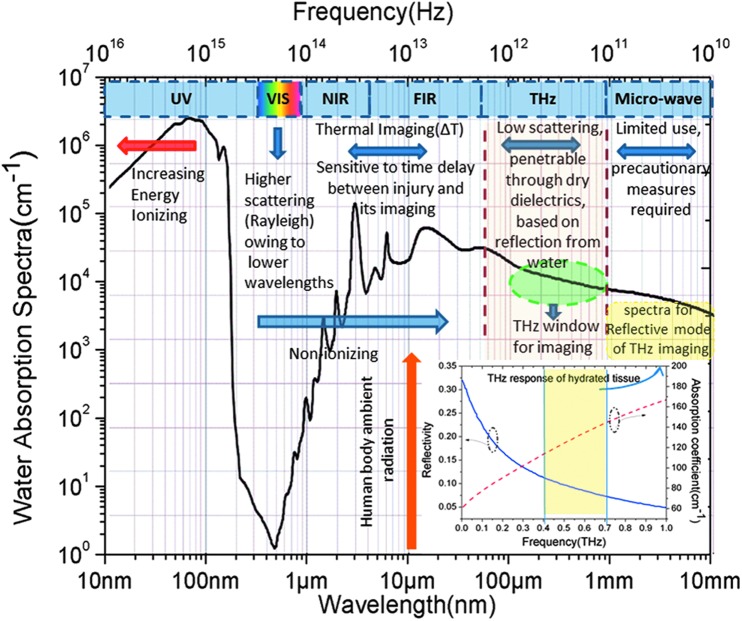
Owing to the limitations observed by the existing technologies, researchers are venturing into the yet fully exploited frequency window, the spectral range lying between the IR/visible and microwave domains. The bridging gap regime of interest essentially stretches from 100 GHz (0.1 THz) to 10 THz. Microwave frequencies known to have inducing thermal effects (owing to its coupling with water molecules), require precautionary measures for biological applications. On the contrary as one goes up in the frequency scale, that is, 100 GHz–10 THz, the low photon energy (Eg = hc/λ) ∼0.4–40 meV making it nonionizing along with a probing wavelength ranging in submillimeter to millimeter makes it potentially promising (as depicted in Fig. 1). The unique properties of THz, that is, its penetrability through dry dielectrics like textiles, synthetics, and high dielectric coefficient of water in this frequency domain (making it more reflective in THz),25,26 acts as a driving factor for realizing it as one of the ideal tools for various biological applications.
Figure 1.
Comparative view of different electromagnetic spectra from a burn imaging perspective (water absorption line generated from Ref.27 and THz response of hydrated tissue generated from Ref.28). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Evolution of THz technology
THz EM spectral window was kept away from any kind of industrial and academic exploration due to the insufficiencies in associated technology until 1980s when THz time domain spectrometer (TDS) got invented.29,30 The last two decades observed a plethora of research developments in this field exploiting its unique properties for various applications,31–33 which also includes biology (well summarized in the review article34). The reasons supporting the intervention into biological discipline can be attributed to various factors. As we are dealing with the structures ranging in the orders of 100 μm to 1 mm, the scattering remains limited to Mie for THz, which otherwise include Rayleigh for lower wavelengths like IR optical causing several higher-order scattering. Higher the scattering, higher the loss of information collected, as the reflected spectra will not converge toward the detector, but rather spread all over remaining uncaptured. Moreover, the submillimeter wavelength giving a resolution of ∼6 dots/mm34 makes it quite appreciable from the imaging point of view. When we are talking about biological tissues and cells, the most abundant content we are interested is water (about 70%). The characteristic high dielectric permittivity of water in THz, making it appreciably reflective, is the property that has been used as the contrast mechanism for the design of different THz biological imaging systems in this frequency regime. The works supporting the endeavor are35,36 where the unevaporated water content of veins was differentiated out from that of dried leaf surface using THz imaging, and a dehydrated tooth was imaged.
The photon energy content being ∼4.1 meV (at 1 THz) that is, way below the typical energy required to ionize or remove any valence electron from the biological molecules, which may otherwise cause a damage to the Biosystems,37 and maintaining the operating window below the vibrational mode resonances (as elaborated in Discussion Section), other possible thermal effects can also be eradicated. Pertaining to these undisruptive characteristics, THz imaging systems have been invariably proposed for various medical applications, and cancer detection,38,39 corneal hydration monitoring,40,41 and skin burn detection are among them.
Interaction of water with THz and proper spectral window selection for imaging
As discussed in the previous section, it is important to understand the interaction of water with THz spectrum to realize the scientific insight of the imaging technique which essentially aims at accurate monitoring of tissue hydration contrast. Different research groups have reported the interaction of water with THz EM waves, and in this section we have tried to provide a conclusive discussion on the factors which decide upon the most suitable THz spectral window to be used for imaging.
One of the unique properties of water is that it forms tetrahedral structures of hydrogen bonds with its neighboring molecules. Several studies have been performed to decipher the dielectric characteristic of water in higher gigahertz to THz frequency window; as the dielectric behavior defines its reflection and absorption dynamics, it is worth discussing pertaining to the scope of the article. As inferred from the elaborative study done by M. Sega and C. Schröder,42 the main dielectric relaxation lies at 15 GHz, while a fast relaxation process resides in the range 0.8–2.4 THz. According to the study reported in Ref.43 the higher frequency relaxation process can be attributed to a torsional vibrational mode at 1.56 THz originating from the bending movement of the intermolecular hydrogen bonds. These relaxations were further demonstrated by double Debye method by many research groups,44,45 which indicated an absorption coefficient as high as 300 cm−1 at 1.5 THz. Thus, it can be derived that a frequency range below 1.5 THz should be preferably selected for imaging purpose, otherwise all the incident THz energy, rather than reflecting back, will get absorbed and the imaging will be inhibited.
As far as imaging is concerned the modalities to be primarily quantified are penetrability or skin depth, scattering, and diffraction limit/spatial resolution. Although reported in Ref.46 that 0.1 THz shows highest reflectivity with sensitivity precise enough to recognize a change of 0.06% of relative change in water concentration, other studies came up with different views. On close inspection of the double Debye model used by Pickwell et al.45 for demonstrating the nonhomogeneous dielectric behavior of water distribution in skin tissue system, it was inferred that transverse electric mode produces contrast higher than transverse magnetic mode for oblique incidences. Based on the study reported in Ref.28 the preferred spectral window offering best imaging is claimed to be 0.4–0.7 THz. To support their findings, they plotted the relative change in THz reflectivity with respect to the percentage change in water by volume in hydrated tissue and observed that lower the frequency, higher the rate of change overserved. 0.15 THz was discarded due to an anomaly observed in the reflective response. A comparative perspective of THz absorption and reflectivity in hydrated tissues has been presented in Fig. 1 as well. As far as scattering is concerned, they took into account two different aspects, one is the angle of incidence, whereas the other is the spot size; while making the grazing angle of incidence narrower reduces the scattering, it also narrows down the spot size. Thus, from a trade-off between the two, they came up with the window 0.4–0.7 THz. A similar range of 0.3–0.9 THz has been supported in Ref.47 as the ideal frequency window, giving a penetration depth of ∼0.75 mm based on its wavelength, to be ventured for skin burn assessment using THz reflective technique.
Developments in THz imaging systems for burn depth evaluation
Before THz imaging can be appreciated as a burn assessment technique, compatible systems need to be developed. The last few decades, exhibiting tremendous progress on material investigation48 with an aim of developing THz as a full-fledged frontier, also contributed toward the subsequent emergence of THz sensing and imaging regime as a widespread research area. Several efforts have been made to engineer more compact, rapid, and precise THz imaging systems, which can conveniently be employed for both in vivo and ex vivo evaluation of skin burns. In this section some of those endeavors have been summarized.
The system developed by the University of California, Los Angeles as detailed out in Ref.28,49 appeared to produce some promising results (discussed in the section “Experimental outcomes and discussion”) outlining its superiority over the other existing techniques. Its photoconductive switch produces THz pulses with a bandwidth of 1 THz having an average power of 46 μW. Off-axis parabolic mirrors are used, to achieve a numerical aperture matching for efficient detection mechanism. The system has a scanning rate of 500 pixels/s with a spatial resolution of ∼700 μm and a spot size of 1.1 mm, sensitive to 0.5% of change in water volume.
Another system reportedly achieved a signal-to-noise ratio of 20 dB.50 The system comprises of ErAs nanoparticle-embedded GaAs thin film as source and a zero-bias Schottky detector, which can operate at a center frequency of 500 GHz with 125 GHz of bandwidth achieving a spatial resolution of 1.5 mm.
The THz TDS51 developed essentially for portable medical evaluation has been reported to be compact enough to be effortlessly carried and made use of at any place of emergency like battlefield hospitals, medical evaluation vehicles, and so on. It is a modified Zomega Mini-Z THz-TDS system, which comes with a custom-designed reflection unit. The system has been reportedly designed to be 15–55 times lighter and 15–80 times smaller than other conventionally used TDS systems. It operates in the frequency range of 0.1–4.0 THz with the reflecting unit ensuring normal incidence of THz beam on to the sample, eliminating any discrepancies brought about by oblique angle of incidence. A high-speed imaging system,52 which has been developed to image a 400 cm2 area in 8 min delineates another endeavor that can also facilitate the medical diagnosis.
Experimental outcomes and discussion
Pertaining to the scope of this article, the outcomes and the observations presented in this section has been essentially kept confined to skin burn detection. The achievements so far reported in this research discipline have been elaborated under the subsections ex vivo and in vivo studies along with a tabular form representation (Tables 1 and 2) to provide a summarized view of the progress in a chronological manner.
Table 1.
Experimental specifics and outcomes of ex vivo studies
| Experimental Conditions | Mode of Imaging | THz Window Used for Imaging | Outcomes | Year | Ref. |
|---|---|---|---|---|---|
| Series of circular burns produced on chicken tissue using argon laser over a region of 3 mm | Reflection | 0.5–1 THz | (a) The relative distortion caused in the damaged tissues was profoundly observed in both amplitude and phase response of the reflected beam, which tended to disappear toward the periphery. | 1999 | 53 |
| (b) Signal-to-noise ratio went down after 1 THz | |||||
| Chicken tissue branded at 350°C | Transmission | Center frequency ∼1.5 THz | Absorbance image clearly showed burn mark indentation. | 2007 | 57 |
| 2.5 mm thick flat sheet of grafted porcine skin branded by pressing a brass brand heated up to 315°C for 3 s | Reflection | A bandwidth of 3 dB, 0.125 THz with center frequency kept at 0.500 THz | (a) Image of the burned skin taken even from beneath five and 10 layers of gauze beautifully captured discernible features. | 2008 | 50 |
| (b) Noninvasive nature of T-ray imaging and its penetrability got demonstrated | |||||
| Full-thickness burn induced on an excised porcine skin by pressing a brass band heated up to 350°C for 5–7 s | Reflection | A bandwidth of 0.125 THz with center frequency maintained at 0.525 THz | (a) The drop of reflectivity at the burnt area was observed to be about 98.4%. | 2011 | 54 |
| (b) SNR of the captured image was reported to be 17 dB | |||||
| Third-degree burn induced on a fresh rat skin by using a brass rod heated in a water bath maintained at 100°C | Reflection | 0.5–0.7 THz | (a) Formation of interstitial edema due to cellular breakdown leading to 30% increase in reflectivity was observed unlike the excised tissues. | 2011 | 55 |
| (b) Density of the discrete structures like microvascular blood capillaries, hair follicles, and sweat glands that influence the THz reflectivity along with water content due to mm-wave scattering was found. | |||||
| (c) Deviation from double Debye model of Terahertz propagation through healthy skin that can add another degree of sensitivity to the image assessment was suggested. |
SNR, signal to noise ratio.
Table 2.
Experimental specifics and outcomes of in vivo studies
| Experimental Conditions | Mode of Imaging | THz Window Used for Imaging | Outcomes | Year | Refs. |
|---|---|---|---|---|---|
| Partial-thickness burn induced by a brass brand heated to 180°C and full thickness by heating it to 220°C. Brass applied onto a flat area on rat's abdomen. Postburn images captured starting from 8 min to 72 h | Reflection | 0.525 THz with an angle of incidence of 14° and a spot size of 1.1 mm | (a) For partial burn, the immediate observation (within 10 min of injury) was tremendous influx of fluid making both the injury and its surrounding edematous, which tends to settle down after 8 h along with an encircling low reflective tissue and the surrounding skin returning back to normal. The shift in water content captured after 24 h indicated the healing process. | 2012 | 58,59 |
| (b) Although for full-thickness burn, similar responses were observed after 10 min, which after 8 h showed some contrasting results. The injured area was dehydrated with pronounced emergence of less reflecting ring. The images taken after 24 h, however, showed some shift in water content, was less pronounced indicating its severity, which hinders rehydration of the tissue confirming its histological analysis of full-thickness burn. | |||||
| (c) Evolution of all the three zones, namely zone of coagulation, zone of stasis, and zone of hyperemia, was captured with high contrast as early as within 1 h of the burn defining its sensitivity. | |||||
| A proposed image processing approach | Reflection | 0.2–1 THz | THz reflectivity not only depends on water content, but also has correlation with the scattering from different microstructural features prevailing at different skin layers. | 2013 | 61 |
| Both partial and full thickness burns induced on rat models for a side by side comparison of the images | Reflection | Center frequency at 0.439THz with a bandwidth of 0.03THz | (a) The false color images showed a decrease in reflectivity at the injury with an increased reflectivity at the surrounding for partial burn. Whereas for second- and third-degree burn, it showed increased reflectivity all throughout the adjacent areas. | 2014 | 60 |
| (b) The image contrast reported to be >200:1 delineating the high spatial resolution of the technique. | |||||
| (c) The system is claimed to be sensitive enough to register the changes in fluid flow occurring within 15 min of burn induction. | |||||
| (d) The results also corresponded to MRI responses. | |||||
| (e) THz imaging system was able to comprehend the degree of burn within 3–4 h of injury |
MRI, magnetic resonance imaging.
Ex vivo studies
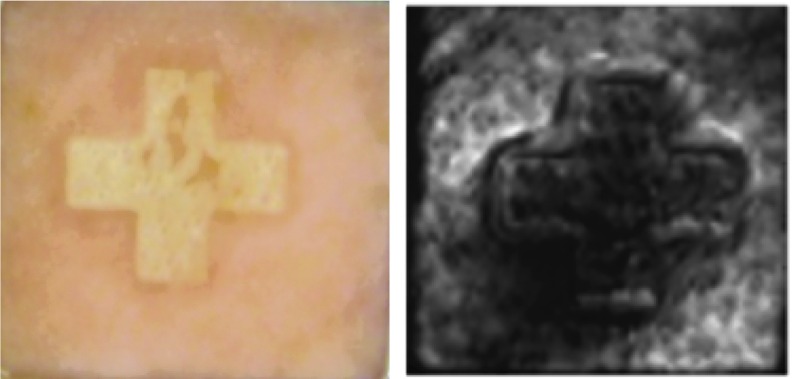
Exploration of THz imaging for skin burn assessment got initiated when a burnt chicken tissue was imaged by THz reflectometer53 for the first time. To provide an imagery view, the captured amplitude of the reflected THz beam was color coded with the darker shade corresponding to less reflection. The attenuation of reflectivity observed at the center of the damaged zone was attributed to the reduction in water content caused by the burning. A Similar drop in reflectivity observed at the burnt zone was also reported in Ref.54 where a full-thickness burn induced on an excised porcine skin was imaged. Figure 3 shows the visible and its corresponding THz image of the burnt skin.
Figure 3.
Visible and ex vivo THz image of a branded (+) excised porcine skin showing drop in reflectivity at the burnt zone (reprinted with permission of authors of Ref.54) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
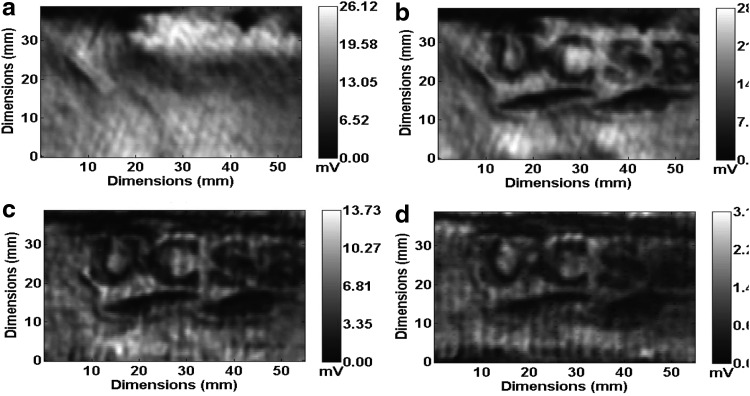
The noninvasive nature and the penetrability of reflective THz T-ray technique were explored in Ref.50 where a grafted piece of porcine skin, which is known to resemble human skin more closely, was branded using a heated UCSB brass logo and was imaged from beneath successive layers of gauze using the same contrast mechanism of varying water concentration. As observed from the images (Fig. 2), quite good discernable features were captured corroborating to its noninvasive nature.
Figure 2.
Ex vivo THz images of branded porcine skin depicting penetrability of the technique: (a) unburned, (b) burned, (c) burned region observed from beneath five, and (d) from 10 layers of gauze (reprinted with permission of authors of Ref.50).
While the above studies reported that burned areas result in the reduction of water leading to lesser THz reflectivity or darker regions in T-ray scanned images, studies by Arbab et al.55 reported completely different findings. When they imaged a burnt fresh rat skin, they observed a significant rise in reflectivity with respect to the control (normal skin) instead. Formation of interstitial edema due to cellular breakdown, which is unlikely to appear in excised skin samples (as in the case of previous studies) along with the difference in temperature used for branding, were attributed to the increase in water content resulting in rise in THz reflection. Moreover, they claimed that apart from water content, scattering caused by different microstructures equally contribute to the THz reflectivity owing to the comparable wavelength of the imaging probe. They proposed that deviation from double Debye model (i.e., frequency dependent dielectric model) of THz propagation through healthy skin, as defined by Pickwell et al.,56 can be used as a yard stick for more sensitive degree of burn assessment.
Although the abovementioned works were all performed in reflection mode, Dougherty et al.57 ventured the transmission mode of THz spectroscopy to image a branded tissue. However, as far as in vivo imaging is concerned, transmission mode appears to be impractical for obvious reasons, thus, all further in vivo studies (discussed in the following subsection, “In vivo studies”) were carried out in reflection mode.
In vivo studies
While the ex vivo studies, as mentioned above, gave a supporting view about the feasibility of using THz imaging for burn degree evaluation, in vivo studies provided stronger consent in realizing it as a potential burn diagnostic tool.
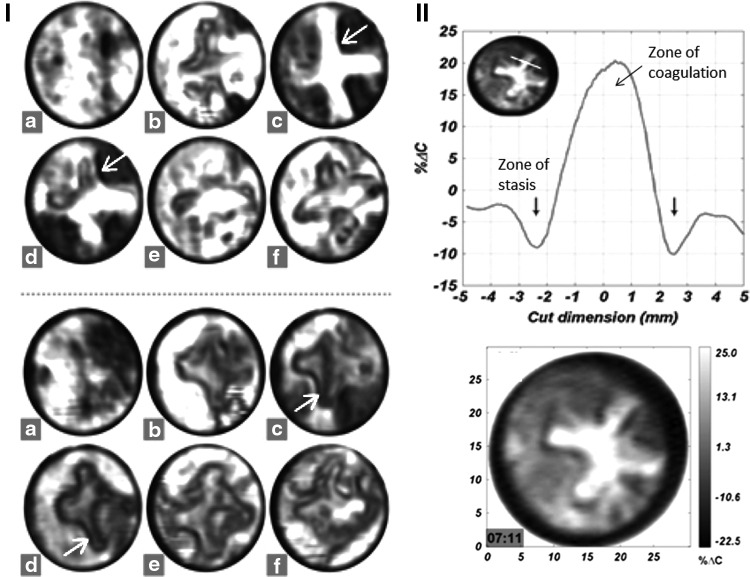
Burn wounds are normally assessed between 24 and 72 h after injury and in vivo studies performed on rat models58,59 reportedly distinguishing the pathological changes for partial- and full-thickness burn noticeably captured over that scanning period signifies the precision of T-ray imaging. Starting from tremendous influx of fluid toward the area of burn (the plus sign), as observed within 10 min of partial burn induction to the emergence of a dark ring of tissue along its periphery (shown in red arrows) after 8 h, to the subsequent shift of water content occurring throughout the healing process of the partial burn as observed after 24, 48, and 72 h are visibly depicted in the captured images (Fig. 4(i): upper). For the full-thickness burn, the pronounced emergence of the low reflecting surrounding tissue after 8 h and lesser shift in water content after 24, 48, and 72 h indicating the irreversible damage are also captured with utter clarity (Fig. 4(i): lower).
Figure 4.
In vivo studies on rat model, (i) partial burn (upper), full-thickness burn (lower) captured at different times after the injury, (a) uninjured, (b) 10 min, (c) 7 h, (d) 24 h, (e) 48 h, and (f) 72 h (modified and reprinted with permission of authors of Ref.59); (ii) upper: cut through of the image, with the x-axis representing the length of cut, shows the evolution of the zone of stasis as 10% dip in reflectivity, and the zone of coagulation as an increased reflectivity by 20%; (ii) lower: quantitative readout of one of the images (reprinted with permission of authors of Ref.58).
On further investigation, the authors were able to decipher the evolution of the three different zones usually associated with a burn wound, namely zone of coagulation situated at the center containing irreversible damaged cells, zone of stasis comprising of dehydrated tissue encircling the zone of coagulation, and the zone of hyperemia forming the outer region with hyperperfused (i.e., huge fluid content) tissues, from the captured THz images. They reportedly claimed that the first time in vivo imaging of the zone of stasis (as observed as a dip of 10% reflectivity in the cut through of the image: Fig. 4(ii): upper) using THz imaging certainly mark its superiority over the other existing techniques like LDI, thermal imaging, and photoacoustic. It is one of the significant findings achieved so far.
On the other hand, Neha et al.60 investigated the distinguishing changes occurring over a time span of 8 h for both partial- and full-thickness burn. The study was performed on a model of 10 rats and the THz images were reported in false color, with red corresponding to higher reflectivity and blue to reduced reflectivity. As shown in Fig. 5, the images are suggestive enough to differentiate between the localized influx (with the burn shown with arrow), as observed in case of partial burn, over the increase of reflectivity occurring all over the adjacent areas, for full-thickness burn.
Figure 5.
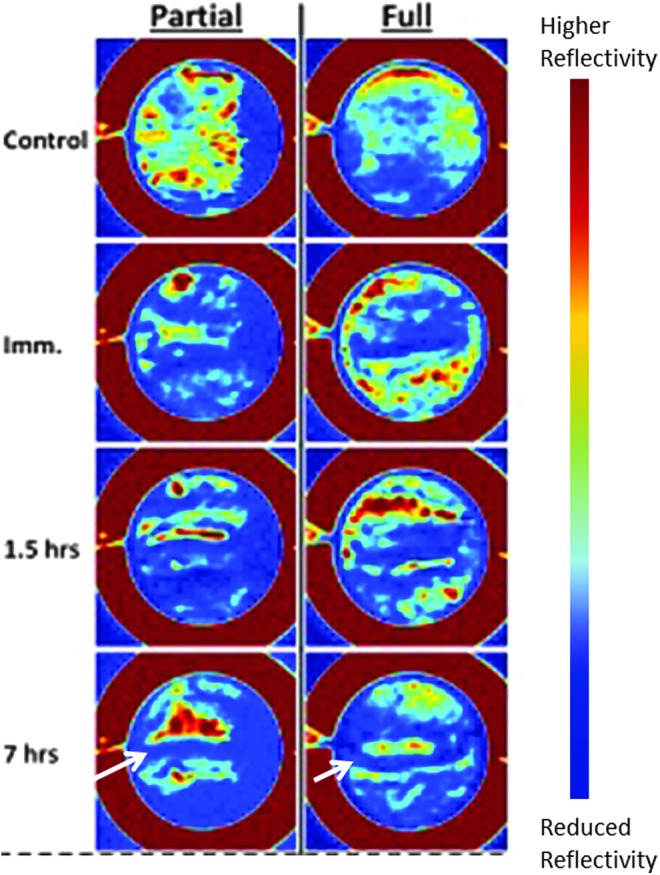
False colored in vivo images captured over 8 h with red indicating higher and blue lower water content (modified and reprinted with permission of authors of Ref.60 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In the same work, the authors further investigated the sensitivity and resolution of THz imaging based on tissue hydration gradient detection by comparing its outcomes with concurrent magnetic resonance imaging (MRI). It was found that MRI response was corroborating to that of THz reflective images. While it took both visual and histological analysis 3 days to confirm its severity, THz imagery was able to determine the degree of burn within 3–4 h of injury, which certainly suggests its preeminence over the existing techniques of analysis.
As an extension of their ex vivo work55 (as discussed in the prior subsection), Arbab et al.61 proposed an image processing approach for in vivo imaging as well. They mentioned that in the case of partial-thickness burn, an irreversible damage is caused to the microvascular and epithelium sites and survival of insufficient number of those structures in the dermis layer may eventually lead to third-degree burn, which, therefore, defines the criticality of clinical discernment between the two, for offering proper treatment. In this study, they have differentiated the second-degree burn from third degree by quantifying the burn extent based on the density of skin structures. Thus, the proposed image processing technique can add further sensitivity to the above-described mechanisms.
Conclusion
In this article we have reviewed all the promising strides observed by this research field with an intention that the gathered information can further contribute toward the evolution of THz imaging as a potential burn assessment tool. A subsequent exploration of accompanied image processing methods to provide a quantitative differentiation of the different burn severities by closely studying the minute nuances captured by the THz beam and correlating them with the physiological–anatomical changes in skin structures can certainly add higher degrees of sensitivity and resolution to the imaging systems. This progress will eventually lead to an improved and efficient approach to address this clinical issue.
Take-Home Messages.
• Limitations of the existing burn depth assessment techniques call for a better solution for this clinical issue.
• Unique properties of THz EM spectra like—penetrability through dry dielectrics (making it more penetrable), nonionizing nature (making it biocompatible), low-scattering (reducing the loss of information thus increasing its precision)—act as the driving factors for realizing THz imaging as a potential burn diagnostic probe with higher degrees of sensitivity and resolution.
• Noninvasiveness, rapid analysis, acceptable resolution, and portability of some systems make THz imaging technique advantageous over the others.
• From the in vivo THz images, one can decipher the evolution of the three different zones usually associated with a burn wound, namely zone of coagulation, zone of stasis, and the zone of hyperemia.
• Development of a more accurately quantifiable THz imaging system capable enough to decipher the demarcation between the shallow partial burn and deep partial-thickness burn (which eventually destroys the skin's regenerative capability) with even higher degrees of sensitivity and resolution will certainly address this clinical issue with promising precision.
Abbreviations and Acronyms
- EM
electromagnetic
- LDI
laser Doppler imaging
- MRI
magnetic resonance imaging
- TDS
time domain spectrometer
- THz
terahertz
Acknowledgments and Funding Sources
This work has been supported by the National Science Foundation under Grant No. 1002380 and by the Department of Defense under the Grant No. W911NF-12-1-0082.
Author Disclosure and Ghostwriting
There is no conflicting financial interest associated. The content presented in this article is solely written by the authors listed, and no other means of writing is involved.
About the Authors
Moumita Dutta, BTech, is a doctoral graduate student in the Department of Electrical and Computer Engineering at the University of Texas at San Antonio. Her field of research includes various electronic material studies, simulation, characterization, circuit design, terahertz spectroscopy, and related multidisciplinary submillimeter sensing and communication applications. Amar S. Bhalla, PhD, is Distinguished Research Professor of Electrical and Computer Engineering at the University of Texas at San Antonio, with research interests spanning over a broad range of ferroelectric, multiferroic, and bio-multiferroic materials and devices. Ruyan Guo, PhD, is Robert E. Clarke Endowed Professor of Electrical and Computer Engineering at the University of Texas at San Antonio. She conducts research in interdisciplinary areas of electronic and optoelectronic materials and devices. More recently her research is focused on multiferroics, energy-converting devices, and piezoelectric resonance controlled phenomena, with potential in sensor, harvester, and biomedical applications.
References
- 1.Watts AM, Tyler MP, Perry ME, Roberts AH, McGrouther DA. Burn depth and its histological measurement. Burns 2001;27:154–160 [DOI] [PubMed] [Google Scholar]
- 2.Miller SF, et al. National burn repository 2007 report: a synopsis of the 2007 call for data. J Burn Care Res 2008;29:862–870; discussion 871 [DOI] [PubMed] [Google Scholar]
- 3.Chung KK, et al. Evolution of burn resuscitation in operation Iraqi freedom. J Burn Care Res 2006;27:606–611 [DOI] [PubMed] [Google Scholar]
- 4.White CE, Renz EM. Advances in surgical care: management of severe burn injury. Crit Care Med 2008;36(7 Suppl):S318–S324 [DOI] [PubMed] [Google Scholar]
- 5.Wolf SE, et al. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Ann Surg 2006;243:786–792; discussion 792–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens BD, et al. Extremity trauma research in the United States Army. J Am Acad Orthop Surg 2006;14(10 Spec No.):S37–S40 [DOI] [PubMed] [Google Scholar]
- 7.Renz EM, Cancio LC. Acute burn care. In: Savitsky E, Eastridge B, eds. Combat Casualty Care: Lessons Learned from OEF and OIF. Department of the Army, Virginia: Office of the Surgeon General, 2012 [Google Scholar]
- 8.Jaskille AD, et al. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res 2009;30:937–947 [DOI] [PubMed] [Google Scholar]
- 9.Singh V, et al. The pathogenesis of burn wound conversion. Ann Plast Surg 2007;59:109–115 [DOI] [PubMed] [Google Scholar]
- 10.Nwariaku FE, et al. Effect of a bradykinin antagonist on the local inflammatory response following thermal injury. Burns 1996;22:324–327 [DOI] [PubMed] [Google Scholar]
- 11.Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ 2004;328:1427–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeng JC, et al. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial. Burns 2003;29:665–670 [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki M, et al. Measurement of burn depths in rats using multiwavelength photoacoustic depth profiling. J Biomed Opt 2005;10:064011. [DOI] [PubMed] [Google Scholar]
- 14.Sayman HB, et al. A method to evaluate microcirculatory vascular patency of full thickness burn in an animal model. Panminerva Med 1999;41:5–9 [PubMed] [Google Scholar]
- 15.Goans RE, Cantrell JH., Meyers F. Ultrasonic pulse-echo determination of thermal injury in deep dermal burns. Med Phys 1977;4:259–263 [DOI] [PubMed] [Google Scholar]
- 16.Watson AC, Vasilescu C. Thermography in plastic surgery. J R Coll Surg Edinb 1972;17:247–252 [PubMed] [Google Scholar]
- 17.Brink J, Sheets P, Dines K, Etchison M, Hanke C, Sadove A. Quantitative assessment of burn injury in porcine skin with high-frequency ultrasonic imaging. Invest Radiol 1986;21:645–651 [DOI] [PubMed] [Google Scholar]
- 18.Iraniha S, Cinat M, Vanderkam V, et al. Determination of burn depth with noncontact ultrasonography. J Burn Care Rehabil 2000;21:333–338 [DOI] [PubMed] [Google Scholar]
- 19.Goertz D, Yu J, Kerbel R, Burns P, Foster F. High-frequency 3-D color-flow imaging of the microcirculation. Ultrasound Med Biol 2003;29:39–51 [DOI] [PubMed] [Google Scholar]
- 20.Pape SA, Skouras CA, and Byrne PO. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 2001;27:233–239 [DOI] [PubMed] [Google Scholar]
- 21.Elamin SE, Dickson JK, Mackie IP. Is Laser Doppler imaging (LDI) a measure of burn depth? Burns 2015;41:413. [DOI] [PubMed] [Google Scholar]
- 22.Still J, Law E, Klavuhn K, Island T, Holtz J, Diagnosis of burn depth using laser-induced indocyanine green fluorescence: a preliminary clinical trial. Burns 2001;27:364–371 [DOI] [PubMed] [Google Scholar]
- 23.Anselmo V, Zawacki B, Infra-red photography as a diagnostic tool for the burn ward. Proc Soc Photo Optic Instr Eng 973;8:181 [Google Scholar]
- 24.Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P, Assessment of burn depth and burn wound healing potential. Burns 2008;33:761–769 [DOI] [PubMed] [Google Scholar]
- 25.Pickwell E, Wallace VP. Biomedical applications of terahertz technology. J Phys D Appl Phys 200; 39:R301–R310 [Google Scholar]
- 26.Siegel PH. Terahertz technology in biology and medicine. IEEE Trans Microw Theory Tech 2004; 52:2438–2447 [Google Scholar]
- 27.Palik ED. Water. In: Palik ED, eds. Handbook of Optical Constants of Solids. Boston, MA: Academic Press, 1991:1067–1077 [Google Scholar]
- 28.Taylor ZD, Singh RS, Bennett DB, et al. THz medical imaging: in vivo hydration sensing. IEEE Trans Terahertz Sci Technol 2011;1:201–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auston DH, et al. cherenkov radiation from femtosecond optical pulses in electro-optic media. Phys Rev Lett 1984;53:1555–1558 [Google Scholar]
- 30.Fattinger C, Grischkowsky D. Point-source terahertz optics. Appl Phys Lett 1988;53:1480–1482 [Google Scholar]
- 31.Dragoman D, Dragoman M. Terahertz fields and applications. Prog Quant Elect 2004;28:1–66 [Google Scholar]
- 32.Xu JZ, Zhang CL, Zhang XC. Recent progress in terahertz science and technology. Progr Nat Sci 2002;12:729–736 [Google Scholar]
- 33.Tonouchi M. Cutting-edge terahertz technology. Nature Photon 2007;1:97–105 [Google Scholar]
- 34.Siegel PH. Terahertz technology in biology and medicine. IEEE Trans Microw Theory Tech 2004;52:2438–2447 [Google Scholar]
- 35.Hu BB, Nuss MC. Imaging with terahertz waves. Optics Lett 1995;20:1716. [DOI] [PubMed] [Google Scholar]
- 36.Arnone DD, et al. Applications of Terahertz (THz) technology to medical imaging. Terahertz Spectrosc Appl 11 1999;3828:209–219 [Google Scholar]
- 37.Wilmink GJ, Grundt JE. Invited review article: current state of research on biological effects of terahertz radiation. J Infrared Millimeter Terahertz Waves 2011;32:1074–1122 [Google Scholar]
- 38.Woodward RM, et al. Terahertz pulse imaging in reflection geometry of human skin cancer and skin tissue. Phys Med Biol 2002;47:3853–3863 [DOI] [PubMed] [Google Scholar]
- 39.Wallace VP, et al. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br J Dermatol 2004;151:424–432 [DOI] [PubMed] [Google Scholar]
- 40.Bennett DB, et al. Terahertz sensing in corneal tissues. J Biomed Opt 2011;16:057003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett D, et al. Assessment of corneal hydration sensing in the terahertz band: in vivo results at 100 GHz. J Biomed Opt 2012;17:97008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sega M, Schröder C. Dielectric and terahertz spectroscopy of polarizable and nonpolarizable water models: a comparative study. J Phys Chem A 2015;119:1539–1547 [DOI] [PubMed] [Google Scholar]
- 43.Yu BL, Yang Y, Zeng F, Xin X, Alfano RR. Reorientation of the H2O cage studied by terahertz time-domain spectroscopy. Appl Phys Lett 2005;86:061912 [Google Scholar]
- 44.Kint JT, Schmuttenmaer CA. Far-infrared dielectric properties of polar liquids probed by femtosecond terahertz pulse spectroscopy. J Phys Chem 1996;100:10373–10379 [Google Scholar]
- 45.Pickwell E, Cole BE, Fitzgerald AJ, Wallace VP, Pepper M. Simulation of terahertz pulse propagation in biological systems. Appl Phys Lett 2004;84:2190 [Google Scholar]
- 46.Sung S, Bennett D, Taylor Z, et al. Reflective measurement of water concentration using millimeter wave illumination. Health Monit Struct Biol Syst 2011
- 47.Bennett DB, et al. Stratified media model for terahertz reflectometry of the skin. IEEE Sens J 2011;11:1253–1262 [Google Scholar]
- 48.Dutta M, Peralta XG, Bhalla A, Guo R. Current status of oxide dielectric materials for terahertz applications—an overview. Integr Ferroelectr 2015;166:108–139 [Google Scholar]
- 49.Bajwa N, Nowroozi B, Sung S, et al. Reflective THz and MR imaging of burn wounds: a potential clinical validation of THz contrast mechanisms. In: Terahertz Emitters, Receivers, and Applications III; Proc SPIE 2012:8496
- 50.Taylor ZD, et al. Reflective terahertz imaging of porcine skin burns. Opt Lett 2008;33:1258–1260 [DOI] [PubMed] [Google Scholar]
- 51.Wilmink GJ, et al. Development of a compact terahertz time-domain spectrometer for the measurement of the optical properties of biological tissues. J Biomed Opt 2011;16:047006. [DOI] [PubMed] [Google Scholar]
- 52.Zimdars D. High speed terahertz reflection imaging. Adv Biomed Clin Diagn Syst III 2005;5692:255–259 [Google Scholar]
- 53.Mittleman DM, et al. Recent advances in terahertz imaging. Appl Phys B Lasers Opt 1999;68:1085–1094 [Google Scholar]
- 54.Tewari P, Taylor ZD, Bennett D, et al. Terahertz imaging of biological tissues. Med Meets Virt Real 2011;18:653–657 [PubMed] [Google Scholar]
- 55.Arbab MH, et al. Terahertz reflectometry of burn wounds in a rat model. Biomed Opt Expr 2011;2:2339–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pickwell E, et al. Simulation of terahertz pulse propagation in biological systems. Appl Phys Lett 2004;84:2190–2192 [Google Scholar]
- 57.Dougherty JP, Jubic GD, Kiser WL. Terahertz imaging of burned tissue. Terahertz Gigahertz Electr Photon VI 2007;6472:N4720–N4720 [Google Scholar]
- 58.Tewari P, et al. In vivo terahertz imaging of rat skin burns. J Biomed Opt 2012;17:040503. [DOI] [PubMed] [Google Scholar]
- 59.Tewari P, et al. Advances in biomedical imaging using THz technology with applications to burn wound assessment. In: Terahertz Technology and Applications V; Proc SPIE 2012:82610T:1–8 [Google Scholar]
- 60.Bajwa N, Sung S, Garritano J, et al. In vivo confirmation of hydration based contrast mechanisms for terahertz medical imaging using MRI. In: Terahertz Emitters, Receivers, and Applications; Proc SPIE 2014;91990U:1–8 [Google Scholar]
- 61.Arbab MH, et al. Terahertz spectroscopy for the assessment of burn injuries in vivo. J Biomed Opt 2013;18:077004. [DOI] [PubMed] [Google Scholar]