Abstract
Dynamic mechanical loading is a strong anabolic signal in the skeleton, increasing osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BM-MSCs) and increasing the bone-forming activity of osteoblasts, but its role in bone metastatic cancer is relatively unknown. In this study, we integrated a hydroxyapatite-containing three-dimensional (3D) scaffold platform with controlled mechanical stimulation to investigate the effects of cyclic compression on the interplay between breast cancer cells and BM-MSCs as it pertains to bone metastasis. BM-MSCs cultured within mineral-containing 3D poly(lactide-co-glycolide) (PLG) scaffolds differentiated into mature osteoblasts, and exposure to tumor-derived soluble factors promoted this process. When BM-MSCs undergoing osteogenic differentiation were exposed to conditioned media collected from mechanically loaded breast cancer cells, their gene expression of osteopontin was increased. This was further enhanced when mechanical compression was simultaneously applied to BM-MSCs, leading to more uniformly deposited osteopontin within scaffold pores. These results suggest that mechanical loading of 3D scaffold-based culture models may be utilized to evaluate the role of physiologically relevant physical cues on bone metastatic breast cancer. Furthermore, our data imply that cyclic mechanical stimuli within the bone microenvironment modulate interactions between tumor cells and BM-MSCs that are relevant to bone metastasis.
Introduction
Breast cancer is the most common cancer among women in the United States1 and the most costly to manage. Although the 5-year survival rate for localized breast cancer is very high (∼99%), prognosis significantly declines (∼24%) when metastasis occurs.1 In fact, nearly 75% of patients with advanced breast cancer develop incurable skeletal metastases2 that increase morbidity and early death due to severe pain, nerve compression, fractures, and other skeletal-related events; therefore, delineating critical factors that modulate interactions of disseminated tumor cells with bone is of significant clinical importance.3
During normal bone remodeling, bone mass is maintained by balancing resorption by osteoclasts and formation by osteoblasts.4 However, in the presence of breast cancer cells, these homeostatic interactions become perturbed, and heightened osteoclastogenesis promotes secondary tumor formation by leading to the release of growth-promoting factors from the degraded bone matrix.5 The current standard of care, including surgery and drugs that target tumor and/or bone-destroying osteoclasts, is only palliative as it merely slows metastatic progression and does not recover lost bone.6–8 Hence, there exists a critical need for an improved understanding of alternative mechanisms that may enable more efficacious treatments of breast cancer bone metastasis.
It is increasingly accepted that tumors modulate the behavior of ostensibly normal cells within their microenvironment to promote malignancy and that mechanical signals play an important role in this process.9–11 However, it is relatively poorly understood what effect the highly dynamic mechanical environment in the skeleton has on the pathogenesis of breast cancer bone metastasis. In the skeleton, cyclic signals due to daily physical activity (e.g., typical walking frequency is ∼1 Hz), rather than static or steady signals, are the primary regulator of remodeling. Increased mechanical loading (e.g., exercise) stimulates osteoblasts and results in net bone formation, while decreased mechanical loading (e.g., bed rest) stimulates osteoclasts and causes bone loss.12,13
Recently, we demonstrated that increasing cyclic mechanical signals in breast cancer cell-bearing bones inhibited secondary tumor growth and maintained cancellous bone mass in vivo.14 In addition, in vitro cyclic compression of three-dimensional (3D)-cultured breast cancer cells revealed that loading did not induce tumor cell death, but rather decreased expression of genes interfering with downstream bone remodeling.14 These data suggested that mechanical loading of the skeleton may reduce metastasis by altering the interplay between tumor and resident bone cells. While most studies focus on osteoclasts as the primary cellular mediators of breast cancer-associated osteolysis, mesenchymal stem cell (MSC)-derived osteogenic cells, including osteoblasts, may be similarly important. In fact, osteoblasts are not only a primary mechanoresponsive cellular element in the skeleton and regulate osteoclast activity and differentiation4,13 but also they change their behavior in the presence of bone metastasis.15–17 To further define how mechanical loading modulates the interplay between bone metastatic breast cancer cells and resident bone cells, it is, therefore, of critical importance to better understand the behavior of tumor-associated MSCs as a function of mechanical loading.
The anabolic effects of increased mechanical signals on osteogenic cells derived from MSCs are well documented both in vivo and in vitro.13 Increased mechanical signals, including fluid flow, mechanical stretching, and compression, enhance commitment of MSCs down the osteogenic lineage and stimulate their bone-forming activity. In the context of bone metastasis, MSCs play an important pro-tumorigenic role in the skeleton by contributing to the formation of a microenvironment that promotes osteolytic bone metastasis and tumor cell proliferation.18 However, whether MSCs retain their pro-tumorigenic function during osteogenic differentiation while being exposed to tumor-derived factors, specifically under the influence of mechanical loading, is not known.
In these studies, we focused on expression of osteopontin (OPN) as our candidate molecule, a bone matrix protein secreted by MSCs and osteoblasts that modulates mineralization and serves as a substrate in the adhesion of osteoclasts and osteoblasts to bone.19,20 OPN is of relevance to bone metastatic cancer, where it is produced by both metastatic breast cancer cells and bone marrow stromal cells, is generally expressed at the interface between tumor cells and the bone, promoting tumor cell adhesion, and regulating osteoclast activity to facilitate osteolytic bone metastasis.21,22 In this study, we describe a 3D in vitro culture model based on mineral-containing poly(lactide-co-glycolide) (PLG) scaffolds14,23–26 that enables us to investigate the combined effects of mechanical loading and breast cancer cell-derived paracrine factors on osteogenic differentiation and OPN expression of bone marrow-derived MSCs.
Methods and Materials
Experimental design
We utilized our previously described 3D in vitro loading setup that applies cyclic mechanical stimuli to cells cultured within mineral-containing 3D porous PLG scaffolds.14 To first verify the ability of MSCs to undergo relevant 3D osteogenic differentiation in these scaffolds, human bone marrow-derived mesenchymal stem cells (BM-MSCs) were cultured in porous PLG scaffolds in the presence and absence of chemical inducers for 21 days (i.e., the time required for osteoblasts to form) and 60 days (i.e., time sufficient for observable osteoid protein deposition27). To assess whether bone mineral impacts BM-MSC differentiation,28 half of the scaffolds were fabricated to contain hydroxyapatite (HA) nanoparticles and half were mineral free. Osteogenic differentiation was quantified through standard markers of functional osteoblasts.
Next, we evaluated if factors secreted by human MDA-MB231 breast cancer cells modulated BM-MSC differentiation. To this end, BM-MSCs were cultured in mineral-containing scaffolds with tumor-conditioned or blank media for 21 days. Finally, we evaluated whether loading of either tumor cells or BM-MSCs impacted the effect of tumor-secreted soluble factors on BM-MSC osteogenic differentiation.
3D culture of BM-MSC
Human BM-MSCs (Lonza or RoosterBio) were cultured using growth media containing 4 mM l-glutamine (Sigma) under standard cell culture conditions (37°C, 5% CO2) and expanded to passage five or population doubling level 20 for use in scaffold seeding.
Scaffolds were prepared from PLG and HA as previously described.24 Briefly, 8 mg of PLG microspheres fabricated from PLG (Evonik Industries) using a double emulsion technique,29 8 mg of HA particles (average diameter of 200 nm; Sigma), and 152 mg of NaCl particles sized 250–400 μm (J.T. Baker) were pressure molded (Carver Press) into disks (1 mm thick, 8 mm diameter). These disks were subsequently subjected to a gas-foaming/particulate leaching technique that results in surface exposure of the incorporated mineral.29 Scaffolds were incubated with 70% ethanol for 30 min, washed 4× with sterile PBS, and seeded with 1–1.5 × 106 BM-MSCs per scaffold. Cell-seeded scaffolds were maintained on an orbital shaker at 37°C and 5% CO2 for 3 days before initiation of experiments to allow for cell adhesion and equilibration. For groups undergoing differentiation, BM-MSC culture media included 50 μM ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycerophosphate. Control groups received differentiation basal media without osteogenic factors.
In vitro mechanical loading
For BM-MSC loading experiments, BM-MSCs were treated with tumor-conditioned media (see Generation of tumor-conditioned media) for 1 week and were then randomized into Loaded and Nonloaded (NL) groups. Loaded scaffolds were exposed to cyclic mechanical loading using a compression bioreactor as previously described.14,30 Briefly, 10% peak compression was applied at 1 Hz to each loaded scaffold using a specialized platen for 1 h per day, 3 days per week (MWF), for two additional weeks (total 3 weeks).
Following experiments, scaffolds were photographed for subsequent image analysis of diameter using ImageJ (NIH). Scaffolds were next bifurcated, and all halves were randomized for use in the following assays: total DNA content, intracellular alkaline phosphatase (ALP) enzyme activity, histological analysis, and microcomputed tomography. DNA was isolated with Caron's buffer and quantified by analysis of fluorescence intensity using QuantiFluor (Promega). Intracellular ALP was isolated with passive lysis buffer, and enzyme activity was measured using a p-nitrophenyl phosphate assay (Pierce). Scaffolds were fixed in 4% paraformaldehyde and scanned using microCT (VersaXRM-520; Zeiss, 20 μm resolution) and then were paraffin embedded and sectioned (4 μm thick).
Generation of tumor-conditioned media
MDA-MB231 human breast cancer cells (ATCC) were maintained in complete Dulbecco's modified Eagle's medium (DMEM [Invitrogen] supplemented with 10% fetal bovine serum [FBS, Tissue Culture Biologicals] and 1% penicillin/streptomycin [P/S, Invitrogen]) under standard cell culture conditions (37°C, 5% CO2). To generate two-dimensional (2D) conditioned media, MDAs were plated in T150 flasks and when they reached 90% confluency, their media was replaced with low serum DMEM (1% FBS, 1% P/S) for 24 h. Conditioned media was collected, concentrated 10-fold in an Amicon centrifugal filter unit (MWCO 3 kDa, EMD Millipore), and subsequently diluted 5-fold with appropriate BM-MSC media. To generate 3D conditioned media, 1.5 × 106 MDA-MB231s were seeded into HA-containing scaffolds, which were randomized into Loaded and Nonloaded (NL) groups. Loaded group scaffolds received cyclic mechanical loading as described above for 1 h, after which media was replaced with low serum DMEM for 24 h. 3D-conditioned media was collected, normalized to DNA content, and processed similarly to 2D-conditioned media. For the 2D versus 3D comparison only, both types of conditioned media were normalized to similar DNA content.
Histology
To assess Type I collagen deposition, transverse sections were stained with Masson's Trichrome using standard procedures. Briefly, slides were deparaffinized and hydrated, followed by incubation with Bouin's solution, and sequential staining with Weigert's hematoxylin, Biebrich scarlet-acid fuchsin solution, phosphomolybdic–phosphotungstic acid, and aniline blue solution. To assess osteopontin (OPN) protein, immunohistochemistry was performed using the VECTASTAIN Elite ABC Kit (Vector Laboratories). Briefly, scaffold sections were deparaffinized in xylene and rehydrated before heat-based antigen retrieval in 10 mM citrate buffer, pH 6 for 20 min at 95°C. Sections were treated with 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity and then blocked with normal horse serum for 30 min. Next, sections were incubated with rabbit anti-human OPN polyclonal antibody (AB 1870, EMD Millipore; diluted 1:1500) at 4°C overnight and then incubated with biotinylated horse anti-mouse/rabbit IgG for 1 h. After being rinsed, sections were incubated with avidin-biotinylated peroxidase complex for 30 min and then developed in diaminobenzidine (DAB; Thermo Scientific) solution for 3 min. Nuclei were counterstained using Mayer's hematoxylin (Thermo Scientific) for 20 s and then sections were dehydrated and mounted with Entellan (EMD Millipore).
Gene expression
Gene expression of osteopontin was determined using quantitative real-time polymerase chain reaction (qRT-PCR) and the comparative ΔCT method.31,32 Briefly, mRNA was isolated from scaffolds using the TRIzol extraction method in RNase-free conditions.33 RNA purity and quantity were tested using a spectrophotometer (NanoDrop 1000; Thermo Scientific). 500–1000 ng of RNA was reverse transcribed to cDNA and brought to 5–10 ng/μL with RNase-free water. qPCR was performed using 10 ng of cDNA in a final volume of 20 μL containing 2X SYBR Green (PerfeCTa SYBR Green FastMix, with ROX, Quanta Biosciences). Quantification cycle was determined for osteopontin [5′-TGAGAGCAATGAGCATTCCGATG-3′ (forward) and 5′-CAGGGAGTTTCCATGAAGCCAC-3′ (reverse)] and normalized to the reference gene β-actin [5′-AATGTGGCCGAGGACTTTGATTGC-3′ (forward) and 5′-AGGATGGCAAGGGACTTCCTGTAA-3′ (reverse)]. Results are presented as fold change.
Statistical analysis
The effects of: (1) mineral (HA vs. PLG) and induction (Induced vs. Control), (2) conditioned media ([2D] tumor factors vs. no tumor factors) and induction (Induced vs. Control), (3) 2D- versus 3D-conditioned media and induction (Induced vs. Control), and (4) loaded-conditioned media (Loaded tumor factors vs. Nonloaded [NL] tumor factors) and loaded BM-MSCs (Loaded BM-MSCs vs. NL BM-MSCs) were all assessed using a two-way full factorial ANOVA (JMP v8.0, SAS Institute, Inc.). When the interaction factor was significant, a post hoc Tukey–Kramer test with a Bonferroni correction was conducted; otherwise, experimental groups were pooled for analysis as appropriate to evaluate main effects. Statistical significance was set at α ≤ 0.05. All experiments were replicated two to four times. All data are represented as mean ± standard deviation.
Results
3D BM-MSC osteogenic differentiation is enhanced in the presence of HA
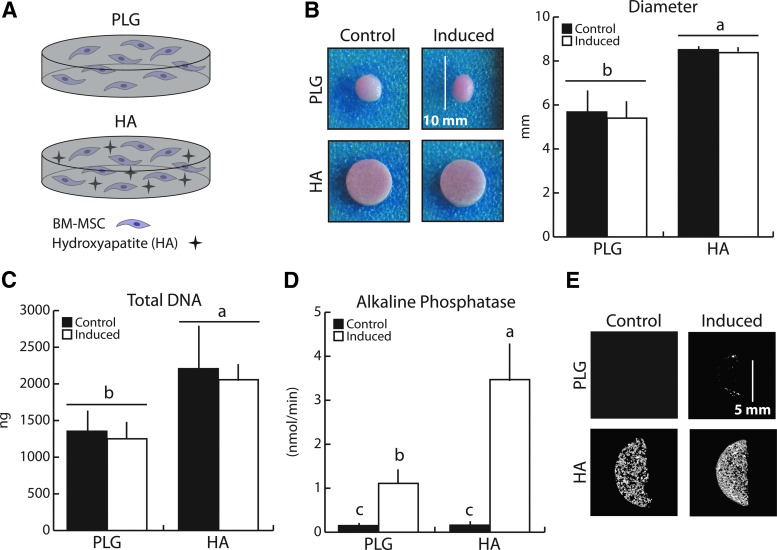
We first verified that chemical induction of BM-MSCs in porous 3D PLG scaffolds resulted in relevant osteogenic differentiation and determined whether incorporation of the bone mineral HA within the scaffolds affected this process (Fig. 1A). Following 21 days of culture, HA-containing scaffolds maintained their initial geometry while the PLG-only scaffolds contracted −35% (pooled PLG vs. pooled HA), independent of differentiation status (Fig. 1B). Similarly, total BM-MSC DNA content was 67% greater in HA versus PLG scaffolds (pooled PLG vs. pooled HA), suggesting superior cellular adhesion, greater proliferation, and/or less apoptosis in the presence of bone mineral (Fig. 1C). Consistent with previous reports by others, BM-MSCs underwent osteogenic differentiation when cultured with chemical inducers, and this was enhanced in HA-containing PLG scaffolds.26 Intracellular ALP enzyme activity increased 21-fold in the HA group (Induced:Control ratio), but only 7.5-fold in the PLG group (ALP was similar between the two Control groups). This resulted in ALP activity being threefold higher in the HA Induced cell-polymer constructs relative to the PLG Induced counterparts (Fig. 1D). Consistent with these results, heightened osteogenic differentiation in the HA Induced versus PLG Induced group resulted in increased mineralization, as evidenced by microCT (Fig. 1E). Taken together, the presence of bone mineral in the 3D culture models created a superior osteogenic environment.
FIG. 1.
Mineral-containing scaffolds enhanced BM-MSC osteogenic differentiation after 21 days relative to control scaffolds. (A) To determine how the presence of HA affects osteogenic differentiation, mineral-free (PLG) and mineral-containing (HA) scaffolds were utilized to differentiate BM-MSCs (1.5 × 106 per scaffold) into osteoblasts. (B) HA scaffolds maintained their initial geometry after 21 days of culture, while control (PLG) scaffolds significantly contracted. (C) BM-MSCs growth in HA scaffolds was greater than that in PLG scaffolds and was independent of differentiation as detected by fluorimetric DNA analysis. (D) ALP enzyme activity increased with osteogenic induction and was enhanced in HA scaffolds. (E) Osteogenic induction resulted in increased mineral content as assessed by microCT, which was greater in HA scaffolds. Same letters (a, b, or c) indicate similar mean values, and groupings with different letters indicate that the difference is significant by post hoc comparison of means with Bonferroni correction (p < 0.05). ALP, alkaline phosphatase; BM-MSC, bone marrow-derived mesenchymal stem cell; HA, hydroxyapatite; BM-MSC, bone marrow-mesenchymal stem cell; PLG, poly(lactide-co-glycolide). Color images available online at www.liebertpub.com/tea
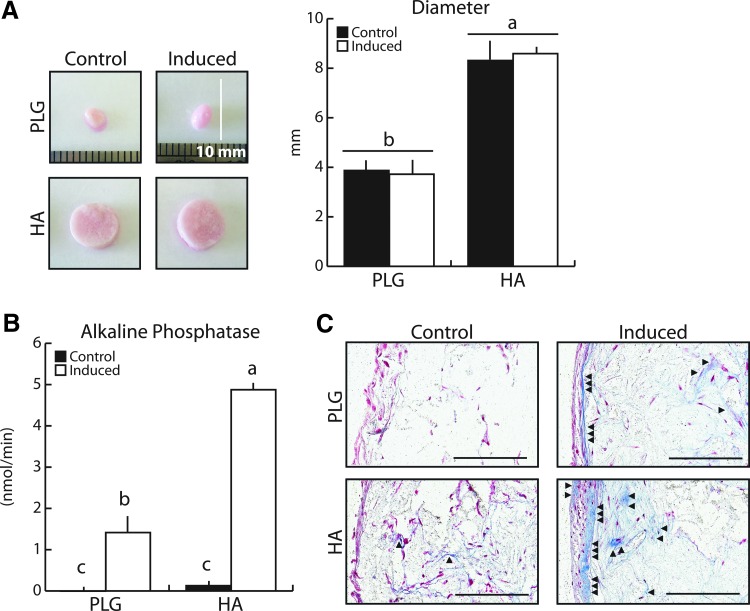
To further verify that 3D culture of BM-MSCs in HA-containing scaffolds over 21 days is representative of cellular behavior over prolonged culture periods, cell-seeded scaffolds were maintained for 60 days, a time frame leading to observable osteoid protein deposition in osteoblast cultures.27 Indeed, HA-containing scaffolds continued to maintain their initial geometry, whereas PLG scaffolds were −55% smaller (pooled HA vs. pooled PLG), independent of differentiation status (Fig. 2A). BM-MSCs also continued to differentiate in both HA and PLG scaffolds under culture with osteogenic media, and the presence of HA enhanced this process long term. ALP activity was 3.5-fold higher in HA Induced cell-polymer constructs relative to PLG Induced groups (Fig. 2B). In the absence of osteogenic inducers, BM-MSCs deposited small amounts of Type I collagen in HA scaffolds (Fig. 2C), which were not visible in PLG Control scaffolds. Furthermore, induced BM-MSCs assembled significantly more collagen in pores, as well as on the scaffold surface, in the HA relative to the PLG scaffolds (Fig. 2C). Taken together, these results suggest that HA promotes osteogenesis of BM-MSCs within 3D culture microenvironments and that 21-day culture periods are appropriate to estimate bone formation processes typically requiring longer culture periods. Therefore, all subsequent studies were performed in HA-containing scaffolds over 21 days.
FIG. 2.
Long-term BM-MSC osteogenic differentiation in mineral-containing scaffolds verified the creation of a bone mimetic microenvironment. (A) After 60 days, HA-containing scaffolds continued to maintain their initial geometry, while PLG scaffolds continued to contract as confirmed by image analysis. (B) Heightened ALP enzyme activity with osteogenic induction was maintained in HA scaffolds. (C) Osteogenic differentiation resulted in greater Type I collagen deposition, as seen with Masson's Trichrome staining (collagen stains blue). The majority of matrix was deposited on scaffold surfaces; however, HA scaffolds also exhibited deposition within interior pores. Arrows indicate collagen deposition. Scale bars = 200 μm. Same letters (a, b, or c) indicate similar mean values, and groupings with different letters indicate that the difference is significant by post hoc comparison of means with Bonferroni correction (p < 0.05). Color images available online at www.liebertpub.com/tea
Breast cancer-derived soluble factors increase 3D BM-MSC osteogenic differentiation
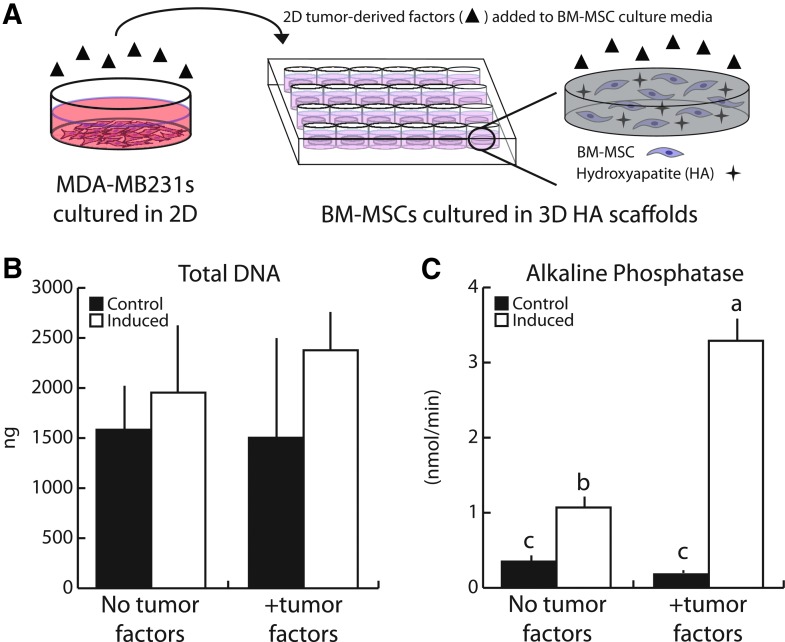
To assess the effect of breast cancer cell-derived soluble factors on the osteogenic capability of bone marrow progenitors, we cultured BM-MSCs in HA-containing scaffolds for 21 days in the presence and absence of media conditioned by 2D-cultured MDA-MB231 (Fig. 3A). No significant differences were observed in total BM-MSC DNA content in any experimental group suggesting that tumor-secreted soluble cues do not significantly affect 3D BM-MSC growth under either control or osteogenic conditions (Fig. 3B). Interestingly, however, ALP activity was enhanced threefold in the presence of tumor-derived factors compared to control conditions lacking tumor-secreted factors (Fig. 3C). This difference in ALP activity was only observed when BM-MSCs were osteogenically induced, while ALP activity in noninduced Control groups was similar in the presence or absence of tumor-conditioned media. These data suggest that tumor-derived soluble factors can augment BM-MSC osteogenic differentiation under 3D microenvironmental conditions.
FIG. 3.
Human breast cancer-derived soluble factors increased BM-MSC osteogenic differentiation. (A) 2D tumor-conditioned media was collected from MDA-MB231 cultured on tissue culture plastic for 24 h in low serum media, after which media was collected, concentrated, and reconstituted with the appropriate BM-MSC culture media. (B) After 21 days, 2D tumor-conditioned media (+tumor factors) did not alter BM-MSC growth patterns as assessed by fluorimetric DNA analysis. (C) Tumor-derived factors enhanced ALP enzyme activity following induction, indicating increased osteogenic differentiation. Same letters (a, b, or c) indicate similar mean values, and groupings with different letters indicate that the difference is significant by post hoc comparison of means with Bonferroni correction (p < 0.05). 2D, two dimensional. Color images available online at www.liebertpub.com/tea
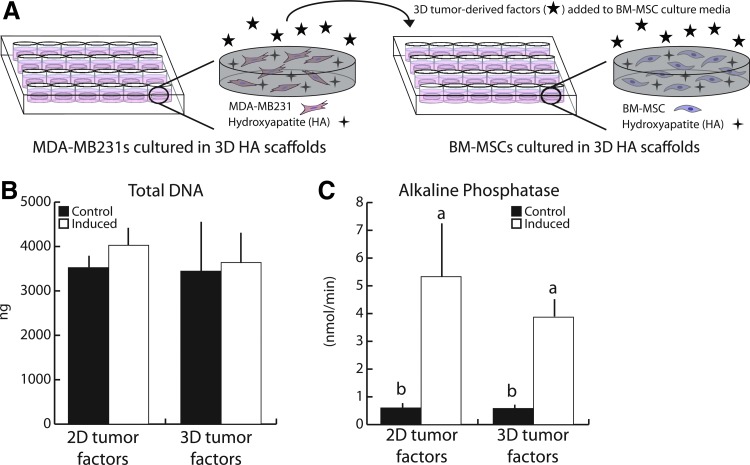
Importantly, tumor cell-secreted factors promoted BM-MSC osteogenic differentiation regardless of whether conditioned media was collected from 2D- or 3D-cultured tumor cells (Fig. 4A). More specifically, neither 2D- nor 3D-conditioned MDA-MB231 media altered BM-MSC growth within the mineral-containing PLG scaffolds, while they both increased ALP enzyme activity of BM-MSCs under inducing conditions to a similar extent (Fig. 4B, C). These results imply that the paracrine pro-osteogenic effects of MDA-MB231 are independent of culture dimensionality. Furthermore, they confirm that cyclic compression of 3D tumor cell cultures and subsequent collection of conditioned media in all subsequent studies will be appropriate to test whether mechanical stimulation alters the pro-osteogenic capability of breast cancer cells.
FIG. 4.
Human breast cancer-derived soluble factors generated in 2D versus 3D had similar effects on BM-MSC osteogenic differentiation. (A) 3D tumor-conditioned media was generated from MDA-MB231 tumor cells (1.5 × 106 per scaffold) cultured in HA-containing scaffolds for 24 h in low serum media, after which media was collected, concentrated, and reconstituted with the appropriate BM-MSC culture media. 2D media was generated as described previously. (B) After 21 days, BM-MSC growth patterns were similar when incubated in 2D versus 3D tumor-conditioned media (tumor factors) as indicated by fluorimetric DNA analysis. (C) Similarly, ALP enzyme activity was comparable between cultures supplemented with 2D and 3D TCM. Same letters (a, b, or c) indicate similar mean values, and groupings with different letters indicate that the difference is significant by post hoc comparison of means with Bonferroni correction (p < 0.05). 3D, three dimensional. Color images available online at www.liebertpub.com/tea
Mechanically loaded tumor cells alter BM-MSC osteopontin expression
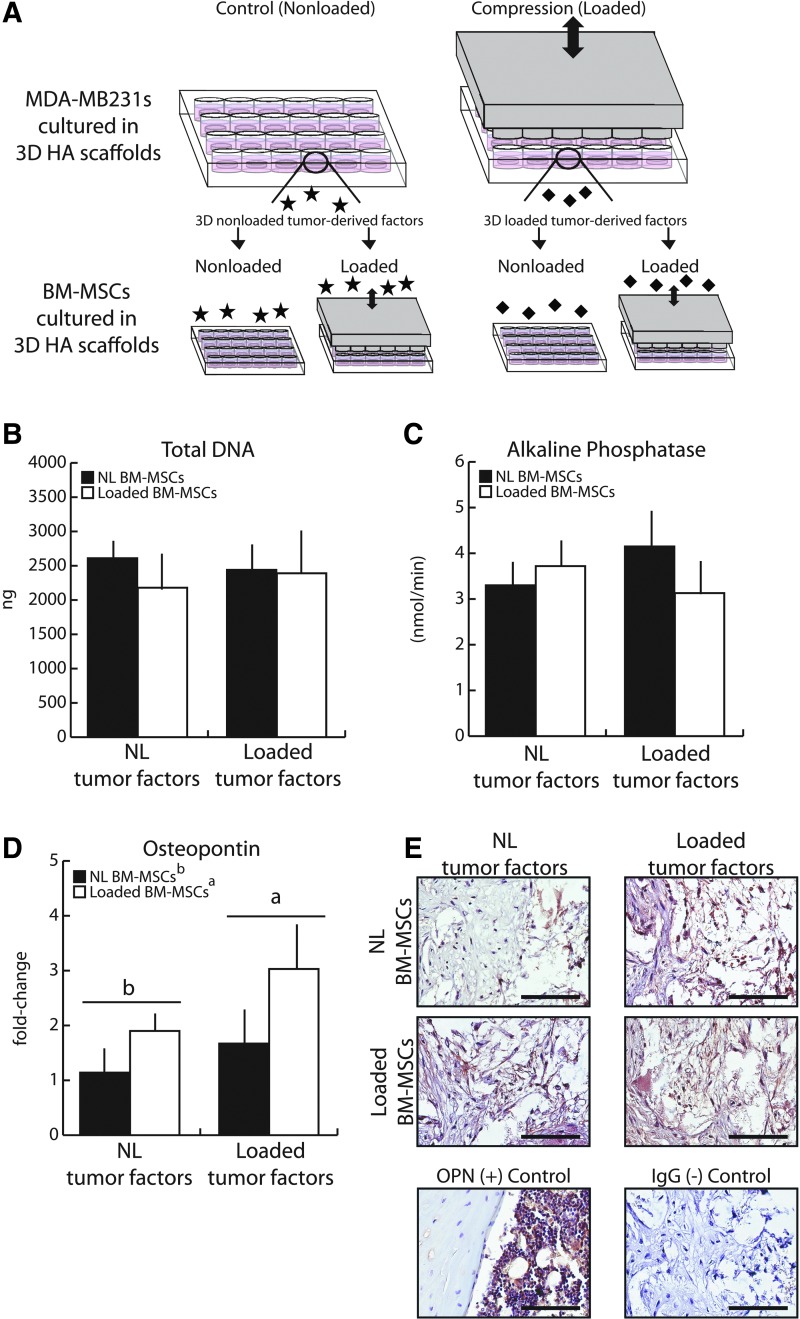
Using conditioned media collected from 3D MDA-MB231 control or cyclically compressed cultures, we next assessed if mechanical stimulation modulates the paracrine signaling that breast cancer cells utilize to influence BM-MSC osteogenic differentiation. To this end, MDA-MB231s were seeded into HA-containing scaffolds and cyclic compression was imposed as previously described for 1 h, and conditioned media was collected 24 h later (Fig. 5A). To more comprehensively test the effect of loading on tumor cell-BM-MSC interactions in the bone microenvironment, we also assessed whether mechanical stimulation of the BM-MSCs themselves impacts their responsiveness to tumor-derived soluble factors (Fig. 5A). Surprisingly, neither total DNA content nor ALP enzyme activity was affected by loading tumor cells and/or by loading BM-MSCs (Fig. 5B, C).
FIG. 5.
Mechanically loading tumor cells affected osteopontin gene expression and protein deposition in BM-MSCs. (A) Loaded and Nonloaded (NL) tumor-conditioned media were generated from MDA-MB231 tumor cells (1.5 × 106 per scaffold) cultured in HA-containing scaffolds that underwent 1 h of cyclic compression (1 Hz, 10% peak strain) and then processed as previously described. Control, nonloaded media was generated similarly. (B) Mechanical loading of tumor cells did not alter downstream growth patterns in BM-MSCs. (C) Similarly, no differences in ALP enzyme activity were detected. (D) Quantitative real-time polymerase chain reaction (qRT-PCR) revealed that mechanical loading increased OPN expression in BM-MSCs, and this effect was more pronounced when BM-MSCs were cultured in conditioned media from loaded tumor cells (Loaded tumor factors). (E) Immunohistochemistry suggested that mechanical loading also stimulated OPN protein deposition by BM-MSCs within scaffolds. Furthermore, BM-MSCs cultured with conditioned media from mechanically loaded versus nonloaded tumor cells resulted in more uniform protein deposition within the scaffolds. Scale bars = 100 μm. Same letters (a or b) indicate similar mean values, and groupings with different letters indicate that the difference is significant by post hoc comparison of means with Bonferroni correction (p < 0.05). Color images available online at www.liebertpub.com/tea
Analysis of the bone extracellular matrix protein OPN, however, revealed differences between the varied conditions. OPN was tested as a candidate molecule secreted by BM-MSCs and osteoblasts because of its additional role in regulating interactions between osteoblasts and breast cancer cells.21,22 qPCR revealed that BM-MSCs increased OPN gene expression in response to mechanical loading by 75% (pooled Loaded groups vs. pooled NL groups) (Fig. 5D). Furthermore, when BM-MSCs received media conditioned by mechanically loaded MDA-MB231s, a trend, but no statistical significance, for increased OPN gene expression was observed, (pooled Loaded TCM vs. pooled NL TCM).
Taken together, mechanical loading of both MDA-MB231s and BM-MSCs resulted in a trend for the greatest stimulation of OPN gene expression, where loading induced an 82% upregulation of OPN gene expression in BM-MSCs receiving Loaded TCM; in comparison, OPN gene expression of BM-MSCs receiving NL TCM only increased 65%. Gene expression results were corroborated by analysis of OPN protein levels, which showed that (1) mechanically loaded BM-MSCs deposited more OPN within the cell-polymer constructs than nonloaded BM-MSCs and (2) that protein distribution within scaffolds was more uniform when conditioned media was collected from loaded versus nonloaded MDA-MB231 cultures (Fig. 5E). Collectively, these results indicate that mechanical loading modulates the interplay between tumor cells and BM-MSCs in the bone microenvironment that may regulate bone metastasis through altered OPN levels.
Discussion
We combined 3D scaffold-based culture models with controlled mechanical stimulation to investigate the impact of cyclic compression on the interplay between breast cancer cells and BM-MSCs as it pertains to bone metastasis. First, we confirmed that BM-MSCs cultured within mineral-containing 3D PLG scaffolds differentiated into mature osteoblasts and that exposure to tumor-derived soluble factors promoted this process. Finally, we revealed that conditioned media collected from mechanically loaded breast cancer cells increased OPN gene expression in BM-MSCs undergoing osteogenic differentiation. Moreover, when mechanical compression was simultaneously applied to BM-MSCs, OPN gene expression was further enhanced. These results suggest that mechanical loading of 3D scaffold-based culture models may be utilized to evaluate the role of physiologically relevant physical cues on bone metastatic breast cancer. Furthermore, our data imply that cyclic mechanical stimuli within the bone microenvironment modulate interactions between tumor cells and BM-MSCs that are relevant to bone metastasis.
While most research on osteolytic breast cancer metastasis focuses on osteoclasts, osteoblasts are similarly critical as they can provide factors triggering osteoclastogenesis.34 Yet breast cancer is generally accepted to inhibit osteogenic differentiation,16,27,35,36 suppress numbers of mature osteoblasts,37–39 reduce bone-forming activity and adhesion,16,36,40 and increase osteoblast apoptosis.15,39 Our results, in contrast, suggest enhanced osteogenic differentiation, which may be related to the stage of osteogenic differentiation at the time of exposure to tumor-derived factors.37,41 While most previous studies reporting negative effects of breast cancer on osteoblastic cells utilized committed preosteoblasts or mature osteoblasts, we tested effects on BM-MSCs.
Interestingly, we did not observe differences in osteogenic differentiation when BM-MSCs were cultured with tumor-conditioned media generated in 2D versus 3D. Given that initial tumor cell adhesion to the bone surface occurs in a pseudo-2D context in vivo, it is possible that tumor cells secrete osteogenic signaling factors independent of substrate dimensionality. However, since our comparison was limited to measurement of ALP activity, we cannot exclude that other markers of osteogenesis are differentially affected by 2D versus 3D tumor cell-conditioned media. This possibility should be evaluated in the future.
The interplay between tumor cells and BM-MSCs may also be affected by the stage of metastasis, in that tumor cells that just homed to bone may yield different effects than ones present within overt secondary tumors. Indeed, a recent report suggests that osteoblast numbers increased in vivo before detectable osteolysis while osteoblast numbers were depressed after osteolysis occurred.37 Cancer cells may also elicit distinct changes in bone cells depending on proximity of the two cell populations. Direct contact with tumor cells can decrease osteoblast numbers while more distant osteoblasts can increase in number possibly due to paracrine signaling,37 which is analogous to our studies using conditioned media. Indeed, these spatial differences may contribute to the observation that breast cancer bone metastases are typically associated with both bone degradation and new formation although the net effect is generally osteolysis.42
Mechanical loading has been long recognized as an anabolic stimulant of bone tissue remodeling through stimulation of osteogenic differentiation of MSCs, commitment of MSCs to the osteoblastic lineage, promotion of osteoblast survival,4,13,43 as well as osteoblast production of bone matrix proteins such as OPN.44 Our results now imply that in the context of bone metastasis, mechanical loading can also enhance the ability of breast cancer cells to stimulate osteoblastic production of osteopontin (OPN).
OPN is generally known as a factor mediating bone cell adhesion to their extracellular matrix,19 but experimental evidence suggests that it may also be priming for breast cancer cell adhesion.21,45 It is possible that loading-induced changes in breast cancer cell-BM-MSC interactions may modulate processes involved in initial stages of secondary tumor formation in bone, but additional work is needed to investigate how mechanical loading affects the ability of breast cancer cells to adhere to the bone matrix, even with increased OPN. For example, cyclic loading modulates integrin expression in osteoblasts,46 and solid stress (e.g., constant compression) modulates integrin expression in breast cancer cells,47 but whether cyclic loading has a similar effect on cancer cells is unclear.
In addition, breast cancer-mediated shifts in osteoblast phenotype have previously been observed. For example, breast cancer cells induced an inflammatory phenotype in committed preosteoblasts in vivo and in vitro, resulting in production of chemoattractant proteins, such as osteoclastogenic interleukin-6 (IL-6).17,48 Whether or not loading affects these differences, which role OPN may play in this process, and if loading-dependent differences in protein secretion are dependent upon the differentiation stage of osteogenic cells are open questions that will need to be addressed in future studies.
When both breast cancer cells and BM-MSCs underwent mechanical loading, OPN gene expression was further increased, suggesting a synergistic effect. However, our previous in vivo studies applying increased mechanical loading of tumor-bearing bones prevented tumorigenesis.14 This discrepancy might be related to the stage of osteoblast differentiation. Specifically, MSCs in the bone marrow have been shown to be pro-tumorigenic,18,41 but they only make up a small fraction of the total bone cell population. Therefore, the downstream effects of increased loading of tumor cells on BM-MSC osteogenic differentiation might be small relative to the effects on committed or mature osteoblasts.
We initially focused on changes in OPN gene and protein expression based on its documented role in both bone matrix formation and metastatic function in breast cancer cells, but other proteins may be playing a similarly important role in mediating the effects of mechanical loading during bone metastasis. For example, transforming growth factor beta (TGF-β) is also a protein known to play a role in both regulation of osteogenic differentiation and osteoblast function,4,49,50 its expression is sensitive to mechanical loading,51 and furthermore, it is a bone matrix protein secreted by osteoblasts that also plays a significant role in osteolytic metastasis.11 Interestingly, TGF-β also regulates OPN expression in osteoblasts49,50 and, thus, could be playing a role in breast cancer cell OPN expression in our studies.
In conclusion, we have established a 3D in vitro model platform that allows studying the impact of cyclic mechanical loading on the relationship between metastatic breast cancer cells and osteoblastic bone cells. We showed that breast cancer cells undergoing compressive loading stimulated osteoblastic gene and protein expression of osteopontin, effects that were further increased when BM-MSCs also received mechanical loading. These results suggest that breast cancer cells may be utilizing BM-MSCs undergoing early osteogenic differentiation to form pro-tumorigenic niches. However, more work is needed to fully characterize the effects of dynamic mechanical forces on bone metastatic tumor pathogenesis and subsequent osteolysis. For example, the studies described here utilized isolated paracrine interactions between tumor cells and BM-MSCs under the influence of mechanical loading, but bone remodeling involves a tight interplay among several cell types beyond BM-MSCs and osteoblasts, including osteoclasts and osteocytes. Future studies will be needed to integrate these additional cell types into our in vitro mechanical loading model, to assess whether direct cell–cell contact may modulate this interplay,52,53 and what functional consequences these interactions have on breast cancer bone metastasis. In addition, whether the effects of dynamic loading on breast cancer cell-BM-MSC interactions observed in these studies are conserved across multiple breast cancer cell lines or patient-derived cells, or whether they differ based on some characteristic of the breast cancer (e.g., subtype), must also be determined.
Acknowledgments
This work was supported by NIH/NCI R01CA173083 (C.F.), Individual Biomedical Research Award by the Hartwell Foundation (M.E.L.), Graduate Assistance in Areas of National Need training grant from the Department of Education (P200A150273, A.E.C.), New York State Stem Cell Science (M.J.L., S.C.M.), Sigma Xi Committee on Grants-in-Aid of Research (S.C.M.), and The Hunter R. Rawlings III Cornell Presidential Research Scholars program (P.V.P., Y.L.). The authors gratefully acknowledge the assistance of the laboratory of Dr. Lawrence Bonassar, Daniel Brooks, Mark Riccio, and Dr. Fred von stein in the Cornell University BRC CT Imaging Facility; and Dr. Nora Springer, Dr. Bo Ri Seo, and Andrew Recknagel for help with histology and histomorphometric analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.DeSantis C.E., Lin C.C., Mariotto A.B., Siegel R.L., Stein K.D., Kramer J.L., Alteri R., Robbins A.S., and Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64, 252, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N., Salem M., Nitz U., Gluz O., and Liedtke C. Personalized treatment of early-stage breast cancer: present concepts and future directions. Cancer Treat Rev 36, 584, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E. Bisphosphonates in breast cancer. Ann Oncol 16, 687, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Manolagas S.C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21, 115, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Guise T.A. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact 2, 570, 2002 [PubMed] [Google Scholar]
- 6.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27, 165, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Green J.R. Antitumor effects of bisphosphonates. Cancer 97, 840, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Stresing V., Daubine F., Benzaid I., Monkkonen H., and Clezardin P. Bisphosphonates in cancer therapy. Cancer Lett 257, 16, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T.V., Sleiman M., Moriarty T., Herrick W.G., and Peyton S.R. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 35, 5749, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Page J.M., Merkel A.R., Ruppender N.S., Guo R., Dadwal U.C., Cannonier S.A., Basu S., Guelcher S.A., and Sterling J.A. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin beta3 and TGF-beta receptor type II. Biomaterials 64, 33, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling J.A., and Guelcher S.A. Bone structural components regulating sites of tumor metastasis. Curr Osteoporos Rep 9, 89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner C.H. Three rules for bone adaptation to mechanical stimuli. Bone 23, 399, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Robling A.G., and Turner C.H. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19, 319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch M.E., Brooks D., Mohanan S., Lee M.J., Polamraju P., Dent K., Bonassar L., van der Meulen M.C., and Fischbach C. In Vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res 28, 2357, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastro A.M., Gay C.V., Welch D.R., Donahue H.J., Jewell J., Mercer R., DiGirolamo D., Chislock E.M., and Guttridge K. Breast cancer cells induce osteoblast apoptosis: a possible contributor to bone degradation. J Cell Biochem 91, 265, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Mendoza-Villanueva D., Zeef L., and Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFbeta-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res 13, R106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussard K.M., Venzon D.J., and Mastro A.M. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J Cell Biochem 111, 1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergfeld S.A., and DeClerck Y.A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 29, 249, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Carvalho R.S., Kostenuik P.J., Salih E., Bumann A., and Gerstenfeld L.C. Selective adhesion of osteoblastic cells to different integrin ligands induces osteopontin gene expression. Matrix Biol 22, 241, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Blair H.C., Robinson L.J., and Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun 328, 728, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Schneider J.G., Amend S.R., and Weilbaecher K.N. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone 48, 54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anborgh P.H., Mutrie J.C., Tuck A.B., and Chambers A.F. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med 14, 2037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischbach C., Chen R., Matsumoto T., Schmelzle T., Brugge J.S., Polverini P.J., and Mooney D.J. Engineering tumors with 3D scaffolds. Nat Methods 4, 855, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Pathi S.P., Kowalczewski C., Tadipatri R., and Fischbach C. A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS One 5, e8849, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathi S.P., Lin D.D., Dorvee J.R., Estroff L.A., and Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 32, 5112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J., Genetos D.C., and Leach J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A 16, 127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhurjati R., Krishnan V., Shuman L.A., Mastro A.M., and Vogler E.A. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin Exp Metastasis 25, 741, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Murphy W.L., Hsiong S., Richardson T.P., Simmons C.A., and Mooney D.J. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials 26, 303, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ennett A.B., Kaigler D., and Mooney D.J. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A 79, 176, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ballyns J.J., and Bonassar L.J. Dynamic compressive loading of image-guided tissue engineered meniscal constructs. J Biomech 44, 509, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen T.D., and Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Arrington S.A., Schoonmaker J.E., Damron T.A., Mann K.A., and Allen M.J. Temporal changes in bone mass and mechanical properties in a murine model of tumor osteolysis. Bone 38, 359, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Chomczynski P., and Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1, 581, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Boyce B.F., and Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473, 139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fong J.E., Le Nihouannen D., and Komarova S.V. Tumor-supportive and osteoclastogenic changes induced by breast cancer-derived factors are reversed by inhibition of {gamma}-secretase. J Biol Chem 285, 31427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer R.R., Miyasaka C., and Mastro A.M. Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clin Exp Metastasis 21, 427, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Brown H.K., Ottewell P.D., Evans C.A., and Holen I. Location matters: osteoblast and osteoclast distribution is modified by the presence and proximity to breast cancer cells in vivo. Clin Exp Metastasis 29, 927, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Krawetz R., Wu Y.E., Rancourt D.E., and Matyas J. Osteoblasts suppress high bone turnover caused by osteolytic breast cancer in-vitro. Exp Cell Res 315, 2333, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Phadke P.A., Mercer R.R., Harms J.F., Jia Y., Frost A.R., Jewell J.L., Bussard K.M., Nelson S., Moore C., Kappes J.C., Gay C.V., Mastro A.M., and Welch D.R. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res 12, 1431, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory L.S., Choi W., Burke L., and Clements J.A. Breast cancer cells induce osteolytic bone lesions in vivo through a reduction in osteoblast activity in mice. PLoS One 8, e68103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molloy A.P., Martin F.T., Dwyer R.M., Griffin T.P., Murphy M., Barry F.P., O'Brien T., and Kerin M.J. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer 124, 326, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Guise T.A., and Mundy G.R. Cancer and bone. Endocr Rev 19, 18, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Thompson W.R., Rubin C.T., and Rubin J. Mechanical regulation of signaling pathways in bone. Gene 503, 179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matziolis D., Tuischer J., Matziolis G., Kasper G., Duda G., and Perka C. Osteogenic predifferentiation of human bone marrow-derived stem cells by short-term mechanical stimulation. Open Orthop J 5, 1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohyama Y., Nemoto H., Rittling S., Tsuji K., Amagasa T., Denhardt D.T., Nifuji A., and Noda M. Osteopontin-deficiency suppresses growth of B16 melanoma cells implanted in bone and osteoclastogenesis in co-cultures. J Bone Miner Res 19, 1706, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Liu L., Zong C., Li B., Shen D., Tang Z., Chen J., Zheng Q., Tong X., Gao C., and Wang J. The interaction between beta1 integrins and ERK1/2 in osteogenic differentiation of human mesenchymal stem cells under fluid shear stress modelled by a perfusion system. J Tissue Eng Regen Med 8, 85, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Demou Z.N. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann Biomed Eng 38, 3509, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Bussard K.M., Okita N., Sharkey N., Neuberger T., Webb A., and Mastro A.M. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clin Exp Metastasis 27, 331, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Fagenholz P.J., Warren S.M., Greenwald J.A., Bouletreau P.J., Spector J.A., Crisera F.E., and Longaker M.T. Osteoblast gene expression is differentially regulated by TGF-beta isoforms. J Craniofac Surg 12, 183, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Lai C.F., and Cheng S.L. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J Biol Chem 277, 15514, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Klein-Nulend J., Roelofsen J., Sterck J.G., Semeins C.M., and Burger E.H. Mechanical loading stimulates the release of transforming growth factor-beta activity by cultured mouse calvariae and periosteal cells. J Cell Physiol 163, 115, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Fong E.L., Wan X., Yang J., Morgado M., Mikos A.G., Harrington D.A., Navone N.M., and Farach-Carson M.C. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77, 164, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sosnoski D.M., Norgard R.J., Grove C.D., Foster S.J., and Mastro A.M. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis 32, 335, 2015 [DOI] [PubMed] [Google Scholar]