Abstract
The potential for biotransformation of weathered hydrocarbon residues in soils collected from two commercial oil refinery sites (Soil A and B) was studied in microcosm experiments. Soil A has previously been subjected to on-site bioremediation and it was believed that no further degradation was possible while soil B has not been subjected to any treatment. A number of amendment strategies including bioaugmentation with hydrocarbon degrader, biostimulation with nutrients and soil grinding, were applied to the microcosms as putative biodegradation improvement strategies. The hydrocarbon concentrations in each amendment group were monitored throughout 112 days incubation. Microcosms treated with biostimulation (BS) and biostimulation/bioaugmentation (BS + BA) showed the most significant reductions in the aliphatic and aromatic hydrocarbon fractions. However, soil grinding was shown to reduce the effectiveness of a nutrient treatment on the extent of biotransformation by up to 25% and 20% for the aliphatic and aromatic hydrocarbon fractions, respectively. This is likely due to the disruption to the indigenous microbial community in the soil caused by grinding. Further, ecotoxicological responses (mustard seed germination and Microtox assays) showed that a reduction of total petroleum hydrocarbon (TPH) concentration in soil was not directly correlable to reduction in toxicity; thus monitoring TPH alone is not sufficient for assessing the environmental risk of a contaminated site after remediation.
Keywords: Soil contamination, Bioremediation, Weathered petroleum hydrocarbon, Bioaugmentation, Biostimulation
Highlights
-
•
Improved biodegradation of weathered hydrocarbon residues.
-
•
Nutrient addition is a key parameter for promoting biodegradation.
-
•
Soil grinding reduced effectiveness of biostimulation.
-
•
Reduction of petroleum hydrocarbons in soil not directly correlable to reduction in toxicity.
1. Introduction
Land contamination from poor historical industrial practices or incidents is a widespread and well recognised environmental issue. In the UK alone, it has been estimated that ca. 300,000 ha of land could be affected by industrial activity leading to contamination (Environment Agency, 2009). Petroleum hydrocarbons are one the most common contaminant, though a wide range of chemicals may be present (Towell et al., 2011). Once released into the environment, petroleum hydrocarbons are subject to abiotic and biotic weathering reactions e.g. physical and biochemical transformations, interactions with soils, that will change their composition and toxicity, and will influence their fate and biodegradation (Brassington et al., 2007, Stroud et al., 2007, Maletić et al., 2011). The extent of these transformations will vary according to the type of petroleum products present, the soil conditions (e.g. organic matter content, soil grain size and clay-type at the sites) (Stroud et al., 2007), and the bioavailability and susceptibility of the different compounds (Stroud et al., 2007, Maletić et al., 2011).
Bioremediation has become the preferred method for the remediation of petroleum hydrocarbon contaminated soils, because it is considered cost effective and sustainable, and can accelerate naturally occurring biodegradation processes through the optimisation of limiting parameters (Vidali, 2001, Coulon et al., 2012). To be effective, it is important to investigate and understand all factors (e.g. soil and contaminant characteristics, bioavailability, stage of weathering) that might effect the efficacy of the process. For example, aliphatic hydrocarbons of intermediate length (ranging between C10 and C25) tend to be readily degradable by microorganisms despite their low solubility, whereas longer chain alkanes (C25-C40), especially those with branched or cyclic chain structures, are more resistant to biological degradation (Maletić et al., 2011).
Heavily weathered hydrocarbons are difficult to biodegrade and have relatively low toxicity, but high residual concentrations can severely alter the physical and chemical properties of the soils, thus reducing soil fertility (Coulon et al., 2012, Wu et al., 2013). Remediation outcomes using biological methods for the treatment of weathered hydrocarbons are often unpredictable, and in some instances contaminated soil may be regarded as ‘untreatable’ via bioremediation (Brassington et al., 2007). Debate around the benefits of bioaugmentation and its capacity to increase the microbial degradation of weathered hydrocarbons after indigenous microorganisms are no longer effective continues, and only a few studies demonstrate continued biodegradation after introduction of specific hydrocarbon degraders (Coulon et al., 2012, Gallego et al., 2001). Biodegradative performance via bioaugmentation can be further improved by the addition of appropriate nutrients; a process referred to as biostimulation (Xu and Lu, 2010). Due to the limited number of studies on the subject, and the complexity of weathered petroleum hydrocarbon products, there is a need for investigation into the potential for bioaugmentation coupled with biostimulation to enhance biotransformation and reduce residual toxicity.
In this study, we investigated the potential for biotransformation of weathered hydrocarbon residues in soil. To do so, we determined whether it was possible to improve biodegradation with the simultaneous application of bioaugmentation and biostimulation on two soil types. Soil A was taken from a windrow where bioremediation had been completed and soil B was taken from a site prior remediation where oil drums had leaked contaminating the soil. Soil A treatment was deemed completed as no further degradation could be achieved. This research provides valuable knowledge concerning chemical and toxicological changes on a soil type not previously investigated and could be used to support the development of bioremediation strategies. Finally, we discuss the relationship between chemical change, toxicity, and total petroleum hydrocarbons (TPH) measurements in the context of risk assessment, highlighting the effects that remediation might have on soil toxicity.
2. Materials and methods
2.1. Physical and chemical characterisation of soil samples
Two different soils collected at a depth of 5–20 cm from two commercial oil refinery sites located in the UK were labelled A and B (to maintain owner anonymity). Soil A is a sandy soil which was heavily contaminated with weathered hydrocarbons (TPH = 50,000 mg kg−1). After 6 month windrow treatment, TPH concentration was decreased to 22,700 mg kg−1 where it was believed no further degradation was possible. Soil B is predominantly clay soil contaminated with weathered hydrocarbons (TPH = 31,500 mg kg−1) taken from a more recently contaminated site where there was no history of any remedial activity. The soils were air-dried for 24 h and sieved through 2 mm mesh to remove stones, plant material, and to facilitate mixing. Prior to air drying the field moisture content was determined in triplicate by oven drying at 105 °C for 24 h. Soils were then stored at 4 °C in the dark before use.
For both soil samples, a routine set of characterisation was carried out. Soil pH was measured using a pH meter (Jenway 3540) in a distilled water slurry (one part soil: two parts water) after a 30 min equilibration period (Black, 1965). Maximum water holding capacity (WHC) was determined in duplicate by flooding the wet weight equivalent of 100 g of dry soil in a filter funnel and allowing it to drain overnight (Black, 1965). Particle size analysis was performed by a combination of wet sieving (sand) and sedimentation (silt and clay), as described by Gee and Baude (1986). The organic matter content as indicated by loss on ignition (LOI) of each soil was measured by combustion at 450 °C in a furnace for 24 h, according to ASTM Method D297487. Total organic carbon (TOC) was analysed by potassium dichromate oxidation, as described by Schnitzer (1982).
For nitrate, phosphate, and ammonium determination, 10 g of soil was first extracted in 0.5 M potassium bicarbonate (adjusted to pH 8.5). The extractant was then analysed by high-performance liquid chromatography (HPLC) for nitrate and phosphate as described by Brenner and Mulvaney (1982) and Olsen and Sommers (1982), respectively. Ammonium was analysed using the colorimetric test described by Reardon et al. (1966).
2.2. Microcosm experiment design
Soil microcosms were established using 700 g of either soil A and B in sterile 1 L, wide-mouth amber glass jars. Four different microcosm conditions for each soil were established and tested in triplicate (Table 1). Soil grinding was done using mortars and pestles made from hard chemical-porcelain ware. The mortars had a lip and were glazed on the outside. The pestles were glazed to the grinding surface. Soil aliquots of about 70 g were ground for about 15 min in the mortar with the pestle to pass the soil through a 42.5 μm sieve. The ground soil aliquots were then combined for additional sample preparation. Nutrients were added in the form of ammonium nitrate and potassium orthophosphate to obtain a C:N:P ratio of 100:1:0.1. The hydrocarbon-degrading inoculum was composed of three bacterial isolates supplied by Remedios Limited (Aberdeen). The bacterial isolates were isolated from an attenuated enrichment culture from No.6 oil impacted soil (Alzahrany, 2011). Two bacterial isolates were related to Pseudomonas sp. (100% match) and one to Klebsiella sp. (99% match). Inoculum was grown using a minimal medium supplemented with diesel (equivalent to 5.5 mg C l−1) as carbon source. The cell concentration added to each microcosm was such as to give 5 × 107 CFU g−1 soil. For each amendment, a few woodchips were added to 10 ml of Bushnell-Haas broth supplemented with 1 g l−1 salicylic acid and 1% ethanol, adjusted to pH 7. The mixture was placed in an orbital shaker at 150 rpm in the dark at 20 °C and left overnight, after which 1 ml was added to 100 ml of fresh medium and grown to a stationary phase (about 24 h, checked by optical density readings at 600 nm). The cell number at stationary phase was 108 cells ml−1. The inoculum solution was then added to the soils at 0.01 ml g−1 dry wt soil to achieve 106 cells g−1 dry wt soil. The moisture content of each microcosm was adjusted to 80% of the soil’s water holding capacity using deionised water. The microcosms were incubated in the dark at 15 °C. High humidity was maintained using damp cotton wool and moisture checked periodically. Each microcosm was mixed weekly and capped loosely to allow oxygen transfer. Soil from each microcosm was sampled at 0, 7, 14, 28, 56, and 112 days for subsequent microorganism respiration monitoring and hydrocarbon analysis.
Table 1.
Microcosm experiment setup.
| Condition | Soil | Amendment/Treatment detail |
|---|---|---|
| Natural attenuation (NA) | Soil A | None - Control |
| Soil B | ||
| Biostimulated (BioS) | Soil A | Amended with NH4NO3 + KH2PO4 |
| Soil B | ||
| Biostimulated and bioaugmented (BioS + BioA) | Soil A | Same as BioS and inoculated with hydrocarbon degrader (5 × 107 cells per g soil dry weight) |
| Soil B | ||
| Ground and Biostimulated (G + BioS) | Soil A | Same as BioS plus soil grinding |
2.3. Microbial respiration monitoring
A soil sample (1 g on dry weight basis) from each microcosm was collected and sealed in a headspace vial. All vials containing microcosm samples were incubated under the same conditions as the microcosms for 24 h. For CO2 analysis, the headspace gas was sampled with a gastight syringe and manually injected into a Cambridge Scientific 200 series gas chromatograph with thermal conductivity detector (GC-TCD; Cambridge Scientific Instruments, Cambridge, UK) using helium as carrier gas at 20 psi (138 kPa). The GC was fitted with a CTR1 concentric packed column (Alltech, USA). The column oven and injector temperature were 110 °C and 125 °C, respectively. The GC was calibrated using a standard CO2 (1% CO2 balanced with N2). Respiration values were determined on a mg CO2 kg soil−1 day−1 basis following subtraction of a blank vial containing atmospheric CO2 only.
2.4. Microbial enumeration
Homogenized soil (2 g) was weighed into a glass Universal bottle and 20 ml of ¼ strength Ringer’s solution was added. Samples were then vortexed for 30 s and sonicated for 1 min and allowed to stand for a further 2 min. A 100 ml aliquot of soil suspension was removed and serially diluted in ¼ strength Ringer’s solution to the appropriate dilution factor (10−5 or 10−4 dilution factor). An aliquot of 10 ml of each dilution series was added in triplicate to ¼ strength Luria Bertani medium to determine heterotrophs and Bushnell-Hass with 1% diesel as the sole carbon source for hydrocarbon-degraders. Samples were incubated at 25 °C for 24–48 h thereafter and colony-forming units (CFUs) enumerated. Results are expressed as CFU g−1 of dry soil.
2.5. Hydrocarbon analysis
Hydrocarbon extraction was performed as described by Risdon et al. (2008). Briefly soils (5 g) were chemically dried with 5 g anhydrous Na2SO4 in 50 ml Teflon centrifuge tubes. Acetone (4 ml) was added and sonicated for 2 min at 20 °C. Acetone (6 ml) and hexane (10 mL) were added to the samples and sonicated for 10 min, followed by manually shaking to mix the solvent and soil. This step was repeated twice followed by centrifugation for 5 min at 750 rpm. After passing the supernatant through a filter column fitted with glass receiver tube, a sequential step series, including resuspension of the samples in 10 ml of acetone/hexane (1:1), sonicated for 15 min at 20 °C, centrifugation for 5 min at 750 rpm, and decantation into a filter column, was repeated twice. The final extract volume was adjusted to 40 ml with a mixture of acetone/hexane (1:1) and homogenized by manual shaking. The silica gel column clean-up was performed by passing the extracts through a column filled with florisil. Total extractable and recoverable petroleum hydrocarbons (TERPH), aliphatic and aromatic fractions were identified and quantified using a Perkin Elmer AutoSystem XL gas chromatograph coupled with a Perkin Elmer Turbomass Gold mass spectrometer operated at 70 eV in positive ion mode. The GC was fitted with a Restek RTX -5MS capillary column (30 m in length, 0.25 mm internal diameter and 0.25 μm coating). Splitless injection with a sample volume of 1 μl was applied. The oven temperature was increased from 60 °C to 220 °C at 20 °C min−1, then to 310 °C at 6 °C/min and held at this temperature for 15 min. The mass spectrometer was operated using the full scan mode (range m/z 50–500) for quantitative analysis of target alkanes and PAHs. For each compound, quantification was performed by integrating the peak at specific m/z. External multilevel calibrations were carried out for both oil fractions, quantification ranging from 0.5 to 2500 μg ml−1 and from 1 to 5 μg ml−1, respectively. Internal standards for the alkanes were nonadecane-d40, triacontane-d62 and naphthalene d8, phenanthracene-d10, chrysene-d12 and perylene d12 (Sigma Aldrich, Dorset, UK). For quality control, a 500 μg ml−1 diesel standard and mineral oil were analysed every 20 samples. In addition, duplicate blank controls were also performed by going through the same extraction procedure but containing no soil. The reagent control was treated following the same procedure as the samples without adding soil sample. The reference material was an uncontaminated soil of known characteristics, and was spiked with a diesel and mineral oil standard at a concentration equivalent to 16,000 mg kg−1.
2.6. Soil toxicity bioassay
Seed germination and Microtox® assay were carried out at the start and the end of the microcosm experiment. The selection of the ecotoxicity assays were based on their ease of execution and representation of different ecological soil organisms. Seed germination tests were performed according to Saterbak et al. (2009). Ten white mustard seeds (Brassica alba) were planted into 20 g of test soil (wet weight) in 120 ml bottle. This was repeated 10 times. The seeds were left to germinate for 4 days at 25 °C in darkness. At the end of each test, if a root was visible, the seed was scored as germinated. Microtox® solid phase test (Microtox® SPT) assay was carried out according to Azur Environmental (1998). Tests were done in triplicate. The soil dilution that inhibits 50% (EC50) of the light output relative to oil-free soil collected nearby the sampling sites was calculated for each oiled samples and expressed as a percent of the pristine sample. Note that Microtox EC50 values decline as toxicity increases. A standard 100 g l−1 phenol solution was used to check the performance of both operator and analytical system and the 95% confidence range was maintained below 15% variation throughout the study.
2.7. Statistical analysis
Statistical analysis of the results such as mean, standard deviation (SD), standard error (SE) and analysis of variance (ANOVA) were performed using Excel and SPSS (version 17, Statistical Product and Service Solutions). Differences in the TERPH, alkanes and PAH concentration between different treatments were compared using ANOVA by Fishers Least Significant Difference (LSD) test. The difference was recognised as significant where P < 0.05.
3. Results and discussion
3.1. Soil characteristics
Soil characterisation provided baseline physical and chemical properties of the two soil samples used in this study (Table 2). TPH concentration in Soil A was measured at 22,700 mg kg−1, and for Soil B, at 31,500 mg kg−1. Both values indicate that the two soils contained elevated concentrations of petroleum hydrocarbons (Zemanek et al., 1997, Tindal, 2005). The ammonium and nitrate levels were undetectable in both soils, phosphate was undetectable in Soil B and measured at a low level (0.0016 mg kg−1) in Soil A. These conditions suggest that both soils could benefit from biostimulation with nutrients. Biodegradation within each soil was not seen to be limited by carbon, nitrogen, pH or moisture conditions as these remained within acceptable ranges (Eweis et al., 1998, Coulon et al., 2012, Wu et al., 2013). Microbial respiration tests (Table 2 and Fig. 1) indicated that an active microbial population was present within both soils prior to the addition of microbial inoculum (Diploick et al., 2009). Other soil properties are shown in Table 2. More detailed soil analysis results including metal concentrations have been reported in a previous study (Towell et al., 2011).
Table 2.
Characteristics of soils A and B.
| Soil A | Soil B | |
|---|---|---|
| TPHa (mg kg−1) | 22,700 ± 589 | 31,500 ± 980 |
| Bulk density (g ml−1) | 0.973 | 0.823 |
| Moisture content in % | 15 ± 2 | 21 ± 4 |
| Moisture content in % at WHCb | 38 ± 6 | 44 ± 8 |
| pH in Water | 6.8 ± 0.3 | 7.5 ± 0.2 |
| pH in 0.01 M CaCl2 | 6.5 ± 0.0 | 6.6 ± 0.0 |
| LOI in % | 12 ± 0.7 | 15 ± 1.2 |
| Organic Carbon in % | 7 ± 2 | 9 ± 3 |
| TOCc(μg g−1) | 168 ± 7 | 280 ± 68 |
| Carbon in % | 9 ± 1 | 8 ± 2 |
| Nitrogen in % | 5 ± 0.5 | 2 ± 1 |
| Microbial respiration (mg CO2 kg−1 soil d−1) | 5.30 | 4.03 |
| Ammonium in % | Not detected | Not detected |
| Nitrate in % | Not detected | Not detected |
| Phosphate in % | 0.0016 | Not detected |
| Total heterotrophs (CFU g−1) | 6.0 × 105 | 5.1 × 106 |
| Total degraders (CFU g−1) | 1.4 × 104 | 1.1 × 105 |
Note: a TPH = Total Petroleum hydrocarbon, b WHC = Water holding capacity, c TOC = Total organic carbon.
Fig. 1.
Mean CO2 release over 112 days (Error bars indicate the standard deviation of triplicate measurements).
3.2. Soil microcosm experiment
3.2.1. Microbial respiration and counts
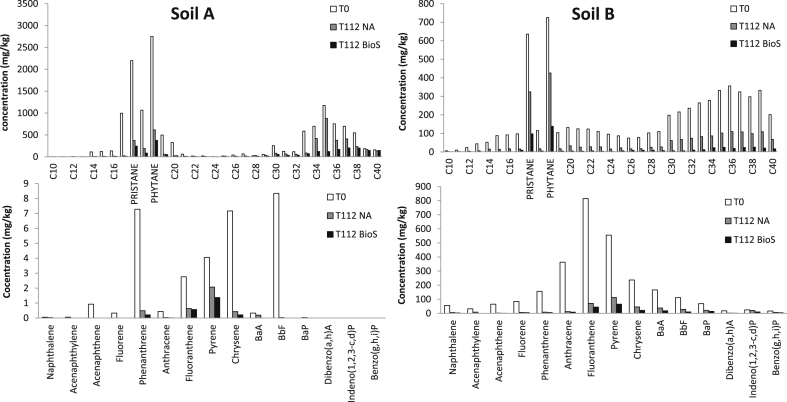
All of the microcosms contained a viable microbial community as demonstrated by respiration rates that were measured by CO2 production from the soil samples (Table 2 and Fig. 1). Soil A (<200 mg CO2 kg soil d−1) had a lower respiration rate than Soil B (∼275 mg CO2 kg soil d−1) and this was likely due to the higher concentration of longer chain hydrocarbons (Fig. 3) and other recalcitrant fractions, as a result of a longer exposure to contamination and weathering. In contrast, Soil B was contaminated more recently and the presence of short chain hydrocarbons and other readily biodegradable compounds (Fig. 3) could be responsible for the initially higher level of CO2 production (Fig. 1). Analysis of the initial soil hydrocarbon concentrations and hydrocarbon fractions support this claim (Table 3).
Fig. 3.
n-alkanes and PAH fingerprints in soil A and B respectively after 112 days of natural attenuation (T112 NA) and biostimulation treatment (T112 BioS).
Table 3.
Mean initial and final aliphatic and aromatic fraction concentration changes in soils A and B after 112 days of treatment.
| Soil | Hydrocarbons | To |
T112 days |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | NA |
BioS |
G + BioS |
BioS + BioA |
|||||||
| mg kg−1 | % loss | mg kg−1 | % loss | mg kg−1 | % loss | mg kg−1 | % loss | ||||
| Soil A | Aliphatic fractions | >C8-C10 | 2.78 | 0.78 | 72 | 0.17 | 94 | 0.76 | 73 | 0.42 | 85 |
| >C10-C12 | 8.56 | 4.42 | 48 | 0.9 | 99 | 6.15 | 28 | 2.82 | 67 | ||
| >C12-C16 | 115 | 37.9 | 67 | 13.9 | 88 | 44.7 | 61 | 26.7 | 77 | ||
| >C16-C35 | 11,000 | 3500 | 68 | 1230 | 89 | 4090 | 63 | 2400 | 78 | ||
| >C35-C40 | 2720 | 818 | 70 | 904 | 67 | 861 | 68 | 991 | 64 | ||
| Total | 13,800 | 4360 | 68 | 2150 | 84 | 5000 | 64 | 3420 | 75 | ||
| Aromatic fractions | >C8-C10 | 0.27 | ND | 100 | ND | – | ND | – | ND | – | |
| >C10-C12 | 1.54 | 0.41 | 73 | 0.34 | 78 | 0.35 | 77 | 0.22 | 86 | ||
| >C12-C16 | 15.5 | 1.9 | 88 | 1.49 | 90 | 3.29 | 79 | 1.15 | 93 | ||
| >C16-C21 | 10.2 | 1.5 | 85 | 0.64 | 94 | 1.68 | 84 | 0.42 | 96 | ||
| >C21-C35 | ND* | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Total | 27.5 | 3.81 | 86 | 2.47 | 91 | 5.32 | 81 | 1.82 | 93 | ||
| Soil B | Aliphatic fractions | >C8-C10 | 2.34 | 0.23 | 90 | 0.14 | 94 | – | – | 0.1 | 96 |
| >C10-C12 | 20.5 | 4.4 | 79 | 1.76 | 91 | – | – | 1.2 | 94 | ||
| >C12-C16 | 198 | 62.7 | 68 | 14.1 | 93 | – | – | 11.2 | 94 | ||
| >C16-C35 | 4840 | 1440 | 70 | 300 | 94 | – | – | 214 | 96 | ||
| >C35-C40 | 1010 | 560 | 45 | 192 | 81 | – | – | 121 | 88 | ||
| Total | 6070 | 2070 | 66 | 508 | 92 | – | – | 348 | 94 | ||
| Aromatic fraction | >C8- C10 | 29.6 | 7.02 | 76 | 5.66 | 81 | – | – | 4.82 | 84 | |
| >C10-C12 | 24.9 | 6.2 | 75 | 5.36 | 79 | – | – | 4.88 | 80 | ||
| >C12-C16 | 1830 | 177 | 90 | 70.3 | 96 | – | – | 82.3 | 96 | ||
| >C16-C21 | 840 | 183 | 78 | 118 | 86 | – | – | 112 | 87 | ||
| >C21-C35 | 43.1 | 10.3 | 76 | 8.45 | 80 | – | – | 5.23 | 88 | ||
| Total | 2770 | 384 | 86 | 208 | 93 | – | – | 209 | 92 | ||
Note: *ND = Not detected.
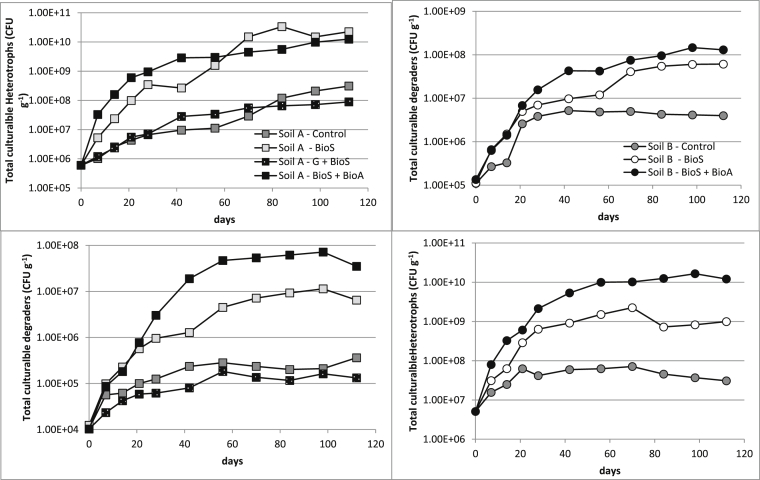
The amendment strategies applied to both soils, except soil grinding + biostimulation, increased by two and three orders of magnitude the numbers of culturable heterotrophs and hydrocarbon degraders (Fig. 2) and this translated into enhanced CO2 production (up to 40% increase after 14 days in Soil A microcosms and up to 23% increase after 14 days in Soil B microcosms) when compared to natural attenuation processes alone (Fig. 1). A significant increase in hydrocarbon degraders was observed within 42 days in both soils (Fig. 2). At the end of experiment, the hydrocarbon degraders number in both soils were three orders of magnitude higher than those in control soils (natural attenuation) and in soil where grinding and biostimulation was applied (105–106 versus 108 CFU g−1 soil). In addition, CO2 production in Soil B stabilised at ∼250 mg CO2 kg soil d−1 for 56 days and then after 100 days gradually stabilised at ∼200 mg CO2 kg soil d−1 (Fig. 1). A similar trend was observed in Soil A where CO2 production stabilised ∼150 mg CO2 kg soil d−1 after 28 days. Where grinding and biostimulation were applied (G + BioS) to Soil A, respiration levels stabilised at ∼120 mg CO2 kg soil d−1 and after 98 days was closer to 100 mg CO2 kg soil d−1 in line with respiration levels measured with the microcosms where natural attenuation was applied (Fig. 1). The TPH levels for Soil A microcosms were similar across the four studied conditions. Thus, this suggests that grinding changed the soil and hydrocarbons intrinsic physico-chemistry that effected both the microbial activity (Fig. 1) and the microbial abundance (Fig. 2).
Fig. 2.
Total culturable heterotroph and degrader numbers over 112 days (Standard deviation was <20% for both CFU counts and not shown on the graphs for clarity).
3.2.2. Petroleum hydrocarbon analysis
Soil B hydrocarbons distribution showed a well-developed series of n-alkanes distribution (Fig. 3). The distribution is heavy-end skewed and bi-modal with a higher proportion of C28-C40 n-alkanes. In contrast soil A hydrocarbons distribution confirms that the hydrocarbon source is weathered (degraded) (Brassington et al., 2010). More specifically, the concentration of aliphatic compounds in Soil A (13,800 mg kg−1) was 2.2 times higher than in Soil B (Table 3). In contrast the concentration of aromatic compounds in Soil B was 100 times higher than in Soil A (Table 3). After treatment, the most prominent residual hydrocarbon fractions in Soil A and Soil B were the aliphatic fractions C16-C35 and C35-C40, and the aromatic fractions C16-C21 and C12-C16, respectively (Table 3 and Fig. 2). The largest reduction in both the aliphatic and aromatic fractions were obtained in the BioS and BioS + BioA microcosms (Table 3, Table 4). While the BioS + BioA combination improved bioremediation performance (compared to natural attenuation alone) the addition of microbes did not necessarily provide additional improvement to the biodegradation process compared to the addition of nutrients alone, suggesting that nutrient addition is a key parameter for promoting biodegradation (Hejazi and Husain, 2004). Similar hydrocarbons loss percentage results were observed in Soil B (Table 3) which had not undergone remediation previously. Further to this, the percentage of degradation (Table 3) and the degradation constant (Table 4) suggests that the higher concentrations of aromatics did not limit the extent of bioremediation performance in soils.
Table 4.
Degradation constant rate of the aliphatic and aromatic fractions.
| Degradation constant rate ka (d−1) |
||||
|---|---|---|---|---|
| Soil A |
Soil B |
|||
| Aliphatic | Aromatic | Aliphatic | Aromatic | |
| Natural attenuation | −0.0084 | −0.0016 | −0.0065 | −0.0021 |
| BioS (Soil A) | −0.0104 | −0.0022 | −0.0086 | −0.0023 |
| Grinding + BioS | −0.0078 | −0.0020 | – | – |
| BioS + BioA | −0.0092 | −0.0023 | −0.0081 | 0.023 |
k was calculated from equation: 1n[TPH] = −kt + b.
Even though Soil A was previously remediated and the soil had undergone considerable weathering, further degradation of the residual hydrocarbons was possible when suitable conditions were provided. As a result, bioremediation end points are variable and will depend on a range of factors, most notably the nutrient levels and the availability of microbes.
In weathered soils, residual hydrocarbons are tightly bound to the soil matrix and can form rigid soil aggregates that can effectively entrap hydrocarbons and limit bioaccessibility (Huesemann et al., 2004). By grinding the soil, the contact surface area and oxygen transfer rates are increased and this increases the chance for microbes and hydrocarbons to come into contact (Craswell and Waring, 1972, Nasser and Mingelgrin, 2012). However, the combination of grinding and BioS was not observed to enhance the mineralisation of the aliphatic and aromatic hydrocarbon fractions compared to the natural attenuation (Table 4). This unexpected result may be due to the disruption of the indigenous microbial consortium caused by soil grinding, as previously suggested by Powlson (1980), or more likely, grinding facilitated the release of bound fractions of hydrocarbons that proved toxic to the microbes (Fig. 4a). This finding reinforces previous findings reported by Wu et al. (2013) and Huesemann et al. (2004) that state it is incorrect to assume that residual hydrocarbons after bioremediation treatment are recalcitrant and therefore they can be left in place without posing an environmental risk.
Fig. 4.
Mean seeds germination percentage (whisker boxes) and total extractable hydrocarbons resolved (aliphatic + aromatic compounds; black squares) at the onset and after 112 days for each treatment in soil A and B (error bars indicate standard error of the triplicate results).
Overall, the extent of biodegradation in both soils was less significant for longer chain hydrocarbons than shorter chain hydrocarbons. This effect became more pronounced for hydrocarbon fractions with an equivalent carbon number over C35 as these compounds are the most recalcitrant to degradation.
3.2.3. Soil ecotoxicology
It is important to evaluate soil ecotoxicology during and after any remediation treatment as it has been shown that a reduction in contaminants alone does not infer a reduction in toxicity (Coulon et al., 2005). Further to this, ecotoxicological tests can provide information on the bioavailability of contaminants present in soil (Molina-Barahona et al., 2005, Dawson et al., 2007). Results of the seed germination were normalised using a clean uncontaminated soil (control) to take into account the germination rate of the seeds used. Visual observations showed that seeds germinated quickly in the uncontaminated soil with an incidence of >90% of seed germination over the experimental period. Whilst seed germination was observed in both contaminated soils and for each treatment, it should be noted that visual observations during the course of the experiment showed that the rate of germination, and subsequently seedling growth, were reduced compared to the un-contaminated soil (control). Thus, even though Soil B, without any specific treatment (natural attenuation), achieved 100% germination at the end of the experiment (112 days) (Fig. 4), the rate and degree of growth were less than that seen in the uncontaminated soil, thus suggesting an ecotoxicological effect.
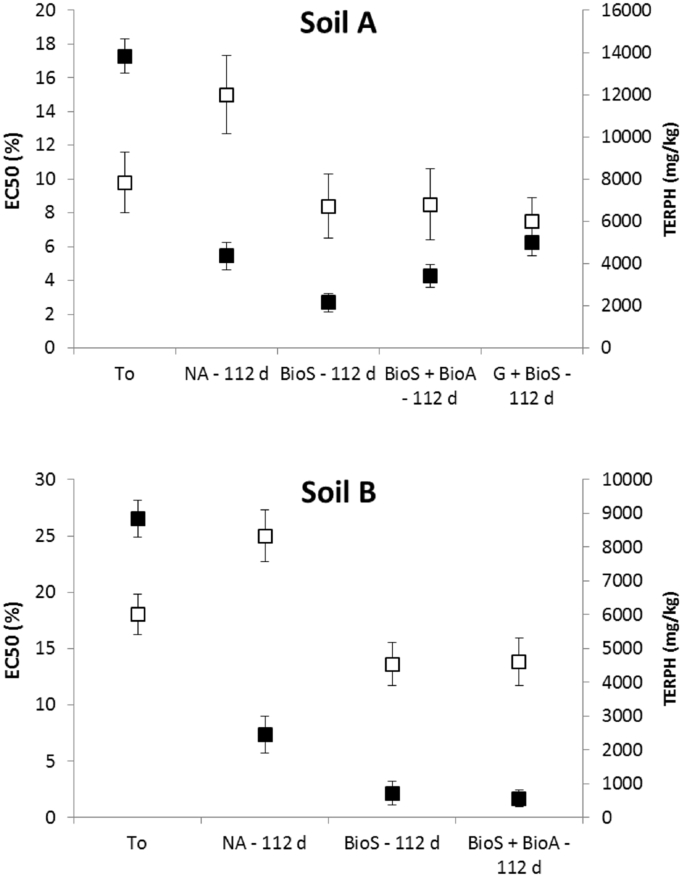
Overall, seed germination and Microtox® SPT showed that the toxicity of treated soils was higher while the natural attenuation approach showed the least change in toxicity (Fig. 4). The seed germination tests showed almost 50% reduction in germination for all three treatments for Soil A and a 40% reduction in the two Soil B treatments. Therefore, although there was a considerable reduction in TERPH, the toxicity of the soils increased (Fig. 4). Such findings have been reported previously, for example, Dorn and Salanitro (2000) reported in a 360-day lab scale bioremediation trial of soils contaminated with crude oil that there was no improvement of seed germination (%) after bioremediation, although a significant degradation of contaminants had occurred. Grinding Soil A for the biostimulation condition offers one explanation for this increased toxicity. It is possible that the grinding process released toxic contaminants that were originally enclosed in soil aggregates or pores, enhancing their bioavailability.
The Microtox® SPT measurements are in good agreement with the findings of the seed germination in terms of ecotoxicity ranking for both soils. The EC50 values decreased with a decline in TERPH (Fig. 5), confirming that remediated soils have higher toxicity compared to original soil conditions and/or soil left to natural attenuation (Fig. 5). These results suggest that the negative shift is due to the compositional changes observed during the active treatments (BioS and BioS + BioA) and the by-product of biodegradation (Roy et al., 2012). In a recent study, Mamindy-Pajany et al. (2012) evaluated the ecotoxicological effects of four contaminated sediments treated with mineral additives using Microtox® assay. In all treated samples, a decrease of contaminants was observed. However, the rank of toxicity was not in accordance with the rank of contamination level, and in two of the less contaminated samples an increased toxicity level was observed. Similar findings have also been reported by Xu and Lu (2010) when using Microtox® SPT to evaluate the ecotoxicity of crude oil contaminated soil after bioremediation.
Fig. 5.
EC50 value (%) (white squares) and TERPH (black squares) at the onset and after 112 days for each treatment in soil A and B (error bars indicate standard error of the triplicate results; toxicity decreases when the EC50 value increases).
In sum, the results suggest that there is no direct correlation between a decrease in total extractable hydrocarbons and a reduction in toxicity. There are several causes for these discrepancies including (i) hydrocarbon bioavailability change during bioremediation treatment, (ii) complex soil-contaminant interactions as well as interactions between residual hydrocarbons rendering them more or less toxic than expected based on additive independent behaviour of toxicants, and (iii) sensitivity of the bioassay to the bioavailable fraction of the residual hydrocarbons as compared to the tightly bound fractions that could be a prominent fraction of the residual hydrocarbons (Roy et al., 2012, Mallick and Dutta, 2008, Thavamani et al., 2015). This is likely due to the more toxic intermediates formed during the biodegradation (Mallick and Dutta, 2008, Ahn et al., 2006).
4. Conclusion
This study confirms it is possible to improve the treatability of weathered hydrocarbons in soil by applying different bioremediation strategies such as bioaugmentation and biostimulation individually or in combination. The rates of biodegradation, however, may be affected by grinding, suggesting that the tightly bound weathered, hydrocarbon fraction can be disrupted, possibly leading to the release of toxic compounds. This observation was supported by monitoring of respiration rates and analysis of soil ecotoxicity, which confirmed that the reduction of the hydrocarbon content in soil, even for weathered hydrocarbons, does not necessarily lower the toxicity of the soil. Thus, assessing the potential biotransformation of weathered hydrocarbons in soil requires careful consideration of a wide range of factors including bioavailability change, and increased concentration of intermediates or biodegradation products during bioremediation treatment. Monitoring TPH alone is therefore not sufficient for determining the environmental risk posed by a contaminated site after remediation. Also, bioavailability is an important factor that can influence the extent of mass reduction achievable by bioremediation. However, the objective of bioremediation should not be mass reduction per se, but risk reduction and management. As such, it is important to consider these aspects in future research.
Acknowledgements
This work was supported by the LINK Bioremediation programme (BIOREM_35), the Environment Agency and the Biotechnology and Biological Sciences Research Council BBSRC (Grant BB/B512432/1). The authors also thank the UK Engineering and Physical Sciences Research Council (EPSRC) for financial support to carry out this work through a CASE award supported by the former FIRSTFARADAY (Environmental Sustainability KTN) partnership (Ref No. 5010978). The views expressed are authors’ alone and may not reflect the views or policies of their employing organisations.
Handling Editor: T Cutright.
References
- Ahn C.K., Woo S.H., Lee D.S., Park J.M. Mathematical evaluation of intermediates accumulation during microbial phenanthrene degradation. Korean J. Chem. Eng. 2006;23:415–418. [Google Scholar]
- Alzahrany H.K. University of Aberdeen; 2011. Molecular, Phenotypic and Functional Assessment of Hydrocarbon Degrading Microbial Communities: Applications in Bioremediation. PhD thesis. 306 pp. [Google Scholar]
- Azur Environmental . 1998. Microtox Acute Toxicity Test Guide, User’s Manual. Carlsbad, USA. [Google Scholar]
- Black C.A. American Society of Agronomy; Madison, WI, USA: 1965. Methods of Soil Analysis, Part 1. Physical and Mineralogical Properties. [Google Scholar]
- Brassington K.J., Hough R.L., Paton G.I., Semple K.T., Risdon G., Crossley J., Hay I., Askari K., Pollard S.J.T. Weathered hydrocarbon wastes: a risk management primer. Crit. Rev. Environ. Sci. Technol. 2007;37:199–232. [Google Scholar]
- Brassington K., Pollard S.J.T., Coulon F. Weathered hydrocarbon biotransformation: implications for bioremediation, analysis and risk assessment. Chapter 5. In: Timmis K., editor. vol. 4. Springer Verlag Berlin Heidelberg, Humana Press; 2010. pp. 2487–2501. (Handbook of Microbiology of Hydrocarbons, Oils, Lipids). ISBN: 978-3-540-77584-3. [Google Scholar]
- Brenner J.M., Mulvaney C.S. Total nitrogen. In: Page A.L., editor. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. American Society of Agronomy; Madison, WI, USA: 1982. pp. 595–624. [Google Scholar]
- Coulon F., Pelletier E., Gourhant L., Delille D. Effects of nutrient and temperature on degradation of petroleum hydrocarbons in contaminated sub-Antarctic soil. Chemosphere. 2005;58:1439–1448. doi: 10.1016/j.chemosphere.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Coulon F., Brassington K.J., Bazin R., Linnet P.E., Thomas K.A., Mitchell T.R., Lethbridge G., Smith J.W.N., Pollard S.J.T. Effect of fertilizer formulation and bioaugmentation on biodegradation and leaching of crude oils and refined products in soils. Environ. Technol. 2012;33:1879–1893. doi: 10.1080/09593330.2011.650221. [DOI] [PubMed] [Google Scholar]
- Craswell E.T., Waring S.A. Effect of grinding on the decomposition of soil organic matter—I. The mineralization of organic nitrogen in relation to soil type. Soil Biol. Biochem. 1972;4:427–433. [Google Scholar]
- Dawson J.J., Godsiffe, Thompson I.P., Ralebitso-Senior T.K., Killham K.S., Paton G.I. Application of biological indicators to assess recovery of hydrocarbon impacted soils. Soil Biol. Biochem. 2007;39:164–177. [Google Scholar]
- Diploick E.E., Mardlin D.P., Killham K.S., Paton G.I. Predicting bioremediation of hydrocarbons: laboratory to field scale. Environ. Pollut. 2009;6:1831–1840. doi: 10.1016/j.envpol.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Dorn P.B., Salanitro J.P. Temporal ecological assessment of oil contaminated soils before and after bioremediation. Chemosphere. 2000;40:419–426. doi: 10.1016/s0045-6535(99)00304-5. [DOI] [PubMed] [Google Scholar]
- Environment Agency . Environment Agency; Almondsbury, Bristol: 2009. Dealing with Contaminated Land in England and Wales – a Review of Progress from 200–2007 with Part 2A of the Environmental Protection Act. 41 pp. [Google Scholar]
- Eweis J.B., Ergas S.J., Chang D.P.Y., Schroeder E.D. McGraw-Hill; Boston: 1998. Bioremediation Principles. [Google Scholar]
- Gallego J.R., Loredo J., Llamas J.F., Vazquez F., Sanchez J. Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation. 2001;12:325–335. doi: 10.1023/a:1014397732435. [DOI] [PubMed] [Google Scholar]
- Gee G.W., Baude J.W. Particle size analysis. In: Klute A., editor. Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods. second ed. American Society of Agronomy; Madison, WI, USA: 1986. pp. 383–411. [Google Scholar]
- Hejazi R.F., Husain T. Landfarm performance under arid conditions. 2. Evaluation of parameters. Environ. Sci. Technol. 2004;38:2457–2469. doi: 10.1021/es026045c. [DOI] [PubMed] [Google Scholar]
- Huesemann M.H., Hausmann T.S., Fortman T.J. Does bioavailability limit biodegradation? A comparison of hydrocarbon biodegradation and desorption rates in aged soils. Biodegradation. 2004;15:261–274. doi: 10.1023/b:biod.0000042996.03551.f4. [DOI] [PubMed] [Google Scholar]
- Maletić S.P., Dalmacija B.D., Roncevic S.D., Agbaba J.R., Perovic S.D.U. Impact of hydrocarbon type, concentration and weathering on its biodegradability in soil. J. Environ. Sci. Health, Part A. 2011;46:1042-1049. doi: 10.1080/10934529.2011.590380. [DOI] [PubMed] [Google Scholar]
- Mallick S., Dutta T.K. Kinetics of phenanthrene degradation by Staphylococcus sp. Strain PN/Y involving 2-hydroxy-1-1naphthoic acid in a novel metabolic pathway. Process Chem. 2008;9:1004–1008. [Google Scholar]
- Mamindy-Pajany Y., Geret F., Roméo M., Hurel C., Marmier N. Ex situ remediation of contaminated sediments using mineral additives: assessment of pollutant bioavailability with the Microtox solid phase test. Chemosphere. 2012;86:1112–1116. doi: 10.1016/j.chemosphere.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Molina-Barahona L., Vega-Logo L., Guerrero M., Rameriez S., Romero I., Vega-Jarquin C., Albores A. Ecotoxicological evaluation of diesel contaminated soil before and after a bioremediation process. Environ. Toxicol. 2005;20:100–109. doi: 10.1002/tox.20083. [DOI] [PubMed] [Google Scholar]
- Nasser A., Mingelgrin U. Mechanochemistry: a review of surface reactions and environmental applications. Appl. Clay Sci. 2012;67–68:141–150. [Google Scholar]
- Olsen S.R., Sommers L.E. Phosphorus. In: Page A.L., editor. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. American Society of Agronomy; Madison, WI, USA: 1982. pp. 403–430. [Google Scholar]
- Powlson D.S. The effects of grinding on microbial and non-microbial organic matter in soil. J. Soil Sci. 1980;3:77–85. [Google Scholar]
- Reardon J., Foreman A., Scarcy R.L. New reactants for the colorimetric determination of ammonia. Clin. Chim. Acta. 1966;14:403–405. doi: 10.1016/0009-8981(66)90120-3. [DOI] [PubMed] [Google Scholar]
- Risdon G., Pollard S.J.T., Brassington K.J., McEwan J.N., Paton G., Semple K., Coulon F. Development of a novel and robust analytical procedure for weathered hydrocarbon contaminated soils within a UK risk based framework. Anal. Chem. 2008;80:7090–7096. doi: 10.1021/ac800698g. [DOI] [PubMed] [Google Scholar]
- Roy M., Khara P., Dutta T.K. Meta-cleavage of hydroxynaphthoic acids in the degradation of phenanthrene by Sphingobium sp. Strain PNB. Microbiology. 2012;158:685–695. doi: 10.1099/mic.0.053363-0. [DOI] [PubMed] [Google Scholar]
- Saterbak A., Toy R.J., Wong D.C.L., McMain B.J., Williams M.P., Dorn P.B., Brzuzy L.P., Chai E.Y., Salanitro J.P. Ecotoxicological and analytical assessment of hydrocarbon-contaminated soils and application to ecological risk assessment. Environ. Toxicol. Chem. 2009;18:1591–1607. [Google Scholar]
- Schnitzer M. Organic matter characterisation. In: Page A.L., editor. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. American Society of Agronomy; Madison, WI, USA: 1982. pp. 581–594. [Google Scholar]
- Stroud J.L., Paton G.I., Semple K.T. Microbe-aliphatic hydrocarbon interactions in soil: implications for biodegradation and bioremediation. J. Appl. Microbiol. 2007;102:1239–1253. doi: 10.1111/j.1365-2672.2007.03401.x. [DOI] [PubMed] [Google Scholar]
- Thavamani P., Smith E., Kavitha R., Mathieson G., Megharaj M., Srivastava P., Naidu R. Risk based land management requires focus beyond the target contaminants – a case study involving weathered hydrocarbon contaminated soils. Environ. Technol. Innov. 2015;4:98–109. [Google Scholar]
- Tindal M. 2005. Environmentally Acceptable Endpoints for Weathered/Aged Petroleum Hydrocarbon CCME CWS Fraction 3. Presentation prepared for: 2005 Soil and Groundwater Issues Forum, Petroleum Technology Alliance Canada, March 23, 2005. Calgary, Alberta. [Google Scholar]
- Towell M.G., Bellarby J., Paton G.I., Coulon F., Pollard S.J.T., Semple K.T. Mineralisation of target hydrocarbons in three contaminated soils from former refinery facilities. Environ. Pollut. 2011;159:515–523. doi: 10.1016/j.envpol.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Vidali M. Bioremediation: an overview. Pure Appl. Chem. 2001;73:1163–1172. [Google Scholar]
- Wu G., Kechavarzi C., Li X., Sui H., Pollard S.J.T., Coulon F. Influence of mature compost amendment on total and bioavailable polycyclic aromatic hydrocarbons in contaminated soils. Chemosphere. 2013;90:2240–2246. doi: 10.1016/j.chemosphere.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lu M. Bioremediation of crude oil-contaminated soil: comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010;83:395–401. doi: 10.1016/j.jhazmat.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Zemanek M.G., Pollard S.J.T., Kenefick S.L., Hrudey S.E. Multi-phase partitioning and co-solvent effects for polynuclear aromatic hydrocarbons (PAH) in authentic petroleum- and creosote-contaminated soils. Environ. Pollut. 1997;98:239–252. [Google Scholar]