Abstract
Background: Among postsurgical and critically ill patients, malglycemia is associated with increased complications. Continuous glucose monitoring (CGM) in the inpatient population may enhance glycemic control. CGM reliability may be compromised by postsurgical complications such as edema or vascular changes. We utilized Clarke Error Grid (CEG) and Surveillance Error Grid (SEG) analysis to evaluate CGM performance after total pancreatectomy with islet autotransplantation.
Materials and Methods: This subanalysis evaluated Medtronic Enlite 2 CGM values against YSI serum glucose in seven post-transplant patients (86% female; 38.6 ± 9.4 years) on artificial pancreas for 72 h at transition from intravenous to subcutaneous insulin. Sensor recalibration occurred for absolute relative difference (ARD) ≥20% x2, ≥30% x1, or by investigator discretion based on trend.
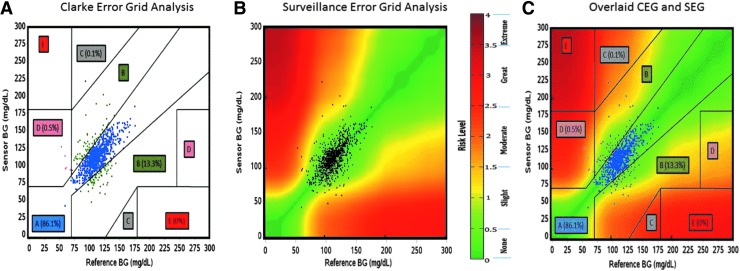
Results: Sensor analysis showed mean absolute relative difference (MARD) of 11.0% ± 11.5%. The sensors were recalibrated 8.3 times/day; active sensor was switched 1.4 times/day. Calibration factor was 7.692 ± 3.786 mg/nA·dL (target = 1.5–20 mg/nA·dL). CEG analysis showed 86.1% of pairs in Zone A (clinically accurate zone) and 99.4% of pairs in Zones A + B (low risk of error). SEG analysis of hypoglycemia/hyperglycemia risk showed 92.22% of pairs in the “no risk” zone, 5.96% of pairs in the “slight lower” risk zone, 1.01% of pairs in the “slight higher” risk zone, and only 0.81% of pairs in the “moderate lower” risk zone.
Conclusions: Overall performance of the Medtronic Enlite 2 CGM in the post-transplant population was reasonably good with “no risk” or “slight lower” risk by SEG analysis and high CGM-YSI agreement by CEG analysis; however, frequent recalibrations were required in this intensive care population.
Introduction
Hyperglycemia, hypoglycemia, and malglycemia (combination of hyperglycemia, hypoglycemia, and increased glycemic variability1) are detrimental to not only the type 1 diabetes (T1D) population but to other groups of nondiabetic patients as well. Among critically ill patients, malglycemia is associated with increased incidence of bacterial infection and sepsis, increased length of hospital stay, and increased overall morbidity and mortality.1–5 Malglycemia is particularly detrimental immediately following allogenic or autologous islet transplantation when islets, which have been stripped off their native blood supply, are reliant on diffusion to supply nutrients and oxygen.6,7 During this time period, overstimulation by hyperglycemia contributes to beta-cell loss.8,9 Usual care for postsurgical, critically ill, and post-transplant patients is to obtain intermittent glucometer whole blood glucose (BG) values every 1–4 h depending on the acuteness of their condition and the clinical protocol.10,11 With continued refinement, improvement, and clinical acceptance of continuous glucose monitoring (CGM) systems, these populations may stand to benefit from ascertainment of continuous rather than intermittent BG values.10–12
CGM systems measure interstitial fluid glucose concentrations through subcutaneously placed glucose-sensing probes operating with a glucose-oxidase enzyme-based technology that is similar to the operation of glucometers.13,14 Current commercially available devices require calibration against concurrent BG values, based on the assumption that glucose concentration in the interstitial fluid is directly related to BG concentration and that this relationship is stable.14 In addition, there is a notable temporal lag between changes in plasma glucose and interstitial glucose, which may range from 4 to 50 min according to some studies.13,15 FDA-approved CGM placement sites are the abdomen and/or the upper buttocks depending on the device manufacturer.16,17 As CGM use extends from relatively healthy out-patient T1D patients to postsurgical and intensive care unit (ICU) patients, there is concern that factors such as postsurgical edema may hinder CGM function. To date, there has been only one published study investigating this concern, which found good performance of the Guardian RT CGM system in 20 children after cardiac bypass surgery.18 In addition, two randomized controlled trials comparing CGM and point-of-care glucose measurements have been conducted and have shown reduced nursing workload and decreased rates of hypoglycemia in the CGM groups compared to the point-of-care groups.19,20 It should be noted that clinicaltrials.gov currently lists 11 additional completed/recruiting studies on CGM use in ICUs.21
Evaluation of the accuracy of a given glucose meter or CGM device has traditionally been performed by error grid analysis. The most widely used error grid is the Clarke Error Grid (CEG).22,23 The CEG describes the clinical accuracy of an experimental BG testing device (BG meter or CGM) against a reference standard across the range of measured glucose values taking into account the measured values, their relative difference, and the clinical significance of this difference.22 The CEG is divided into five zones: Zone A representing glucose values that are clinically accurate with error of <20%, Zone B representing values that are inaccurate by ≥20% but would lead to benign treatment assumptions, Zone C representing values that would result in overcorrecting elevated BG, Zone D representing potentially dangerous failure levels, and Zone E representing totally erroneous measurements.22
Starting in 2012, a multidisciplinary Error Grid Panel developed a new Surveillance Error Grid (SEG) based on questionnaires of 206 clinicians and an advanced mathematical interpretation of the results.24,25 The SEG aims to evaluate the perceived level of risk for hypoglycemia or hyperglycemia due to measurement error associated with glucose measurement errors.25 Risk for each is coded on a scale from 0 to 4 with 0 representing no risk, 1 representing slight risk, 2 representing moderate risk, 3 representing great risk, and 4 representing extreme risk.24 Rather than being defined by discrete zones as with the CEG, the SEG uses a gradual spectrum of risk within each zone defined by a range of risk scores.24 In this way, SEG analysis allows for a more complex, but more complete, analysis of the risk of errors in CGM or glucometer readings beyond simply a percentage agreement to a validated risk assessment of the potential clinical errors associated with real-world measurement errors.
This project represents a substudy of a larger randomized controlled clinical trial to evaluate the safety and efficacy of a closed loop (CL) artificial pancreas (AP) system in patients after total pancreatectomy with islet autotransplantation (TPIAT).26 This study marked the first clinical trial using the Medtronic Enlite 2 subcutaneous glucose sensors as part of a CL system. It was also one of the first studies to use CGM in a postsurgical patient population and in patients with some level of endogenous islet function. Transient abdominal and peripheral edema was anecdotally observed by the researchers during this pilot study with possible low signal strength as a result. As such, we decided to investigate the accuracy and calibration frequency of the Medtronic Enlite 2 sensor in patients after TPIAT, using both the classical CEG and the new SEG methods. This represents the first application of the SEG to CGM analysis.
Materials and Methods
Patient population
Participants in this substudy were adult (age 21–64 years), nondiabetic patients with chronic pancreatitis undergoing TPIAT at the University of Minnesota (UMN) between February 2014 and March 2015. Patients with pre-existing diabetes mellitus by the American Diabetes Association criteria, those who required acetaminophen (which is known to produce erroneous CGM measuremnts27) or corticosteroids, and those with significant psychiatric disease or developmental delay impacting ability to provide informed consent were excluded.
The clinical trial was approved by the UMN Investigational Review Board (IRB; #1307 M37923); informed consent was obtained from all participants. As the study involves the use of an investigational device, an Investigational Device Exemption (IDE) was obtained from the United States Food and Drug Agency (FDA; #G130178). The study is registered with clinicaltrials.gov (NCT01945138).
Total pancreatectomy and islet autotransplantation procedure
All participants underwent total pancreatectomy as previously described28–30 along with infusion of the patient's own islet cells into a tributary of the portal vein, or if elevated portal pressures prevented infusion of all the islets intraportally, the remaining islets were transplanted into the peritoneal cavity. Immediately after surgery, all patients were started on a continuous intravenous (IV) insulin infusion protocol with the goal of maintaining BG in the range of 80–125 mg/dL. As per the standard management protocol for TPIAT recipients at our institution, patients had no oral intake after surgery or during the subsequent study period due to postsurgical gastroparesis; all nutrition was delivered by continuous enteral feeds administered through a jejunal tube. IV insulin was discontinued and subcutaneous (SQ) insulin started once patients reached full enteral nutrition (generally 4–8 days after surgery).
All patients in this subanalysis received SQ insulin as directed by the Medtronic ePID 2.0 Control Tool System. Participants in this group wore a Medtronic Paradigm REAL-Time insulin pump loaded with insulin as part, as well as two Enlite 2 Glucose Sensors attached to MiniLink REAL-Time Transmitters. One sensor was active, providing glucose values to the CL algorithm, and the other was a backup. Which sensor was active and which was backup was able to be switched at the investigator's discretion. Sensor glucose levels were calculated based on a calibration factor estimated from the slope, a linear regression, with intercept set at 0, of plasma glucose and filtered sensor current with a fixed sensor delay time of 10 min to account for delayed BG shifts between the IV and interstitial compartments.13,15 The Proportional-Integral-Derivative (PID) portion of the controller then used a series of equations to determine the insulin requirements for a given minute based on the sensor data and updated the rate of insulin delivery every minute in real time.
CGM methods
The study protocol called for sensor recalibration for two values in a row with an absolute relative difference (ARD) of ≥20% or one value with an ARD of ≥30%. Device recalibration and switching of the active sensor were also permissible at the investigator's subjective discretion based on consistent trend of ARD above or below the reference values. To evaluate CGM performance in this pilot study, a post hoc analysis was conducted of CGM bias, mean absolute relative difference (MARD), calibration values, recalibration frequency, active sensor changes, and CEG and SEG analysis. CEG analysis was conducted using standard regional bounds defined by Clarke et al.23,31 Methods for creating a CEG using current software can be found online.31 SEG analysis was performed with an Excel Macro using bounds defined by Kovatchev et al.25,32 Additional MARD analysis was conducted by computing the MARD as a function of the reference glucose level and then determining a best-fit fourth-order polynomial to express this nonlinear relationship as previously described by Rodbard.33 The effect of the rate of change of glucose on MARD was also investigated by comparing the MARD against categorical rates of change of sensor glucose as described by Pleus et al.34
Recalibration frequency analysis
As this study was not originally designed to minimize the frequency of recalibrations, additional analysis was conducted to investigate the necessity of recalibration more frequently than every 3 h in this population. CGM data were divided into two segments: time where recalibration occurred more frequently than once every 3 h and time where recalibration occurred at intervals of 3 h or greater. These segments were analyzed for differences in MARD, calibration factor, and % time in each zone for both the CEG and SEG.
Data analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at UMN.35 Data are presented as mean ± standard deviation or as percentage (95% confidence interval), except where otherwise noted. Analysis was conducted using PC SAS (version 9.4). Significance testing was conducted with two-sided Student's t-test assuming equal variance or with chi-square testing for categorical variables.
Results
Patient population, demographics, and pretransplant history
CGM data from the seven patients randomized to the CL AP system were used in this analysis, as this subset were the patients with every 30-min reference glucose values for device accuracy comparison. Included patients consisted of one man and six women with an average age of 38.6 ± 9.4 years. Demographic and pretransplant characteristics are presented in Table 1.
Table 1.
Patient Characteristics, Pretransplant Measures, and Islet Yield
| Age (years) | 38.6 ± 9.4 |
| Sex, M/F (% male) | 1/6 (14) |
| Wt at Tx (kg) | 66.1 ± 10.3 |
| BMI at Tx (kg/m2) | 25.3 ± 4.5 |
| Hemoglobin A1c (%) | 5.1 ± 0.2 |
| Fasting BG (mg/dL) | 89 ± 6 |
| Fasting C-peptide (ng/mL) | 2.0 ± 0.6 |
| Peak MMTT C-peptide (ng/mL) | 6.4 ± 2.2 |
| Islet yield (IEq/kg) | 6619 ± 3357 |
| Intraportal (IEq/kg) | 5930 ± 2577 |
| Intraperitoneal (IEq/kg) | 689 ± 1423 |
| Days on drip (days) | 5.1 ± 1.1 |
| Avg BG on insulin drip (mg/dL) | 115 ± 4 |
| Primary etiology of CP | |
| Identified genetic mutation (PRSS1, SPINK1, CFTR) | 3 (43%) |
| Mechanical dysfunction (pancreatic divisum, sphincter of oddi dysfunction, annular pancreas) | 3 (43%) |
| Idiopathic pancreatitis | 1 (14%) |
BG, blood glucose.
CGM analysis
Subanalysis of sensor performance of the Enlite 2 sensors in the seven patients was conducted correlating every 30-min reference YSI BG values with calibrated sensor readings 10 min later to account for interstitial glucose lag, using 990 paired YSI-CGM sets over seven patients totaling 21 days of observations. Overall, sensor analysis showed MARD of 11.0% ± 12.2%, with substantial variability across different patients (Table 2). Excluding the first 12 h after sensor placement, the sensors were recalibrated an average of 8.3 times/day and the active sensor was switched an average of 1.4 times/day (Table 3). During the first 12 h, the sensors were calibrated an average of 6.9 times or once every 1.75 h. Average calibration factor across all patients was 7.692 ± 3.786 mg/nA·dL with an acceptable range being 1.5–20 mg/nA·dL. The CGM requiring calibration in the middle of this range indicates a more optimal and accurate performance, whereas calibration values above this range indicate poor signal strength observed by the CGM and likely erroneous measurements.
Table 2.
CGM Bias and MARD
| Bias (mg/dL) | ARD (%) | |||||
|---|---|---|---|---|---|---|
| Patient | Mean | SD | SEM | Mean | SD | SEM |
| CLTPIAT01 | 0.6 | 12.6 | 1.1 | 8.83 | 7.81 | 0.68 |
| CLTPIAT03 | −3.3 | 17.1 | 1.4 | 10.45 | 10.27 | 0.85 |
| CLTPIAT05 | −0.5 | 24.3 | 2.1 | 16.59 | 17.16 | 1.46 |
| CLTPIAT06 | 7.6 | 12.6 | 1.0 | 10.9 | 9.32 | 0.76 |
| CLTPIAT09 | −1.3 | 16.7 | 1.4 | 10.94 | 10.62 | 0.91 |
| CLTPIAT11 | 4.7 | 13.7 | 1.2 | 10.18 | 9.09 | 0.77 |
| CLTPIAT13 | −0.1 | 13.7 | 1.1 | 9.08 | 16.21 | 1.34 |
| Overall | 1.2 | 16.6 | 0.5 | 11.0 | 12.2 | 0.39 |
CGM, continuous glucose monitoring; MARD, mean absolute relative difference; SD, standard deviation.
Table 3.
CGM Calibration Data
| Cal factor (mg/nA × dL) | |||||
|---|---|---|---|---|---|
| Patient | Average | SD | SEM | Recalibrations per 24 ha | Active sensor changes per 24 ha |
| CLTPIAT01 | 5.597 | 0.652 | 0.010 | 9.2 | 1.6 |
| CLTPIAT03 | 11.115 | 9.923 | 0.151 | 5.6 | 0.4 |
| CLTPIAT05 | 7.492 | 4.550 | 0.069 | 14.0 | 2.8 |
| CLTPIAT06 | 9.495 | 2.531 | 0.039 | 7.6 | 0.0 |
| CLTPIAT09 | 11.091 | 7.042 | 0.109 | 12.4 | 2.4 |
| CLTPIAT11 | 4.500 | 0.886 | 0.013 | 5.6 | 1.6 |
| CLTPIAT13 | 4.552 | 0.916 | 0.014 | 3.6 | 1.2 |
| Average | 7.676 | 5.703 | 0.033 | 8.3 | 1.4 |
Over the 72-h study period excluding the initial 12 h.
Traditional CEG analysis was conducted as displayed in Figure 1A and Table 4. With regard to the CEG analysis, sensor performance had 86.1% of all pairs in Zone A (clinically accurate measurements) and 99.4% of all pairs in Zones A or B (clinically accurate or no risk from error). SEG analysis is displayed in Figure 1B and Table 5. This analysis showed 92.22% of values in the dark green “no risk” zone (no risk for hypo- or hyperglycemia as a result of measurement errors), 5.96% of values in the light green “slight lower” risk zone, 1.01% of values in the yellow “slight higher” risk zone (very low risk and low risk for hypo- or hyperglycemia as a result of measurement errors), and only 0.81% of values in the light orange “moderate lower” risk zone (moderate risk of hypo- or hyperglycemia as a result of measurement errors). No values were in the moderate higher, greater, or extreme risk zones, which would indicate risk to the patient as a result of measurement errors. A composite of the CEG and SEG analysis methods is displayed in Figure 1C.
FIG. 1.
CGM Error Grid analysis. (A) CEG analysis: Zone A represents glucose values that are clinically accurate with error of <20%, Zone B represents values that are inaccurate by ≥20% but would lead to benign treatment assumptions, Zone C represents values that would result in overcorrecting elevated BG, Zone D represents potentially dangerous failure levels, and Zone E represents totally erroneous measurements. (B) SEG analysis: Clinical risk for errors in measurement is coded continuously from 0 to 4 with 0 representing no risk, 1 representing slight risk, 2 representing moderate risk, 3 representing great risk, and 4 representing extreme risk. (C) Overlaid CEG and SEG analysis: The discrete CEG and the continuous SEG are overlain for direct visualization of their relative presentations of the CGM data. BG, blood glucose; CEG, Clarke Error Grid; CGM, continuous glucose monitoring; SEG, Surveillance Error Grid.
Table 4.
Clarke Error Grid Zones
| Region | ||||||
|---|---|---|---|---|---|---|
| EGA metrics | A | B | C | D | E | Total |
| Points | 852 | 132 | 1 | 5 | 0 | 990 |
| % | 86.1 | 13.3 | 0.1 | 0.5 | 0.0 | 100.0 |
| % A + B | 99.4 | |||||
Zone A values are clinically accurate with error of <20%, Zone B values are clinically inaccurate by ≥20% but would lead to benign treatment assumptions, Zone C values would result in overcorrecting elevated BG, Zone D values are potentially dangerous failure levels, and Zone E values are totally erroneous measurements.
Table 5.
Surveillance Error Grid Zones
| Degree of risk | Absolute value | Color | No. of hypo glycemia | No. of hyperglycemia | Total | Hypoglycemia (%) | Hyperglycemia (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| None | 0–0.5 | D. Green | 466 | 404 | 913 | 47.07 | 40.81 | 92.22 |
| Slight lower | >0.5–1.0 | L. Green | 34 | 25 | 59 | 3.43 | 2.53 | 5.96 |
| Slight higher | >1.0–1.5 | Yellow | 6 | 4 | 10 | 0.61 | 0.40 | 1.01 |
| Moderate lower | >1.5–2.0 | L. Orange | 5 | 3 | 8 | 0.51 | 0.30 | 0.81 |
| Moderate higher | >2.0–2.5 | D. Orange | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Great lower | >2.5–3.0 | L. Red | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Great higher | >3.0–3.5 | D. Red | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Extreme | >3.5 | Brown | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
Degree of clinical risk for hypo/hyperglycemia as a result of measurement error is coded on a scale from 0 to 4 with 0 being no risk and 4 being extreme risk.
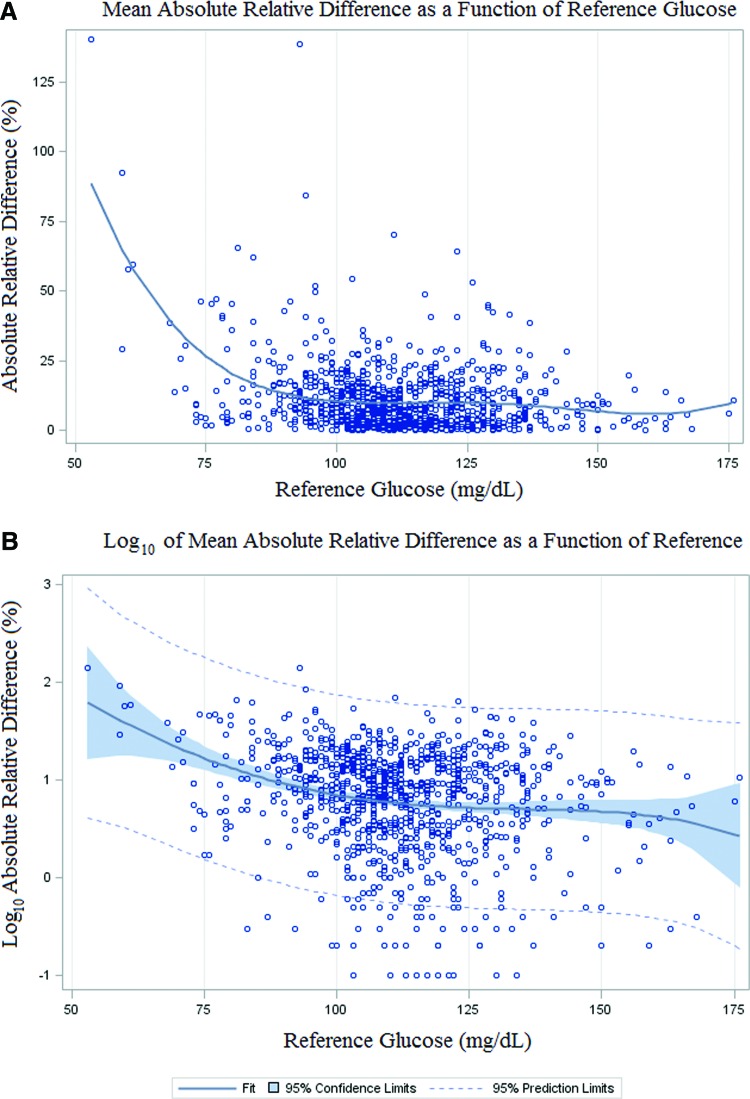
Further CGM analysis investigated the MARD as a function of the reference glucose level (Fig. 2.) by plotting a fourth-order best-fit regression curve to the data. This analysis shows good linear fit of the CGM values through the target range of 70–140 mg/dL, but nonlinear fit with glucose values in the hypoglycemic range. MARD analysis broken up by segments of ≤70, 70–140, and ≥140 mg/dL showed very large MARD (57.2% ± 41.7%) for the hypoglycemic range with better MARD values for the target (10.8% ± 11.1%) and hyperglycemic (7.2% ± 6.4%) ranges (Table 6). In addition, the rate of change of sensor glucose was broken into eight categories and the MARD for each category was determined (Table 7). This analysis showed very poor MARD for rapidly falling BG with rate of change <−3 mg/dL/min (MARD = 19.8% ± 16.1%), but more reliable values for flat and even rapidly rising rates of change.
FIG. 2.
MARD as a function of reference glucose: (A) Best-fit regression: The ARD between CGM glucose and reference glucose is plotted as a function of reference glucose values. A fourth-order best-fit regression curve is displayed to emphasize the nonlinear nature of this relationship. Measurements with an ARD of 0 were included in regression analysis. (B) Log10 of MARD: Confidence limits for the regression curve and prediction limits are displayed for the Log10 of the MARD to display the uncertainty at high and low glycemic extremes. Measurements with an ARD of 0 were not included as their Log10 is undefined (4 of 991 measurements). ARD, absolute relative difference; MARD, mean absolute relative difference.
Table 6.
Absolute Relative Difference by Reference Glucose Segments
| Reference glucose range (md/dL) | No. of observations | Mean ARD (%) | ARD SD (%) | ARD SE (%) |
|---|---|---|---|---|
| ≤70 | 8 | 57.2 | 41.7 | 14.8 |
| 70–140 | 930 | 10.8 | 11.1 | 0.4 |
| ≥140 | 52 | 7.2 | 6.4 | 0.9 |
ARD, absolute relative difference; SE, standard error.
Table 7.
MARD and SD in Each Rate-of-Change Category
| Rate of Change (mg/dL/min) | MARD (%) | SD ARD (%) | n |
|---|---|---|---|
| <−3 | 19.8 | 16.1 | 20 |
| ≥−3 to ≤−2 | 12.0 | 5.4 | 12 |
| >−2 to ≤−1 | 10.2 | 12.9 | 59 |
| >−1 to ≤0 | 11.1 | 11.3 | 457 |
| >0 to ≤1 | 10.3 | 11.7 | 362 |
| >1 to ≤2 | 12.8 | 21.2 | 46 |
| >2 to ≤3 | 10.1 | 7.0 | 13 |
| >3 | 9.7 | 9.2 | 21 |
Recalibration frequency analysis
Comparison of time segments with recalibration frequencies of less than and more than 3-h intervals is presented in Table 8. The same sensors were used during both time segments, and this is thus a comparison of those time periods, and not of the sensors themselves. This analysis shows that all aspects of sensor performance were significantly worse during periods of frequent recalibration supporting the need for CGM recalibration during these times. The average BG values were not significantly different between the <3 and >3-h recalibration segments (111.9 ± 4.8 vs. 114.86.5; P = 0.3525); however, the glycemic variability, as evidenced by standard deviation in glucose, was significantly more during the <3-h recalibration period compared to the >3-h recalibration period (22.8 ± 6.4 vs. 16.6 ± 4.0; P = 0.0489).
Table 8.
Calibration Frequency Segment Analysis
| Recalibration <3 hours | Recalibration ≥3 hours | P | |
|---|---|---|---|
| MARD (%), average + SD | 15.0 ± 17.1 | 9.3 ± 8.8 | <0.0001 |
| CGM glucose mean (mg/dL) (%), average + SD | 111.9 ± 4.8 | 114.8 ± 6.5 | 0.3525 |
| CGM glucose SD (mg/dL) (%), average + SD | 22.8 ± 6.4 | 16.6 ± 4.0 | 0.0489 |
| Calibration factor (mg/nA·dL) (%), average + SD | 8.38 ± 6.07 | 7.46 ± 5.60 | 0.0215 |
| CEG zone (%) | <0.0001 | ||
| A | 77 | 90 | |
| B | 22 | 10 | |
| C | 0.3 | 0.0 | |
| D | 1 | 0.3 | |
| SEG risk zone (%) | <0.0001 | ||
| None | 85.47 | 95.1 | |
| Slight lower | 10.14 | 4.18 | |
| Slight higher | 2.03 | 0.58 | |
| Moderate lower | 2.36 | 0.14 | |
| Moderate higher | 0.00 | 0.00 | |
| Great lower | 0.00 | 0.00 | |
| Great higher | 0.00 | 0.00 | |
| Extreme | 0.00 | 0.00 |
Discussion
An important aspect of CL system performance is continuous glucose monitor performance. This technology has been mainly designed for outpatient use among healthy patients with T1D and is not currently approved for use in hospitalized patients. In this study, CGMs were used in a critically ill postsurgical population. CGM devices are recommended to be worn on the abdomen or the upper buttocks as an alternate site for some devices. Concern arose among the investigators during this pilot investigation that transient edema could be markedly effecting the CGM sensor strength and contributing to more frequent recalibrations in this population. For this reason, a subanalysis was conducted among the seven CL patients (selected as these patients had every 30-min reference glucose values to use for comparison) looking at CGM performance during the 72-h investigational period.
A traditional CEG was constructed looking at the relative agreement between calibrated sensor reading and reference YSI serum BG. These data demonstrated that with recalibration, Enlite 2 sensor performance in this population was very good with >85% of values in Zone A and >99% of values in Zones A or B. SEG analysis showed a similar continuous risk profile with >98% of values in the “no risk or “slight lower” risk zones. Further analysis of MARD between the reference and CGM values revealed correlation at 11.0% MARD overall, which is in the middle of the range reported by similar studies that have shown an MARD of 7%–15%.10,36,37 Segmented analysis of MARD as a function of reference glucose showed very large MARD in the hypoglycemia range, although relatively few values were used to make this determination. Analysis of MARD by rate of change of sensor glucose showed large MARD for rapidly falling BG, but surprisingly good values for flat and even rapidly rising sensor glucose values.
An additional variable that merits consideration in this context is the supervisory rigor required to achieve this level of system correlation. While the accuracy of the CGM was excellent under the study conditions, a high degree of supervisory rigor was required to achieve this level of correlation. Medtronic recommends a calibration factor for the Enlite 2 in this system between 1.5 and 20 mg/nA·dL and recommends recalibration for two values in a row with an ARD of ≥20% or one value with an ARD of ≥30%. In clinical practice, healthy outpatients with diabetes typically perform a calibration 2–3 times/day. To more closely replicate true clinical utility, sensors were placed in this study just before SQ transition rather than a day in advance, as is done in many other studies. Sensors were frequently recalibrated during the first 12 h, considered the initial “settling time” for the sensor. After the first 12 h, sensors in this study were recalibrated an average of 8.3 times/day or roughly once every 3 h, including overnight. Furthermore the active sensor was switched 1.4 times/day. In outpatient use, patients typically recalibrate their sensors 2–3 times/day or once every 8–12 h and wear only one sensor that is changed every 7 days. The level of rigor in this study achieved a very good calibration factor of 7.692 mg/nA·dL across the entire investigational period.
A significant limitation of this study is that it was a post hoc investigation of pilot data, and no protocols were in place during the study to minimize recalibrations or active sensor changes. Analysis of recalibration time segments suggests that, however, frequent recalibration was necessary during certain time periods of the study. This analysis showed that during periods of high glycemic variability, as evidenced by significantly larger standard deviation of sensor glucose, more frequent calibration was required. As such, the level of physician and nursing supervision and involvement in this pilot and development project is not sustainable for a commercialized device. Further work on CGM and AP use in the post-TPIAT and other postsurgical populations will require more rigorous criteria for recalibrations and testing of systems using only one CGM device, with calibration limited to 2–4 times/day. As AP and CGM system refinement continues to an outpatient T1D population, improved systems will likely provide greater benefit to the postsurgical and islet transplant populations as well.
Only within the past 1–2 years have published studies investigated the role of CGM technology in postsurgical populations, most often cardiac surgery patients. A recent study from Siegelaar et al. investigated CGM use after cardiac surgery.38 They found MARD values of 11% and 14% for the Navigator and Guardian sensors investigated. They placed sensors on the abdomen the day before surgery and calibrated devices according to the manufacturers' instructions. No information on calibration factors was provided. Saur et al. also investigated CGM after cardiac surgery using the Symphony CGM system and found 99.6% of readings in Zones A and B with an MARD of 12.3%.10 They calibrated the sensors every 4 h. Schuster et al. have also recently published an analysis of CGM data in a broader surgical ICU population and found an MARD of 15.9% with 71.3% of values in Zone A and 98.9% of values in Zones A and B.39 Their study used only three calibrations per day. Overall, the findings from this study reveal similar MARD and Zone A and B percentages although with a higher calibration frequency than other recent studies.
Conclusions
The overall performance of the Medtronic Enlite 2 CGM in the post-TPIAT population was reasonably good with “no risk” or “slight lower” risk by SEG analysis and high CGM-YSI agreement by CEG analysis, indicating a correlation between CGM and reference glucose with no or very low risk of producing hypo- or hyperglycemia. This high degree of agreement required a significant amount of investigator supervision and recalibration in this pilot trial. However, considering that these were critically ill patients being treated in the intensive care or step-down unit, calibrating the sensor every 3 h may not be an excessive requirement. Future work may involve the use of the new Enlite 3, Dexcom G5, or FreeStyle Libre CGM devices in this population with the goal of less frequent calibration. Expanded use of CGM technology in postsurgical populations is likely to continue and full understanding of these devices is necessary to achieve optimal benefit for patients in this setting.
Acknowledgments
The authors thank our patients for participation in this project. They also acknowledge the dedicated support of the research nursing staff, in particular, Marnee Brandenberg and Nancee Nichols; and the dedicated medical staff on hospital units 4D and 7A. They also thank Martin Cantwell and Dr. Natalie Kurtz from Medtronic who developed the custom algorithm for this study and provided teaching and training on the experimental device, Dr. Yongyin Wang and Dr. John Shin from Medtronic who assisted with CEG SAS analysis, James Hodges who provided guidance on statistical analysis, and Dr. Stuart Weinzimer who assisted with the FDA IDE master file.
Author Disclosure Statement
The authors have no conflicts of interest to disclose except as described below regarding funding for this project. Funding for this study was provided by the Vikings Children's Research Fund and the Clinical Translational Sciences Institute (CTSI) pilot study award at the University of Minnesota (UMN). Medtronic Diabetes provided supplies as part of the investigator-initiated grant. The UMN CTSI is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Hammer MJ, Casper C, Gooley TA, et al. : The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009;15:344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storey S, Von Ah D: Impact of malglycemia on clinical outcomes in hospitalized patients with cancer: a review of the literature. Oncol Nurs Forum 2012;39:458–465 [DOI] [PubMed] [Google Scholar]
- 3.Benfield T, Jensen JS, Nordestgaard BG: Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia 2007;50:549–554 [DOI] [PubMed] [Google Scholar]
- 4.Krinsley JS: Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 2004;79:992–1000 [DOI] [PubMed] [Google Scholar]
- 5.Kreutziger J, Schlaepfer J, Wenzel V, Constantinescu MA: The role of admission blood glucose in outcome prediction of surviving patients with multiple injuries. J Trauma 2009;67:704–708 [DOI] [PubMed] [Google Scholar]
- 6.Hathout E, Chan NK, Tan A, et al. : In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr Transplant 2009;13:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speier S, Nyqvist D, Cabrera O, et al. : Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finzi G, Davalli A, Placidi C, et al. : Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastruct Pathol 2005;29:525–533 [DOI] [PubMed] [Google Scholar]
- 9.Nacher V, Merino JF, Raurell M, et al. : Normoglycemia restores beta-cell replicative response to glucose in transplanted islets exposed to chronic hyperglycemia. Diabetes 1998;47:192–196 [DOI] [PubMed] [Google Scholar]
- 10.Saur NM, England MR, Menzie W, et al. : Accuracy of a novel noninvasive transdermal continuous glucose monitor in critically ill patients. J Diabetes Sci Technol 2014;8:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch IB: Improvements in our understanding of diabetes mellitus. Mayo Clin Proc 2013;88:907–909 [DOI] [PubMed] [Google Scholar]
- 12.Rice MJ, Coursin DB: Continuous measurement of glucose: facts and challenges. Anesthesiology 2012;116:199–204 [DOI] [PubMed] [Google Scholar]
- 13.Basu A, Dube S, Slama M, et al. : Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013;62:4083–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossetti P, Bondia J, Vehi J, Fanelli CG: Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors (Basel) 2010;10:10936–10952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Dube S, Veettil S, et al. : Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol 2015;9:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA. MiniMed 530G System—P120010. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm372176.htm (accessed September14, 2015)
- 17.FDA. FDA approves pediatric use of Dexcom's G4 Platinum continuous glucose monitoring system. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm384495.htm (accessed September14, 2015)
- 18.Piper HG, Alexander JL, Shukla A, et al. : Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics 2006;118:1176–1184 [DOI] [PubMed] [Google Scholar]
- 19.Boom DT, Sechterberger MK, Rijkenberg S, et al. : Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care 2014;18:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzinger U, Warszawska J, Kitzberger R, et al. : Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care 2010;33:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov www.clinicaltrials.gov/ct2/results?term=cgm+icu&Search=Search (accessed November16, 2015)
- 22.Clarke WL, Cox D, Gonder-Frederick LA, et al. : Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987;10:622–628 [DOI] [PubMed] [Google Scholar]
- 23.Clarke WL: The original Clarke Error Grid Analysis (EGA). Diabetes Technol Ther 2005;7:776–779 [DOI] [PubMed] [Google Scholar]
- 24.Klonoff DC, Lias C, Vigersky R, et al. : The surveillance error grid. J Diabetes Sci Technol 2014;8:658–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovatchev BP, Wakeman CA, Breton MD, et al. : Computing the surveillance error grid analysis: procedure and examples. J Diabetes Sci Technol 2014;8:673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forlenza GP, Nathan BM, Moran AM, et al. : Successful application of closed-loop artificial pancreas therapy after islet autotransplantation. Am J Transplant 2016;16:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maahs DM, DeSalvo D, Pyle L, et al. : Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015;38:e158–e159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellin MD, Sutherland DE: Pediatric islet autotransplantation: indication, technique, and outcome. Curr Diab Rep 2010;10:326–331 [DOI] [PubMed] [Google Scholar]
- 29.Forlenza GP, Chinnakotla S, Schwarzenberg SJ, et al. : Near-euglycemia can be achieved safely in pediatric total pancreatectomy islet autotransplant recipients using an adapted intravenous insulin infusion protocol. Diabetes Technol Ther 2014;16:706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farney AC, Najarian JS, Nakhleh RE, et al. : Autotransplantation of dispersed pancreatic islet tissue combined with total or near-total pancreatectomy for treatment of chronic pancreatitis. Surgery 1991;110:427–437 [PubMed] [Google Scholar]
- 31.Wang Y, Shin J: Creating Clark Error Grid with SAS/GRAPH, and the SAS/GRAPH Annotate Facility, and the SAS Macro Applications. SAS Global Forum 2013;133-2013:1–11 [Google Scholar]
- 32.Wakeman CA: Surveillance Error Grid software. http://diabetestechnology.org/SEGsoftware/ (accessed August26, 2015)
- 33.Rodbard D: Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol 2014;8:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pleus S, Schoemaker M, Morgenstern K, et al. : Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J Diabetes Sci Technol 2015;9:801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zschornack E, Schmid C, Pleus S, et al. : Evaluation of the performance of a novel system for continuous glucose monitoring. J Diabetes Sci Technol 2013;7:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jina A, Tierney MJ, Tamada JA, et al. : Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol 2014;8:483–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegelaar SE, Barwari T, Hermanides J, et al. : Microcirculation and its relation to continuous subcutaneous glucose sensor accuracy in cardiac surgery patients in the intensive care unit. J Thorac Cardiovasc Surg 2013;146:1283–1289 [DOI] [PubMed] [Google Scholar]
- 39.Schuster KM, Barre K, Inzucchi SE, et al. : Continuous glucose monitoring in the surgical intensive care unit: concordance with capillary glucose. J Trauma Acute Care Surg 2014;76:798–803 [DOI] [PubMed] [Google Scholar]