Abstract
Development and maintenance of a vascular network are critical for bone growth and homeostasis; strategies that promote vascular function are critical for clinical success of tissue-engineered bone constructs. Co-culture of endothelial cells (ECs) with mesenchymal stem cells (MSCs) and exposure to 10% cyclic tensile strain have both been shown to regulate osteogenesis in isolation, but potential synergistic effects have yet to be explored. The objective of this study was to expose an MSC-EC co-culture to 10% cyclic tensile strain to examine the role of this mechanical stimulus on MSC-EC behavior. We hypothesized that paracrine signaling from ECs would stimulate osteogenesis of MSCs, and exposure to 10% cyclic tensile strain would enhance this anabolic signal. Human umbilical vein ECs and human bone marrow-derived MSCs were either monocultured or co-cultured at a 1:1 ratio in a mixed osteo/angiogenic medium, exposed to 10% cyclic tensile strain at 1 Hz for 4 h/day for 2 weeks, and biochemically and histologically analyzed for endothelial and osteogenic markers. While neither 10% cyclic tensile strain nor co-culture alone had a significant effect on osteogenesis, the concurrent application of strain to an MSC-EC co-culture resulted in a significant increase in calcium accretion and mineral deposition, suggesting that co-culture and strain synergistically enhance osteogenesis. Neither co-culture, 10% cyclic tensile strain, nor a combination of these stimuli affected endothelial markers, indicating that the endothelial phenotype remained stable, but unresponsive to the stimuli evaluated in this study. This study is the first to investigate the role of cyclic tensile strain on the complex interplay between ECs and MSCs in co-culture. The results of this study provide key insights into the synergistic effects of 10% cyclic tensile strain and co-culture on osteogenesis. Understanding mechanobiological factors affecting MSC-EC crosstalk will help enhance strategies for creating vascularized tissues in tissue engineering and regenerative medicine.
Introduction
The aim of bone tissue engineering is to regenerate functional bone using a combination of biomaterials, cells, and signals (growth factors, mechanical stimuli, etc.) to overcome drawbacks of current treatments. Bone tissue engineering strategies have primarily focused on designing constructs with properties similar to native bone in terms of composition and mechanical properties. However, current strategies neglect the development and formation of a vascular network, a critical component of bone growth and homeostasis,1,2 and suffer from donor site morbidity, immune rejection, high cost, and poor integration.2,3 With such tissue-engineered constructs, both in vitro and in vivo studies have shown that a lack of nutrient supply and waste removal in the core of the engineered constructs results in necrosis.4,5
Establishing a vascular network is a critical step in improving current bone tissue engineering strategies. A vascular network supplies cells with nutrients and oxygen, while also removing waste products, which is especially important in large 3D constructs. Endothelial cells (ECs) are also known to play a key role in bone development, establishing an environment suitable for osteogenesis during endochondral ossification. One strategy to develop a vascular network is to culture bone-forming cells (e.g., osteoblasts, mesenchymal stem cells [MSCs]) with vascular cells (ECs and progenitors).1,6 Co-culture of human ECs and MSCs has been found to increase the alkaline phosphatase activity (an early osteogenic marker) and mineralization when the two cell types are in direct contact; conditioned media fail to produce such results.7–14 Furthermore, direct contact of ECs and MSCs has been shown to upregulate several angiogenic markers.10,13–17 Together, these results document that direct contact between ECs and MSCs can modulate both angiogenesis and osteogenesis. Paracrine signaling is also hypothesized to play a role in the stimulation of angiogenesis and osteogenesis in co-cultures, but the specific factors regulating these interactions are not well understood. ECs are thought to secrete bone-modulating factors, such as bone morphogenetic protein 2 (BMP2), endothelin 1, interleukins (IL1 and IL6), and nitric oxide, while osteoprogenitors and osteoblasts are thought to release vascular regulating factors such as vascular endothelial growth factor (VEGF).6–8,15,16,18–20
In addition to biochemical signaling between vascular and bone-forming cells in vivo, bone vascular systems are naturally exposed to mechanical stimuli. Mechanical cues are known to regulate the differentiation of MSCs and, specifically, enhance osteogenesis.21–23 Previous work has demonstrated that exposure to 10% cyclic tensile strain not only increases osteogenesis of MSCs but also increases expression of angiogenic factors,24–26 suggesting that mechanical loading may simultaneously influence both angiogenesis and osteogenesis. Hemodynamic forces apply both fluid shear stresses and tensile strains to EC lining blood vessels.27–29 Cyclic tensile strain specifically has been shown to alter tubule formation,30–32 migration,30,31 proliferation,33,34 alignment,32,35 and auto/paracrine signaling.28,31,33,34 While the role of mechanical stimulation on EC behavior has been well established in the literature, the role of 10% cyclic tensile strain on osteogenic paracrine signaling has not been directly investigated.
The in vivo bone vascular environment is complex due to the presence of multiple cell types, biochemical cues, geometric organization, and biophysical stimuli. While co-culture and mechanical stimulation have been studied in isolation, the interplay between these two stimuli is yet to be investigated. Applying both stimuli simultaneously to 2D cultures is more biomimetic than applying them in isolation and can act as an intermediate step toward more complex 3D cultures. The results of this study could provide key insights into bone–vascular interactions that could potentially enhance bone tissue engineering strategies. Therefore, the objective of this study was to examine the effects of 10% cyclic tensile strain applied to a co-culture of MSCs and ECs on angiogenesis and osteogenesis. We hypothesized that co-culture and 10% cyclic tensile strain would synergistically enhance both angiogenesis and osteogenesis.
Materials and Methods
Cell isolation and expansion
Excess human bone fragments were obtained during elective procedures from three female donors (aged 15–25 years old) at the University of North Carolina-Chapel Hill hospitals (IRB exemption protocol: 10-0201). Human MSCs were isolated from the tissue using a method based on enzymatic digestion and substrate adherence, as previously described.24,36 Briefly, bone fragments were washed with phosphate-buffered saline (PBS) containing 100 U/mL penicillin-100 μg/mL streptomycin (P/S; Corning). The fragments were then diced into small pieces and digested in a collagenase XI solution (3 mg/mL) at 37°C for 3 h. Cells were then filtered through a 100-μm cell strainer, centrifuged at 500 g for 5 min, and plated in T-75 flasks in a complete growth medium (CGM) consisting of alpha-MEM (Gibco®), 10% fetal bovine serum (Gemini Bio-Products), and 100 U/mL penicillin-100 μg/mL streptomycin (P/S; Corning, Inc.) in a humidified atmosphere of 37°C and 5% CO2. After 24 h, the nonadherent cells were washed out, and the cultures were expanded to passage 3 or 4.
Human umbilical vein endothelial cells (abbreviated as ECs throughout the rest of the article) from pooled donors (Lonza) were seeded on 0.5% gelatin-coated flasks (Nunc) and expanded to passage 2 or 3 in endothelial growth media (EGM) consisting of CGM, 30 μg/mL endothelial complete growth supplement (Sigma), and 100 μg/mL heparin sodium salt (Alfa Aesar) in a humidified atmosphere of 37°C and 5% CO2.
Co-culture validation and optimization
Various media formulations and culture surfaces were tested in three preliminary experiments to develop an optimal co-culture procedure for both osteogenesis and angiogenesis. In all experiments, a total of 50 × 103 cells per well in a six-well plate were cultured. First, to test the effects of media composition on cell viability, ECs, MSCs, or a co-culture of MSCs and ECs (MSC-EC) were cultured for 7 days in either EGM or a mixed osteogenic-endothelial medium (OEM) consisting of EGM, 50 mM ascorbic acid (Sigma), 100 μM dexamethasone (Sigma), and 1 M β-glycerophosphate (Sigma) in 0.5% gelatin-coated tissue culture plastic (TCP). Second, to determine the optimal surface coating for EC viability and phenotypic stability, ECs were cultured for 14 days in EGM on BioFlex® plates (Flexcell International) modified with different surface coatings: amino groups, pronectin, laminin, or collagen type I. Third, to determine the optimal surface coating for co-culture viability and osteogenesis, MSCs alone and MSC-EC co-cultures (1:1 ratio) were cultured in OEM for 14 days on either laminin or collagen type I-coated BioFlex plates.
Application of 10% cyclic tensile strain
Three groups were exposed to 10% cyclic tensile strain; ECs alone, MSCs alone, and MSC-EC co-cultures. ECs were seeded onto six-well collagen type I-coated BioFlex plates and maintained in EGM for 3 days to allow time to attach and spread, which was found to be required for EC viability in a pilot study (data not shown). Subsequently, MSCs were seeded, and the medium was changed to OEM for all groups for the duration of the study. A total of 50 × 103 cells were seeded in each well (either 50 × 103 of a single cell type or 25 × 103 each of both cell types for a 1:1 ratio in the MSC-EC group). One day after MSC seeding, cells were exposed to 10% cyclic tensile strain using the Flexcell® Tension Plus™ System (Flexcell International) at 1 Hz for 4 h/day for 2 weeks (Fig. 1). Cell morphology was assessed using an Olympus CKX41 microscope at 20× magnification on days 3, 7, 10, and 14 of loading.
FIG. 1.
To enhance EC attachment, ECs were cultured in EGM for 3 days before co-culture with MSCs or exposure to 10% cyclic tensile strain. From days 0 to 14, ECs, MSCs, and MSC-EC co-cultures were all cultured in OEM and either exposed to 10% cyclic tensile strain (1 Hz for 4 h/day) or left in static conditions. EC, endothelial cell; EGM, endothelial growth medium; MSC, mesenchymal stem cell; OEM, osteogenic-endothelial medium. Color images available online at www.liebertpub.com/tea
Biochemical analyses
On days 3, 7, 10, and 14 alamarBlue® solution (AbD Serotec, Inc.) was mixed into the wells (90% media, 10% alamarBlue; n = 3), and the cells were incubated at 37°C. After 3 h, the alamarBlue/medium mixture was collected, and absorbance readings were taken at 570 and 600 nm (GENios microplate reader with Magellan 5 Software; Tecan). Percent reduction in alamarBlue levels, indicating a proliferative activity, was determined from the absorbance readings according to the manufacturer's instructions.
A Hoechst DNA fluorescence assay was performed to quantify DNA on day 14 (n = 3). Cells were rinsed with PBS and digested with papain (125 μg/mL; Sigma) in 0.1 M sodium phosphate (Sigma), 5 mM l-cysteine-HCl (Sigma), and 0.05 M EDTA (Fisher; pH 6.0) at 60°C in a shaking water bath for 18 h. Type 1 calf thymus highly polymerized DNA (Sigma) was used to generate a standard curve. Briefly, a 0.2 μg/mL Hoechst 33258 (Ex/Em 352/461 nm) solution was added to each standard and sample, and fluorescence measurements were obtained using the plate reader. To isolate the contributions of each cell type to the total DNA content in the co-culture groups, the average percentage of cells expressing the endothelial markers CD31 and vascular endothelial cadherin (VE-Cdh) was calculated from images (at least four); the DNA values obtained from the assay described above were adjusted based on this percentage to estimate a DNA value for the MSCs in the MSC-EC group.
Total calcium concentration was quantified using the Calcium Liquicolor kit (Stanbio Laboratory; n = 3). Briefly, on day 14, cells were rinsed with PBS and digested in 0.5 N HCl overnight at 4°C. Samples were centrifuged at 1000 g for 2 min, and the supernatant was analyzed as per the manufacturer's instructions. CaCl2 from the kit was used to generate a standard curve. Absorbance readings were taken at 550 nm using the plate reader. Calcium data were normalized to the MSC DNA content in each well.
Histology and immunohistochemistry
On day 14, wells (n = 2) were rinsed in PBS, fixed in 10% zinc-buffered formalin (VWR) for 30 min, and rinsed with PBS. The cell-laden membranes were removed from the BioFlex plates and cut into quarters. To assess mineral deposition a 2% Alizarin Red S (Fisher) solution was applied to each sample for 5 min, rinsed with deionized water, and imaged using a Leica DM5500B microscope at 20×.
To assess endothelial phenotypic stability, additional fixed samples were blocked in a blocking buffer (1% BSA and 0.1% Tween 20) for 30 min. Samples were then incubated in a blocking buffer containing either mouse monoclonal CD31 FITC conjugate (1:50) or rabbit polyclonal VE-Cdh (1:200) primary antibodies (Abcam). The VE-Cdh specimens were rinsed with PBS and incubated in a blocking buffer containing goat anti-rabbit Alexa 488 (Abcam; 1:200) for 1 h. The ProLong gold antifade reagent with DAPI (Life Technologies) was used to mount coverslips on the samples, which were subsequently imaged using a Leica DM5500B microscope at 20 × .
Statistical analyses
Statistical analyses were performed using Prism (version 6.07; GraphPad Software). Biochemical results were averaged across all three donors and expressed in the form of mean ± standard deviation. Differences due to media formulation or culture surface were calculated using a Student's t-test. Differences due to co-culture and strain were determined using two-way ANOVA with a Tukey post-hoc test. A level of p < 0.05 was considered significant.
Results
Media and culture surface composition are key factors in the development of an MSC-EC co-culture
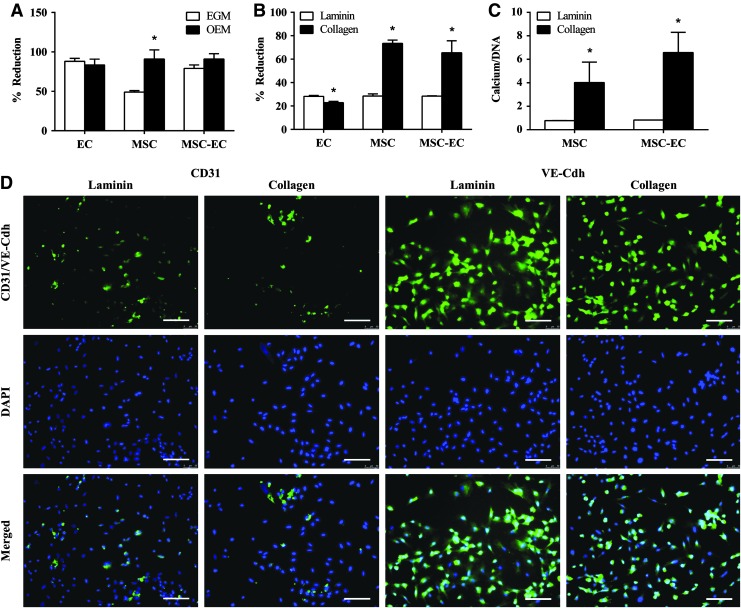
A variety of media formulations and culture surfaces have previously been used in co-cultures of ECs and MSCs (Table 1). In this study, we tested EGM and OEM on cells cultured on TCP and various coatings of BioFlex culture plates. On 0.5% gelatin-coated TCP, ECs and MSC-EC co-cultures were equally viable when cultured with either EGM or OEM. However, MSCs had significantly higher viability in OEM than EGM (Fig. 2A). Calcium accretion was also higher in OEM than EGM for both MSCs alone and MSC-EC co-cultures (data not shown). Due to the negative effects of EGM on MSC viability and osteogenesis, OEM was used throughout the rest of the study unless otherwise noted.
Table 1.
Parameters of Direct-Contact Co-culture Models of Human ECs and MSCs
| Study | Cell sources | Ratio (MSC:EC) | Media components | Substrate |
|---|---|---|---|---|
| Villars (2000) | HUVEC | 1:3 | IMDM + 10% FCS | TCP |
| hMSC | ||||
| Xue (2004) | HUVEC | 5:1 | Mixed, osteogenic factor free | TCP |
| hMSC | ||||
| Kaigler (2005) | HDMEC | 1:1; 5:1; 10:1 | Mixed, osteogenic factor free | TCP |
| hMSC | ||||
| Rouwkema (2006) | HUVEC | 1, 2, 5, 10, 15, 30, or 50% EC | Endothelial, osteogenic, or mixed | 3D spheroids |
| hMSC | ||||
| Grellier (2009) | HUVEC | 2:1 | IMDM + 10% FBS | TCP |
| hMSC | ||||
| Tao (2009) | IEC | 1:1 | Osteogenic | TCP |
| hMSC | ||||
| Aguirre et al. (2010) | BM-EPC | 1:1 | IMDM + 10% FBS | Fibronectin-coated TCP |
| hMSC | ||||
| Correia et al. (2011) | HUVEC | 1:2 | Mixed with osteogenic factors | Fibrin hydrogel |
| hMSC | ||||
| Saleh et al. (2011) | HUVEC | 1:1 | Mixed, osteogenic factor free | 3D spheroids |
| hMSC | ||||
| Kim et al. (2013) | HUVEC | 1:1 | Osteogenic | Nanopatterned polyurethane acrylate |
| hASC | ||||
| Freeman et al. (2015) | HUVEC | 1:1 | Mixed with osteogenic factors | Pellet |
| hMSC | ||||
| Current study | HUVEC | 1:1 | Endothelial, or mixed with osteogenic factors | Collagen type I and laminin-coated BioFlex® plates |
| hMSC |
BM-EPC, bone marrow-derived endothelial progenitor cell; EC, endothelial cell; FBS, fetal bovine serum; FCS, fetal calf serum; hASC, human adipose-derived stem cell; HDMEC, human dermal microvascular endothelial cell; hMSC, human bone marrow-derived mesenchymal stem cell; HUVEC, human umbilical vein endothelial cell; IEC, induced endothelial cell; IMDM, Iscove's modified Dulbecco's medium; TCP, tissue culture plastic.
FIG. 2.
(A) Osteogenic endothelial growth medium (OEM) was found to enhance MSC viability relative to EGM, while maintaining viability of ECs and MSC-EC co-cultures. While a laminin coating slightly increased EC viability (B) and endothelial markers CD31 and VE-Cdh staining (D), a collagen type I coating greatly enhanced MSC and MSC-EC viability (B) and osteogenesis, (C) and thus was chosen for all experiments. Scale bars, 100 μm. *p < 0.05 for black versus white bars. Color images available online at www.liebertpub.com/tea
Once a media formulation was confirmed, cell viability and angio/osteogenic markers were assessed on BioFlex plates modified with different surface coatings: amino groups, pronectin, laminin, or collagen type I. Amino- and pronectin-coated plates were not conducive to EC proliferation (data not shown). Relative to the collagen type I coating, the laminin coating significantly increased EC viability (an increase in % reduction is indicative of an increase in cell activity and viability) and enhanced the expression of endothelial markers CD31 and VE-Cdh (Fig. 2B, D). However, collagen type I significantly enhanced MSC and MSC-EC viability and significantly increased calcium accretion in both groups (Fig. 2B, C). Therefore, the benefits of a collagen type I coating to MSC and MSC-EC viability and osteogenic potential outweighed the slight decreases in EC viability and CD31/VE-Cdh staining compared with the laminin coating. Collagen type I-coated BioFlex plates were exclusively used for the remainder of the experiments.
ECs remain phenotypically stable in response to 10% cyclic tensile strain and co-culture with MSCs
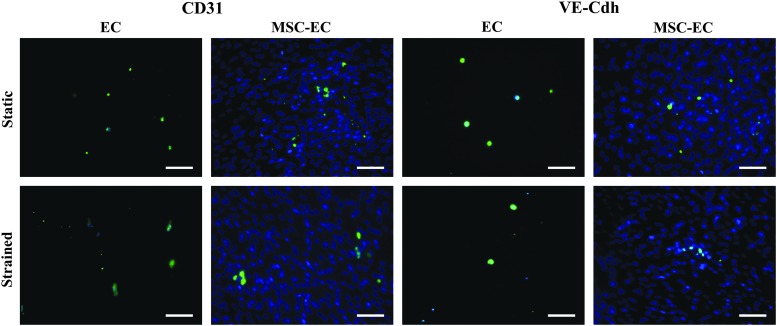
ECs exhibited a spread-out, cuboidal morphology (Fig. 3). While ECs alone did not align perpendicular to loading like the majority of observed cells in the MSC and MSC-EC co-culture groups, 10% cyclic tensile strain did seem to elongate ECs (Fig. 3). As expected, ECs in monoculture expressed both CD31 and VE-Cdh. ECs continued to express CD31 and VE-Cdh, both when exposed to 10% cyclic tensile strain and in co-culture with MSCs (Fig. 4), showing that their phenotype was preserved.
FIG. 3.
Representative images demonstrating that ECs elongated in response to 10% cyclic tensile strain, while MSCs and MSC-EC co-cultures elongated and aligned perpendicular to the direction of strain. Scale bars, 100 μm (200 μm for inserts). Color images available online at www.liebertpub.com/tea
FIG. 4.
Representative images demonstrating that ECs maintained expression of CD31 and VE-Cdh in co-cultures with MSCs and in response to 10% cyclic tensile strain, indicating that EC phenotypic stability was preserved. Scale bars, 100 μm. Color images available online at www.liebertpub.com/tea
Ten percent cyclic tensile strain and co-culture synergistically enhance osteogenesis in MSCs
MSCs appeared elongated and spindle shaped and, in both monoculture and co-culture, aligned perpendicular to the direction of loading (Fig. 3). No significant changes in cell viability were observed either in response to co-culture with ECs or with exposure to 10% cyclic tensile strain in any of the three donors (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). In MSCs alone, 10% cyclic tensile strain had no significant effect on calcium accretion relative to the static condition, although it did appear to enhance mineral deposition (Fig. 5). Similarly, static co-culture had no significant effect on calcium accretion relative to static MSCs alone, although mineral deposition again appeared enhanced. However, MSC-EC co-cultures loaded with 10% cyclic tensile strain had significantly enhanced calcium accretion and mineral deposition than either static co-cultures or loaded MSC alone (Fig. 5). These synergistic effects were consistent with two additional donors (histological results from Donor 3 are shown as representative; Supplementary Fig. S2).
FIG. 5.
(A) Calcium accretion normalized to DNA and (B) mineral deposition were synergistically enhanced by co-culture and application of 10% cyclic tensile strain. Scale bars, 100 μm. *p < 0.05. Color images available online at www.liebertpub.com/tea
Discussion
Co-culture with ECs and 10% cyclic tensile strain, in isolation, were previously shown to enhance osteogenesis in MSCs.1,24–26 We hypothesized that co-culturing MSCs with ECs and applying 10% cyclic tensile strain would synergistically enhance osteogenesis and angiogenesis and would be a key first step to improving current bone tissue engineering strategies. To conduct the analysis, we first had to determine optimal co-culture conditions (e.g., media formulation and culture surface) that would enable both EC/MSC viability and angiogenic/osteogenic potential. We then examined both monocultures and co-cultures of MSCs and ECs, kept either in static/unloaded conditions or exposed to 10% cyclic tensile strain, and monitored changes in cellular viability, cellular morphology, osteogenic markers, and endothelial markers. MSCs appeared to align perpendicular to the direction of loading, ECs maintained phenotypic stability, and both co-culture and 10% cyclic tensile strain synergistically enhanced osteogenesis, while having no observable effect on angiogenesis. While our hypothesis was correct in regard to the synergistic effects of co-culture and 10% cyclic tensile strain on osteogenesis, we must reject our hypothesis with regard to angiogenesis as neither stimulus was found to affect endothelial behavior. Together these data suggest that exposing MSC-EC co-cultures to 10% cyclic tensile strain may be a useful strategy for enhancing bone tissue engineering strategies.
While utilizing co-cultures of MSCs and ECs as a strategy to enhance angiogenesis and osteogenesis is not unique, the optimal conditions for enhancing vascularization of bone tissue engineering constructs are not well established, and current strategies to promote vascularization are not very successful. A variety of cell sources of ECs and MSCs have been used, as well as different cell ratios, media formulations, and culture surfaces (Table 1). In addition to a lack of consensus on the optimal conditions for co-culture, most studies do not discuss how specific conditions were chosen. A more open discussion of why different conditions are used, and how these conditions impact cell function, would propel this area of bone tissue engineering.
We chose a 1:1 ratio of MSCs:ECs based on previous research, demonstrating that a 1:1 ratio produced greater osteogenesis than did a 1:5 or 1:10 ratio.8 We also used a direct co-culture method, as multiple studies have shown that direct co-culture has a stronger effect on osteogenesis than using conditioned media from ECs.7,37 Determining an optimal media formulation is crucial for the best performance of MSC-EC co-cultures; several studies used an osteogenic factor-free media,7,9,15,18,37 while others used a mixed media with both endothelial and osteogenic factors.10,17 In this study, the addition of osteogenic factors significantly enhanced MSC viability and osteogenesis yet had no significant effect on EC viability. More research is needed to determine optimal media that support both angiogenesis and osteogenesis in vitro and improve vascularization and performance of bone tissue engineering constructs in vivo.
The surface that the cells are cultured on is an important, yet often overlooked, factor regulating EC and MSC behavior. In native endothelium, collagen type I and laminin have a “fire” and “ice” functionality; collagen type I leads to a breakdown in EC attachments and induces capillary morphogenesis, while laminin decreases morphogenesis and maintains a mature endothelium.38 In this study, while laminin better supported EC viability and CD31 and VE-Cdh staining, collagen type I better supported MSC viability and osteogenesis. We chose to use collagen type I in our system, as its benefits on osteogenesis and MSC viability outweighed the slight decreases seen in EC viability. However, while we were limited to the surface coatings compatible with our loading device, further optimization of the surface to promote both angiogenesis and osteogenesis could have profound effects on developing a biomimetic scaffold.
Surprisingly, neither co-culture nor loading alone had a significant effect on EC or MSC behavior. EC phenotype remained stable in both co-culture with MSCs and/or when exposed to 10% cyclic tensile strain, as evidenced by the constant CD31 and VE-Cdh staining. While ECs alone did not appear to align along or perpendicular to the direction of 10% cyclic tensile strain, they did appear to elongate, a behavior that has been associated with capillary morphogenesis.38,39 Co-culture of MSCs with ECs did not significantly enhance calcium accretion or mineral deposition. Furthermore, previous work in our laboratory established that the application of 10% cyclic tensile strain to MSCs enhances osteogenesis, yet this stimulus did not significantly affect osteogenesis in two out of three donors in this study. One possibility is that certain donors are more responsive to mechanical loading than others, an explanation supported by our previous work reporting individual differences observed with this mechanical stimulus and in response to chemical stimuli.40,41 However, in our prior studies, 10% cyclic tensile strain consistently enhanced osteogenesis of MSCs.24–26 We believe the more likely explanation is that the endothelial supplements to the media decreased the ability of MSCs to respond to loading. Specifically, heparin decreases osteogenesis of MSCs42 and potentially could have decreased the ability of MSCs to differentiate in response to 10% cyclic tensile strain.
While neither co-culture nor loading alone significantly affected EC or MSC behavior, the concurrent application of 10% cyclic tensile strain to a co-culture of MSCs and ECs synergistically enhanced osteogenesis, while having no observable effect on angiogenesis. In all three donors tested, 10% cyclic tensile strain significantly enhanced calcium accretion and mineral deposition in co-cultures of MSCs and ECs relative to static co-cultures or strained monocultures of MSCs. Previous studies demonstrated that 10% cyclic tensile strain not only enhances osteogenesis but also influences angiogenesis through VEGF and fibroblast growth factor 2 (FGF2) signaling.24,25,43 Furthermore, ECs enhance osteogenesis through BMP2 signaling,13,37,44,45 and BMP2 gene expression in ECs is increased in the presence of VEGF.45 Therefore, while we found no apparent changes in CD31 or VE-Cdh staining, the likely possibility remains that 10% cyclic tensile strain may have enhanced VEGF signaling from MSCs, and subsequently increased the release of growth factors, such as BMP2, from ECs to enhance osteogenesis. Further work is needed to determine the specific signaling pathways regulating MSC-EC interactions in response to 10% cyclic tensile strain.
In summary, we demonstrated that co-culture and 10% cyclic tensile strain synergistically enhanced osteogenesis; however, neither stimuli had a significant effect on angiogenesis. This study is the first to investigate the angiogenic and osteogenic effects of applying 10% cyclic tensile strain to a co-culture of MSCs and ECs simultaneously. We also found that collagen type I coatings better supported osteogenesis, while laminin coatings better supported angiogenesis. Future studies should focus on optimizing the co-culture system to increase its efficacy as a therapeutic strategy to regenerate bone. Due to the presence of multiple cell types, common mechanistic analyses, such as gene expression and growth factor release profiles, remain an unsolved challenge for co-culture investigations. ECs and MSCs cannot be unequivocally identified on the basis of cell morphology and either, or both, could secrete products into the media, making it challenging to parse out the relative contributions of each cell type to the observed results. Similarly, the current standards for measuring angiogenesis and tissue vascularization include techniques such as tubule formation; however, these techniques are typically performed in monocultures of ECs in 3D systems.46 The 2D co-culture system used in this study precluded use of these techniques. Future studies should focus on methods to overcome these technical challenges. More investigations into the specific signaling pathways regulating this synergistic response could provide potential pharmacologic targets to enhance vascularization in tissue-engineered bone constructs. Overall, this study demonstrated that the intricate bone–vascular interactions depend on both biochemical and biophysical cues, and leveraging these interactions holds great potential to enhance bone tissue engineering and regenerative medicine therapeutic strategies.
Supplementary Material
Acknowledgments
This study was supported by the William R. Kenan Jr. Institute for Engineering, Technology and Science NC State/UNC Strategic Alliance for Translational and Regenerative Medicine. Pilot Research Grant, NIH/NIBIB 1R03EB008790 (E.G.L.), and NSF/CBET 1133427 (E.G.L.). A special thanks to Dr. Andrew Dudley in the Department of Cell Biology and Physiology at UNC-Chapel Hill for his invaluable guidance on EC culture.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nguyen L.H., Annabi N., Nikkhah M., Bae H., Binan L., Park S., et al. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev 18, 363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanczler J.M., and Oreffo R.O.C. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15, 100, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Salgado A.J., Coutinho O.P., and Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci 4, 743, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Santos M.I., and Reis R.L. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 10, 12, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Sheehy E.J., Mesallati T., Kelly L., Vinardell T., Buckley C.T., and Kelly D.J. Tissue engineering whole bones through endochondral ossification: regenerating the distal phalanx. BioRes Open Access 4, 229, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D.S., Miura M., Demura H., and Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology 138, 2953, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Villars F., Bordenave L., Bareille R., and Amédée J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem 79, 672, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Kaigler D., Krebsbach P.H., West E.R., Horger K., Huang Y.-C., and Mooney D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J 19, 665, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Xue Y., Xing Z., Hellem S., Arvidson K., and Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online 8, 34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman F.E., Haugh M.G., and McNamara L. An in vitro bone tissue regeneration strategy combining chondrogenic and vascular priming enhances the mineralisation potential of MSCs in vitro whilst also allowing for vessel formation. Tissue Eng Part A 21, 1320, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Tao J., Sun Y., Wang Q., and Liu C. Induced endothelial cells enhance osteogenesis and vascularization of mesenchymal stem cells. Cells Tissues Organs 190, 185, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Kim H.N., Lim K.-T., Kim Y., Pandey S., Garg P., et al. Synergistic effects of nanotopography and co-culture with endothelial cells on osteogenesis of mesenchymal stem cells. Biomaterials 34, 7257, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Saleh F.A., Whyte M., and Genever P.G. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater 22, 242, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Rouwkema J., Boer J.D., and Blitterswijk C.A.V. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 12, 2685, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Aguirre A., Planell J.A., and Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun 400, 284, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Teoh S.-H., Chong M.S.K., Lee E.S.M., Mattar C.N.Z., Randhawa N.K., et al. Vasculogenic and osteogenesis-enhancing potential of human umbilical cord blood endothelial colony-forming cells. Stem Cells Dayt Ohio 30, 1911, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Correia C., Grayson W.L., Park M., Hutton D., Zhou B., Guo X.E., et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One 6, e28352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grellier M., Ferreira-Tojais N., Bourget C., Bareille R., Guillemot F., and Amédée J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 106, 390, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Lin R.-Z., Moreno-Luna R., Li D., Jaminet S.-C., Greene A.K., and Melero-Martin J.M. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci 111, 10137, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem 55, 304, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Steward A.J., and Kelly D.J. Mechanical regulation of mesenchymal stem cell differentiation. J Anat 227, 717, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loboa E.G., Beaupré G.S., and Carter D.R. Mechanobiology of initial pseudarthrosis formation with oblique fractures. J Orthop Res 19, 1067, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kelly D.J., and Jacobs C.R. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res Part C Embryo Today Rev 90, 75, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Charoenpanich A., Wall M.E., Tucker C.J., Andrews D.M.K., Lalush D.S., Dirschl D.R., et al. Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng Part A 20, 67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charoenpanich A., Wall M.E., Tucker C.J., Andrews D.M.K., Lalush D.S., and Loboa E.G. Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng. Part A 17, 2615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumanasinghe R.D., Bernacki S.H., and Loboa E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng 12, 3459, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol Rev 75, 519, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuma N., Duzgun S.A., Ikeda M., Kito H., Akasaka N., Sasajima T., et al. Endothelial cell response to different mechanical forces. J Vasc Surg 32, 789, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Dewey J.C.F., Bussolari S.R., Gimbrone J.M.A., and Davies P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103, 177, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Von Offenberg Sweeney N., Cummins P.M., Cotter E.J., Fitzpatrick P.A., Birney Y.A., Redmond E.M., et al. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 329, 573, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Yung Y.C., Chae J., Buehler M.J., Hunter C.P., and Mooney D.J. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci 106, 15279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto T., Yung Y.C., Fischbach C., Kong H.J., Nakaoka R., and Mooney D.J. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng 13, 207, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Li W., and Sumpio B.E. Strain-induced vascular endothelial cell proliferation requires PI3K-dependent mTOR-4E-BP1 signal pathway. Am J Physiol Heart Circ Physiol 288, H1591, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Upchurch G.R., Leopold J.A., Welch G.N., and Loscalzo J. Nitric oxide alters human microvascular endothelial cell response to cyclic strain. J Cardiovasc Pharmacol Ther 3, 135, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Iba T., and Sumpio B.E. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res 42, 245, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi Y., Sekiya I., Yagishita K., Ichinose S., Shinomiya K., and Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 104, 2728, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kaigler D., Wang Z., Horger K., Mooney D.J., and Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res 21, 735, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Davis G.E., and Senger D.R. Endothelial extracellular matrix biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97, 1093, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., and Senger D.R. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J 18, 457, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Bodle J.C., Teeter S.D., Hluck B.H., Hardin J.W., Bernacki S.H., and Loboa E.G. Age-related effects on the potency of human adipose-derived stem cells: creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng Part C Methods 20, 972, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson A.D., Marvel S.W., Bernacki S.H., Banes A.J., Aalst J., and Loboa E.G. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng 37, 955, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Hemeda H., Kalz J., Walenda G., Lohmann M., and Wagner W. Heparin concentration is critical for cell culture with human platelet lysate. Cytotherapy 15, 1174, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Kasper G., Dankert N., Tuischer J., Hoeft M., Gaber T., Glaeser J.D., et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells Dayt Ohio 25, 903, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Li W.M., Huang W.Q., Huang Y.H., Jiang D.Z., and Wang Q.R. positive and negative haematopoietic cytokines produced by bone marrow endothelial cells. Cytokine 12, 1017, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Bouletreau P.J., Warren S.M., Spector J.A., Peled Z.M., Gerrets R.P., Greenwald J.A., et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 109, 2384, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Simons M., Alitalo K., Annex B.H., Augustin H.G., Beam C., Berk B.C., et al. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: a scientific statement from the American Heart Association. Circ Res 116, e99, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.