Abstract
We have described the development of capsid-modified next-generation AAV vectors for both AAV2 and AAV3 serotypes, in which specific surface-exposed tyrosine (Y), serine (S), threonine (T), and lysine (K) residues on viral capsids were modified to achieve high-efficiency transduction at lower doses. We have also described the development of genome-modified AAV vectors, in which the transcriptionally inactive, single-stranded AAV genome was modified to achieve improved transgene expression. Here, we describe that combination of capsid modifications and genome modifications leads to the generation of optimized AAV serotype vectors, which transduce cells and tissues more efficiently, both in vitro and in vivo, at ∼20–30-fold reduced doses. These studies have significant implications in the potential use of the optimized AAV serotype vectors in human gene therapy.
Introduction
Recombinant aav vectors have proven to be safe in a number of phase I/II/III clinical trials, having shown clinical efficacy in five human diseases.1–9 However, in some cases, the use of large vector doses to achieve therapeutic benefits has also been shown to trigger the host immune response to the capsid proteins.10 In the first clinical for the potential gene therapy of hemophilia B, AAV2 serotype vectors failed to express therapeutic levels of human factor IX (hF.IX) at a dose of 1013 vg in a patient. When the dose was increased to 1014 vg, AAV2 vectors did express the therapeutic level of hF.IX. However, such a high vector dose also induced a cytotoxic T-cell (CTL) response against the capsid proteins.11–13 In a subsequent clinical trial with AAV8 serotype vectors, clinical efficacy was observed, but once again, at the highest dose, and AAV8 vectors also induced an immune response in two patients.5,6 Thus, the vector-induced toxicity appears to correlate directly with the vector dose. It is, therefore, desirable to develop strategies to achieve clinical efficacy with AAV vectors at significantly reduced doses. In such a pursuit, we developed the next-generation AAV serotype vectors, in which specific surface-exposed tyrosine (Y), serine (S), threonine (T), and lysine (K) residues were mutagenized to achieve enhanced transduction efficiency at reduced vector doses.14–16
In addition to the capsid-modified AAV vectors, we have also described the generation of genome-modified vectors, with which improved transgene expression from single-stranded AAV (ssAAV) vectors can be achieved.17 In the present studies, we wished to examine whether combining the capsid modifications and genome modifications might lead to the generation of optimized AAV vectors capable of increased transduction at further reduced doses. To this end, ssAAV2 and ssAAV3 serotype vectors were generated using the strategy depicted schematically in Figure 1. Here, we provide experimental evidence that optimized AAV serotype vectors do indeed transduce cells and tissues more efficiently, both in human cell lines in vitro and in murine models in vivo, at doses with which little transgene expression is achieved with the first-generation AAV serotype vectors. These studies should have significant implications in the potential use of the optimized AAV serotype vectors in human gene therapy.
Figure 1.
Schematic representation of the first-generation (A, C), capsid-modified (B), genome-modified (D), and capsid+genome-modified AAV (E) vectors. Surface-exposed tyrosine (Y), serine (S), threonine (T), and lysine (K) residues, and their replacement with phenylalanine (F) and valine (V) residues are depicted by red and green symbols, respectively. The negative regulatory sequences within the D-sequence in the viral ITRs are denoted by the red bars.
Materials and Methods
Cells and reagents
Human embryonic kidney cell line, HEK293, and human hepatocellular carcinoma cell line, Huh7, were described previously,18,19 and maintained as monolayer cultures in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Sigma) and antibiotics (Lonza).
Production of recombinant AAV vectors
Recombinant capsid-optimized AAV2 (Y444 + 500 + 730F+T491V quadruple-mutant [QM]) and AAV3 (S663V+T492V double-mutant) vectors containing the firefly luciferase gene (Fluc) flanked by either the unmodified wild-type (WT) or the D-sequence-deleted AAV2-ITRs (LC1 or LC2) driven by the chicken β-actin promoter (CBA) were generated by triple transfection as described previously, with modification.20 Briefly, HEK293 cells were transfected using polyethyleneimine (PEI; linear, MW 25,000; Polysciences, Inc.). Seventy-two hours posttransfection, cells were harvested and vectors were purified by iodixanol (Sigma) gradient centrifugation and ion exchange column chromatography (HiTrap SP/Q HP columns; 5 ml; GE Healthcare). Virus was then concentrated and buffer exchanged into phosphate-buffered saline (PBS) in three cycles using centrifugal spin concentrators (Apollo; 150 kDa cutoff, 20 ml capacity; CLP). To determine genome titers, 10 μl of purified virus stocks was incubated with DNase I (Invitrogen) at 37°C for 2 hr, and then with Proteinase K (Invitrogen) at 55°C for an additional 2 hr. The reaction mixture was purified by phenol/chloroform, followed by chloroform extraction. Packaged DNA was precipitated overnight with ethanol in the presence of 20 μg glycogen (Invitrogen). DNase I-resistant AAV2 particle titers were determined by qPCR assays. SYBR GreenER PCR Master Mix (Invitrogen) and the following primer pairs specific for the CBA promoter were used: F-5′-TCCCATAGTAACGCCAATAGG-3′; R-5′-CTTGGCATATGATACACTTGATG-3′.
Recombinant AAV vector transduction assays in vitro
Recombinant AAV vector transduction was performed as previously described.21 Briefly, human Huh7 cells were transduced with 1 × 103 vg/cell with QM-ssAAV2-WT-Fluc, QM-ssAAV2-LC1-Fluc, QM-ssAAV2-LC2-Fluc, DM-ssAAV3-WT-Fluc, DM-ssAAV3-LC1-Fluc, or DM-ssAAV3-LC2-Fluc vectors and incubated for 72 hr. Transgene expression was determined by Luciferase Assay System (mean ± SD) as described previously.22
Whole-body bioluminescence imaging in vivo
All animal experiments were approved by the University of Florida Institutional Animal Care and Use Committee. All procedures were done in accordance with the principles of the National Research Council's Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering. Ten-week-old C57BL/6 male mice (Jackson Laboratory) were injected intravenously with 5 × 109 vg/animal of WT or mutant ssAAV2-Fluc vectors (n = 3). NSG mice were xenotransplanted with human liver tumors as previously described23 and were injected intravenously with 3 × 109 vg/animal of WT or mutant ssAAV3-Fluc vectors (n = 3). Luciferase activity was analyzed 2 weeks postinjection using a Xenogen IVIS Lumina System (Caliper Life Sciences).24 Briefly, mice were anesthetized with 2% isofluorane and injected intraperitoneally with luciferin substrate (beetle luciferin; Caliper Life Sciences) at a dose of 150 μg/g of body weight. Mice were placed in a light-tight chamber and images were collected at 5 min after the substrate injection. Images were analyzed by the Living Image 3.2 software (Caliper Life Sciences) to determine relative signal intensity.
Statistical analysis
Results are presented as mean ± SD. Differences between groups were identified using a grouped-unpaired two-tailed distribution of Student's t-test. p-Values <0.05 were considered statistically significant.
Results
Optimized AAV2 and AAV3 serotype vectors efficiently transduce human cells in vitro at low doses
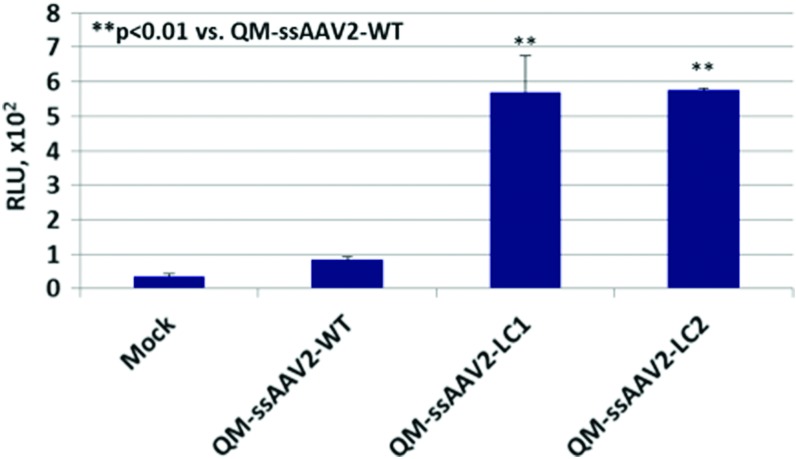
We have previously described that a capsid-modified Y444 + 500 + 730F+T491V quadruple-mutant (QM) AAV2 vector is highly efficient, as it is capable of largely circumventing proteasomal degradation, and as a consequence, ∼90% of the vector successfully traffics to the nucleus.15 We have also described that for the genome-modified AAV2 vectors, LC1 and LC2, in which one of the D-sequences in the viral inverted terminal repeats (ITRs) was deleted, the efficiency of transgene expression from the single-stranded genomes was significantly increased.17 Thus, as depicted schematically in Figure 1, we generated the following set of QM-ssAAV2 vectors containing the firefly luciferase (Fluc) reporter gene flanked by either the unmodified ITRs (QM-ssAAV2-WT-Fluc), or with D-sequence−deleted ITRs (QM-ssAAV2-LC1-Fluc and QM-ssAAV2-LC2-Fluc). A human hepatocellular carcinoma (HCC) cell line, Huh7, was transduced with each of the 3 optimized AAV2 vectors deliberately at a relative low MOI of 1000 vg/cell under identical conditions, and transgene expression was determined 72 hr posttransduction. As can be seen in Figure 2, even with the capsid-optimized QM-ssAAV2 vector, only a low-level transgene expression was detected at such a low MOI. Interestingly, however, with both QM-ssAAV2-LC1-Fluc and QM-ssAAV2-LC2-Fluc vectors, up to 6-fold increase in transgene expression was achieved.
Figure 2.
Comparative analysis of the transduction efficiency of capsid-modified and capsid+genome-modified AAV2 serotype vectors. Human Huh7 cells were either mock-transduced, or transduced with the indicated ssAAV2-Fluc vectors at an MOI of 1 × 103 vg/cell. Fluc gene expression was determined 72 hr posttransduction. **p < 0.01 vs. QM-AAV2 vectors containing unmodified single-stranded DNA genomes. Color images available online at www.liebertpub.com/hgtb
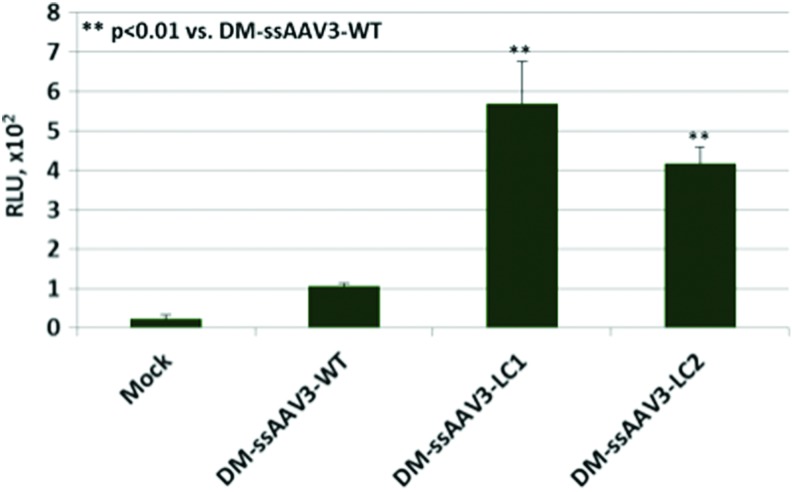
These studies were extended to include the AAV3 serotype vectors. In our previously published studies, we identified the S663V+T492V double-mutant (DM) AAV3 vector as the most efficient vector in transducing human liver cancer cell lines.25 Thus, the following sets of DM-ssAAV3-Fluc vectors were generated: DM-ssAAV3-WT-Fluc; DM-ssAAV3-LC1-Fluc; and DM-ssAAV3-LC2-Fluc. Human Huh7 cells were transduced with each of the 3 optimized AAV3 vectors, once again, deliberately at a relative low MOI of 1000 vg/cell under identical conditions, and transgene expression was determined 72 hr posttransduction as described above. These results are shown in Figure 3. It is evident that whereas only a low level of transgene expression was obtained with the DM-ssAAV3-WT-Fluc vector, the transduction efficiency of both DM-ssAAV3-LC1-Fluc and DM-ssAAV3-LC2-Fluc vectors was increased by up to 6-fold at such a low MOI.
Figure 3.
Comparative analysis of the transduction efficiency of capsid-modified and capsid+genome-modified AAV3 serotype vectors. Human Huh7 cells were either mock-transduced, or transduced with the indicated ssAAV3-Fluc vectors at an MOI of 1 × 103 vg/cell. Fluc gene expression was determined 72 hr posttransduction. **p < 0.01 vs. DM-AAV3 vectors containing unmodified single-stranded DNA genomes. Color images available online at www.liebertpub.com/hgtb
Efficient transduction of mouse and human cells by optimized AAV2 and optimized AAV3 serotype vectors can also be achieved at low doses in murine models in vivo
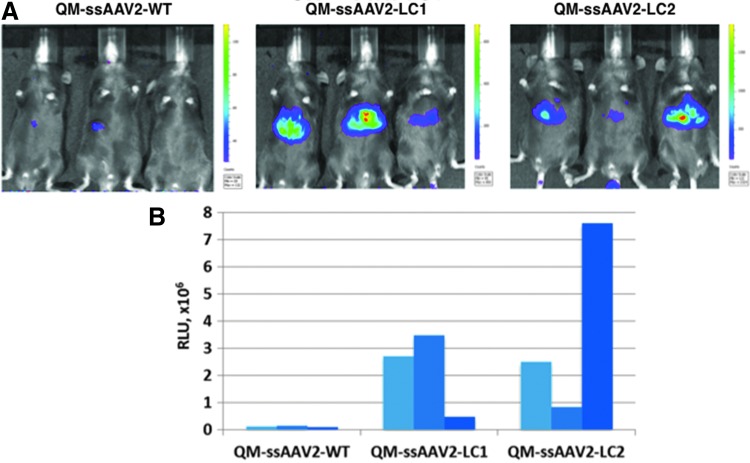
We next evaluated the transduction efficiency of the optimized AAV2 vectors in a murine model in vivo. C57BL/6 mice (n = 3 for each group) were injected via the tail vein with a relatively low dose of 5 × 109 vg of each vector and the levels of expression of Fluc gene were assessed two weeks postinjection by whole-body bioluminescence imaging. These results are shown in Figure 4. Whereas transgene expression mediated by the QM-ssAAV2-WT-Fluc vectors was barely detectable, up to ∼6–10-fold increase in transgene expression in the liver was readily detectable in mice injected with QM-ssAAV2-LC1-Fluc and QM-ssAAV2-LC2-Fluc vectors, although some variability among the mice was noted, which would be expected, given that such a low dose of the vector were administered.
Figure 4.
In vivo imaging of Fluc gene expression after tail vein injection of various indicated ssAAV2 vectors. C57BL/6 mice were injected with 5 × 109 vg/animal of each vector expressing the firefly luciferase gene. Live images were obtained 2 weeks after vector administration to determine the luciferase activity. The visual output represents the number of photons emitted/second/cm2 as a false color image in which the maximum is red and the minimum is blue (A). Quantitation of the signal intensity in each individual mouse (B).
We also extended these studies to include optimized AAV3 serotype vectors. Since we and others have previously reported that AAV3 vectors do not transduce any murine cells, tissues, or organs, we utilized an NSG mouse xenograft model, in which human liver tumors are grown after subcutaneous injection of human liver cancer lines.26 NSG mice bearing human liver tumors (n = 3 for each group) were injected via the tail vein with a relatively low dose of 3 × 109 vg of each vector intravenously and Fluc gene expression was determined 3 days postinjection by whole-body bioluminescence imaging as described above. These results, shown in Figure 5, document ∼5–8-fold increase in the transgene expression in 1 of 3 mice injected with DM-ssAAV3-LC1-Fluc vectors, and in 2 of 3 mice injected with DM-ssAAV3-LC2-Fluc vectors, which was restricted to human liver tumors. Transgene expression mediated by DM-ssAAV3-WT-Fluc vectors was barely detectable in all three mice. Again, some variability was observed, given that such a low dose of the vector was administered. Taken together, these data nonetheless document that it is indeed feasible to achieve improved transduction efficiency by optimized AAV at significantly reduced vector doses.
Figure 5.
In vivo imaging of Fluc gene expression after tail vein injection of various indicated ssAAV3 vectors. NSG mice xenotransplanted with Huh7 cells were injected with 3 × 109 vg/animal of each vector expressing the firefly luciferase gene. Live images were obtained 3 days after vector administration to determine the luciferase activity. The visual output represents the number of photons emitted/second/cm2 as a false color image in which the maximum is red and the minimum is blue (A). Quantitation of the signal intensity in each individual mouse (B).
Discussion
Although AAV is a nonpathogenic virus, there is little doubt that, not unlike other pathogenic viruses, AAV did not evolve to be used as a vector for the purposes of gene delivery.27 Thus, the use of the first generation of AAV vectors, although remarkably successful in a number of phase I/II/III clinical trials, not surprisingly, did lead to an undesirable outcome, that is, a cytotoxic T-cell-mediated immune response to viral capsid proteins in two clinical trials for the potential gene therapy of hemophilia B with AAV2 and AAV8 serotype vectors, respectively, especially when relatively high vector doses were used.5,11 Thus, we have made the argument that the AAV capsids must be modified in such a way that the host immune system does not target the administered vectors. In other words, in order to evade the host immune system, the vector needs to be different from the virus. Furthermore, the single-stranded nature of the AAV genome, which is transcriptionally inactive, also renders the first generation of ssAAV vectors less than optimal, although it is quite remarkable that clinical efficacy has been achieved with the first-generation ssAAV vectors in at least five human clinical trials.1–9
The elegant work of Samulski and colleagues has overcome this barrier with the development of double-stranded self-complementary AAV (scAAV) vectors, but the packaging capacity of these vectors is compromised in that expression cassettes exceeding ∼2.5 kb are excluded.28,29 Although the successful use of scAAV8-F.IX vectors in the gene therapy of hemophilia B trial is a clear testament to this approach,6 it is unlikely to be applicable to hemophilia A, the incidence of which is significantly higher, as the F.VIII therapeutic gene far exceeds the packaging capacity of scAAV vectors. Thus, it would appear that at least two significant limitations associated with the first generation of AAV vectors need to be overcome: (1) elimination/diminution of the vector-induced host cell immune response, and (2) achievement of therapeutic levels of transgene expression from expression cassettes encapsidated in ssAAV vectors.
Over the past nearly two decades, we have invested substantial efforts to overcome these two limitations of the first-generation AAV vectors. By modifying the capsids, we have generated the next-generation AAV vectors in which specific surface-exposed tyrosine (Y), serine (S), threonine (T), and lysine (K) residues were mutagenized, and various permutations and combinations of these mutations thereof, to significantly augment the transduction efficiency of these vectors, both in vitro and in vivo, by circumventing proteasome-mediated degradation.14–16,30 At least one of these vectors has also shown to be less immunogenic31 and, in a recent phase I clinical trial, shown efficacy for the potential gene therapy of Leber's hereditary optic neuropathy.32 We have also described strategies involving genome modifications that lead to the generation of ssAAV vectors with which high efficiency of transgene expression can be achieved.17
In the present studies, we combined the two strategies to generate the optimized AAV vectors. Reassuringly, the combination of the two strategies further augmented the transduction efficiency up to ∼6-fold, compared with that of the most efficient AAV2 and AAV3 serotype vectors containing single-stranded expression cassettes. These observations further suggest that this approach can be successfully applied to any and all AAV serotype vectors.
Despite the obvious advances, our studies have a few limitations in that we did not evaluate the efficacy of the optimized AAV serotype vectors in primary human cells in vitro, and in our in vivo studies, only a reporter gene and a limited numbers of animals were used. Additional studies with larger cohorts are warranted to address the issue of interanimal variations as well as to determine the minimal effective doses of therapeutic optimized AAV serotype vectors. However, it is tempting to speculate that the optimized AAV serotype vectors are likely to be less immunogenic, but this predication must await experimental validation. Ultimately, only future clinical trials in humans will validate the potential utility of optimized AAV serotype vectors in human gene therapy.
Acknowledgments
This work was supported in part by Bankhead-Coley New Investigator Research Grant and Alex's Lemonade Stand Young Investigator Award (to C.L.); Public Health Service grants R01 HL-097088 and R21 EB-015684 from the National Institutes of Health (to A.S.); and grants from the Children's Miracle Network (to C.L. and A.S.).
Author Disclosure
A.S. holds issued patents related to AAV vectors that have been licensed to various AAV gene therapy companies. All other authors declare no competing financial interests.
References
- 1.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 2.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwu WL, Muramatsu S, Tseng SH, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med 2012;4:134ra61. [DOI] [PubMed] [Google Scholar]
- 8.Gaudet D, Methot J, Dery S, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther 2013;20:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014;383:1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mingozzi F, High KA. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 12.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–422 [DOI] [PubMed] [Google Scholar]
- 13.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A 2008;105:7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslanidi GV, Rivers AE, Ortiz L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: The final threshold? PLoS One 2013;8:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslanidi GV, Rivers AE, Ortiz L, et al. High-efficiency transduction of human monocyte-derived dendritic cells by capsid-modified recombinant AAV2 vectors. Vaccine 2012;30:3908–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling C, Wang Y, Lu Y, et al. Enhanced transgene expression from recombinant single-stranded D-sequence-substituted adeno-associated virus vectors in human cell lines in vitro and in murine hepatocytes in vivo. J Virol 2015;89:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Gregory RI. Identification of small molecule inhibitors of Zcchc11 TUTase activity. RNA Biol 2015;12:792–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YH, Wang Y, Yusufali AH, et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med 2014;12:483–494 [DOI] [PubMed] [Google Scholar]
- 20.Dong B, Moore AR, Dai J, et al. A concept of eliminating nonhomologous recombination for scalable and safe AAV vector generation for human gene therapy. Nucleic Acids Res 2013;41:6609–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Dong B, Firrman J, et al. Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery. Hum Gene Ther Methods 2014;25:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling C, Wang Y, Feng YL, et al. Prevalence of neutralizing antibodies against liver-tropic adeno-associated virus serotype vectors in 100 healthy Chinese and its potential relation to body constitutions. J Integr Med 2015;13:341–346 [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Li W, Zhou Y, et al. The overexpression and nuclear translocation of Trx-1 during hypoxia confers on HepG2 cells resistance to DDP, and GL-V9 reverses the resistance by suppressing the Trx-1/Ref-1 axis. Free Radic Biol Med 2015;82:29–41 [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Tian L, Shen H, et al. DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene 2013;32:4845–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling C, Wang Y, Zhang Y, et al. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther 2014;25:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng B, Ling C, Dai Y, et al. Development of optimized AAV3 serotype vectors: Mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012;19:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava A. Adeno-associated virus: The naturally occurring virus versus the recombinant vector. Hum Gene Ther 2016;27:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarty DM, Fu H, Monahan PE, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 2003;10:2112–2118 [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Ma HI, Li J, et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 2003;10:2105–2111 [DOI] [PubMed] [Google Scholar]
- 30.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuer WJ, Schiffman JC, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: Initial results. Ophthalmology 2016;123:558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]