Abstract
Serum Lp(a) is a CAD risk factor in persons of European ancestry. Levels are 2-3 fold higher in African Americans (AA) but reported associations with coronary artery disease (CAD) have been inconsistent. The relationship of Lp(a) with the extent and severity of subclinical coronary plaque has not been described in African Americans. We screened 269 apparently healthy African Americans for risk factors and coronary plaque using advanced coronary computed tomographic angiography. Total coronary plaque (TCP), noncalcified plaque (NCP), and calcified plaque (CCP) volumes (mm3) were quantified using a validated automated method. Lp(a) was measured by ELISA. Multivariable modeling was performed with adjustment for traditional CAD risk factors and intrafamilial correlations. Mean age was 51±11 years and 64% were female. Plaque was present in 41%. Lp(a) was independently associated with total plaque volume [log(TCP+1)] (p=0.04), 3 vessel and/or left main involvement (p=0.04), and at least one stenosis >50% (p=0.006). Best fit regression analyses showed subjects with Lp(a)>40 mg/dl were 3-fold more likely to have 3-vessel and/or LM disease (95% CI:1.4-6.8, p=0.005) and 4-fold more likely to have stenosis >50% (95% CI: 1.3-15.0, p=0.02). In subjects with plaque (N=110), multivariable models showed Lp(a) level was significantly and independently associated with TCP (p=0.009), NCP (p=0.01), CCP (p=0.003), and affected vessel length (p=0.01). In conclusion, high Lp(a) is strongly associated with coronary plaque volumes, extent, and severity in apparently healthy AA. High levels of Lp(a) may be particularly important in the pathogenesis of CAD in AA.
Keywords: Lipoproteins, coronary disease, plaque, imaging, epidemiology
INTRODUCTION
To more accurately assess the relation of Lp(a) with preclinical coronary atherosclerosis in younger African Americans, who have significantly more noncalcified coronary plaque (NCP) than calcified coronary plaque (CCP),1 we designed this study to examine the association of Lp(a) with the volume, extent, and severity of coronary plaque using advanced multidetector computed tomographic angiography (CTA) in healthy asymptomatic African Americans from early-onset CAD families.
Methods
The population consisted of 269 participants in the Early Noninvasive Imaging for Genetic Mechanisms of Atherosclerosis (ENIGMA) substudy of the larger ongoing Genetic Study of Atherosclerosis Risk (GeneSTAR), a prospective study designed to characterize genetic and biological factors associated with cardiovascular disease phenotypes in families with early-onset CAD. Probands <60 years of age with documented acute myocardial infarction, unstable angina with coronary revascularization, or acute angina with angiographic evidence of a flow-limiting stenosis of >50% diameter in at least one coronary artery were identified during hospitalization and excluded. Apparently healthy siblings and the offspring of the probands and siblings were eligible if they were 30 to 75 years of age and had no known personal history of CAD. Siblings and offspring were excluded if they had systemic autoimmune disease, known allergy to iodinated contrast media, or chronic kidney disease. The study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants gave informed consent.
Participants underwent a comprehensive risk factor screening following a 12-hour overnight fast. Medical history and current medication use were elicited. A physical exam was performed by a study physician. Height was determined using a fixed stadiometer and weight was measured on a balance beam scale with the subject wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight(kg)/height(m)2. Current cigarette smoking behavior was assessed by self-report and verified by expired carbon monoxide (CO) levels of ≥8 ppm. Blood pressure was measured according to the American Heart Association guidelines three times over the course of the day. Hypertension was defined as an average blood pressure ≥140 mmHg systolic, or ≥90 mmHg diastolic, and/or use of an antihypertensive drug. Blood was obtained and total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using the United States Centers for Disease Control standardized methods.2 Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula3 for persons with triglyceride levels up to 400 mg/dl. Direct measurement of LDL cholesterol using ultracentrifugation was used for persons with triglyceride levels ≥ 400 mg/dl (n=2). Glucose concentration was measured using the glucose oxidase method; type 2 diabetes was defined as a physician diagnosed history, a fasting glucose level ≥ 126 mg/dl, and/or use of prescribed hypoglycemic medications. After blood collection, plasma samples were sent to the University of Maryland Cytokine Core for analysis. Lp(a) was analyzed using a Human Lp(a) ELISA kit (AlerCHEK, Inc., Springvale, ME). This assay employs a solid phase capture sandwich ELISA that measures human Lp(a) independent of isoform size. The intraassay coefficient of variation has been shown to be 4-8%.
All participants underwent coronary CTA using a newest generation dual-source multi-detector scanner (SOMATOM Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Because of the high temporal resolution and excellent image quality of the scanner, beta-blockade was not necessary for reducing the heart rate.4 A noncontrast scan was first performed to determine the coronary artery calcium volume. Subsequently coronary CTA was performed to examine the presence, location, composition, and severity of any coronary plaque. Approximately 80 mL of isosmolar contrast agent (320 mg iodine/mL) was injected at 6 mL/s. Prospective ECG-gating was used in patients with low, steady heart rates (<65 bpm) and little heart rate variability. For patients with variable heart rates or heart rates >65 bpm, retrospective gating with dose modulation was used. Tube potential was selected on a per patient basis by the performing technologist assessment of patient size; 100 kV was used for patients that were not overweight or obese, otherwise 120 kV was used. We reconstructed 0.75-mm thick axial slices at 0.4-mm intervals with a B26 kernel; 10 reconstructions were done at 10% increments in the R-R interval. All scans were evaluated with the CT radiologist blinded to the participants’ risk factor profiles. The coronary arterial tree was segmented according to the standard American Heart Association classification, and the segments were investigated for plaque and luminal narrowing. Any focal stenoses >50% in severity were identified with the use of quantitative software (COR Analyzer System, Rcadia Medical Imaging, Haifa, Israel)5 and verified by the expert reader.
The volume of CCP was measured on a workstation (Leonardo Multimodality Workstation, Syngo, Siemens Medical Solutions, Malvern, PA) using noncontrast images. Regions of interest were placed over each of the coronary arteries and a threshold of >130 HU was used for determining per vessel volumes of CCP (mm3) using standard validated methods.6 Vessel CCP volumes were summed for a total CCP volume. For each affected coronary segment, NCP volumes (mm3) were quantified using AUTOPLAQ (Cedars-Sinai Medical Center, Los Angeles, CA), as previously described.7 This automated method of NCP measurement has high interobserver correlation (r=0.97),8 and has been previously validated against intravascular ultrasound (IVUS).7 To quantify each affected segment, CTA images were examined in multiplanar format and proximal and distal limits of the plaque were manually marked. Control points defining the lumen center-line were placed. Subsequent NCP plaque quantification was then fully automated using adaptive algorithms that are scan specific per individual.7 Segmental NCP volumes were summed for a total NCP volume per vessel, including the left main (LM), left anterior descending (LAD), left circumflex (LCX), and right coronary arteries (RCA). The vessel specific volumes were summed for a total NCP volume. Total coronary plaque (TCP) was calculated as the sum of CCP + NCP. Additionally, affected coronary segment length with any plaque was automatically quantified and summed for total vessel length affected (mm).
Standard descriptive analyses were used to examine distributions of sociodemographic and CAD risk factor variables by race and the absence or presence of coronary plaque. The Kolmogorov-Smirnov statistic was used to test for normality of continuous variables. Given the skewed distribution of Lp(a), log(Lp(a)) was used to compare subjects with and without plaque. In subjects with any coronary plaque the correlation of Lp(a) with total, calcified, and noncalcified plaque volumes was examined. Mixed multivariable regression models were performed predicting transformed plaque volumes as log(plaque volume) as well as affected vessel length accounting for nonindependence within families as a random effect. Covariates in the model included age, sex, the presence of hypertension and/or diabetes, current smoking behavior, HDL cholesterol, LDL cholesterol, triglycerides, BMI, education level, and Lp(a). To examine the association of Lp(a) with plaque presence and volume in the entire population, TCP volumes were transformed as log [TCP + 1], given the non-normal distribution and presence of zero plaque in many individuals. Mixed multivariable regression analysis was performed using the same covariates. Logistic regressions predicting the presence of three vessel and/or left main disease as well as the presence of at least one stenosis >50% were performed separately with the same dependent variables using Lp(a) as a continuous variable. Maximum likelihood analyses were performed for each model to determine the best fit Lp(a) risk level for which odds ratios were determined.
Results
Apparently healthy individuals (N=269) from 147 families with the onset of CAD <60 years of age agreed to participate. Study subjects were siblings (n=166) of the index patient or adult offspring of the index patient or the siblings (n=103). The sample was 64.3% female with a mean age of 51.3±11.1 years. All were healthy and without any chest pain or angina-equivalent symptoms. Overall, 40.9% of the subjects had subclinical coronary plaque, including 5.6% with exclusively NCP without any CCP. Population demographics and CAD risk factors and the absence or presence of coronary plaque are shown in Table 1. The presence of coronary plaque was significantly associated with older age, hypertension, diabetes, and triglyceride level. Median Lp(a) level was higher in subjects with plaque compared to no plaque but this difference was not statistically significant. There were no significant sex differences in Lp(a) level in subjects with or without plaque.
Table 1.
Population Characteristics by Absence or Presence of Coronary Plaque (N=269)*
| Variable | No Plaque (N=159) |
Plaque (N=110) |
p-value |
|---|---|---|---|
| Age (years) | 47.1 ± 10.8 | 57.4 ± 8.2 | <0.0001 |
| Men | 28.9% | 45.4% | 0.005 |
| Hypertension | 43.4% | 71.8% | <0.0001 |
| Diabetes mellitus | 12.6% | 25.4% | 0.007 |
| Current smoker | 23.9% | 28.2% | 0.43 |
| Statin use | 14.5% | 38.2% | <0.0001 |
| LDL cholesterol (mg/dl) | 109.9 ± 36.8 | 117.3 ± 38.9 | 0.11 |
| HDL cholesterol (mg/dl) | 58.5 ± 15.4 | 58.5 ± 17.2 | 1.00 |
| Triglycerides (mg/dl) | 91.1 ± 48.7 | 111.2 ± 80.5 | 0.01 |
| Body-mass index (kg/m2) | 31.8 ± 7.2 | 31.6 ± 6.0 | 0.87 |
| Education (years) | 13.2 ± 2.4 | 13.0 ± 2.7 | 0.77 |
| Lp(a) (mg/dl)† | 19.5 [14.2,40.0] | 25.8 [13.9,49.7] | 0.13 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein; Lp(a) = lipoprotein(a)
Continuous variables presented as mean ± 1 standard deviation
Median [Interquartile range]
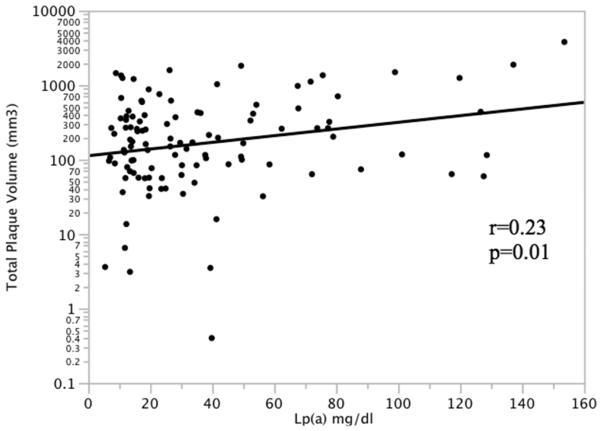
In participants with coronary plaque (N=110), serum Lp(a) was significantly correlated with total coronary plaque volume (Figure 1). Lp(a) was also significantly correlated with noncalcified plaque volume (r=0.20, p=0.04), calcified plaque volume (r=0.31, p=0.002), and total affected vessel length (r=0.25, p=0.01). Multivariate regression models accounting for intrafamilial correlation were performed in subjects with coronary plaque predicting each plaque phenotype using the covariates age, sex, hypertension, diabetes, current smoking, HDL cholesterol, LDL cholesterol, triglycerides, BMI, education, and Lp(a) level (Table 2). Age and Lp(a) were significant predictors of total plaque volume. Age, male sex, and Lp(a) were significant predictors of noncalcified plaque volume. Age, hypertension, and Lp(a) were significant predictors of calcified plaque volume. Finally, age and Lp(a) were independently associated with total affected vessel length. Using the entire population (N=269), multivariate regression models predicting the transformed total coronary plaque volume [log(TCP+1)] was performed using the same covariates. Lp(a) level was significantly and independently associated with TCP (p=0.04), as was older age (p<0.0001) and male sex (p=0.0003).
Figure 1.
Correlation of serum Lp(a) with total coronary plaque volume in individuals with plaque (N=110)
Table 2.
Mixed multivariate regression models predicting total plaque volumes and affected vessel length in subjects with coronary plaque (N=110)
| log(total plaque volume) | log(noncalcified volume) | log(calcified volume) | Affected length (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Beta ± SE | p-value | Beta ± SE | p-value | Beta ± SE | p-value | Beta ± SE | p-value | |
| Age | 0.056 ± 0.017 |
0.002 | 0.031 ± 0.014 |
0.02 | 0.090 ± 0.023 |
0.0002 | 1.553 ± 0.593 |
0.01 |
| Male sex | 0.154 ± 0.156 |
0.33 | 0.333 ± 0.123 |
0.008 | 0.011 ± 0.197 |
0.96 | 9.730 ± 5.411 |
0.08 |
| Hypertension | 0.099 ± 0.155 |
0.52 | 0.013 ± 0.119 |
0.91 | 0.527 ± 0.206 |
0.01 | 1.347 ± 5.266 |
0.80 |
| Diabetes mellitus | 0.125 ± 0.178 |
0.49 | 0.060 ± 0.135 |
0.65 | 0.037 ± 0.231 |
0.87 | 5.067 ± 5.994 |
0.40 |
| Current smoker | −0.017 ±0.169 |
0.92 | −0.115 ± 0.133 |
0.39 | −0.077 ± 0.221 |
0.73 | 2.021 ± 5.855 |
0.73 |
| LDL-C (mg/dl) | −0.002 ± 0.004 |
0.53 | −0.002 ± 0.003 |
0.44 | 0.003 ± 0.005 |
0.57 | −0.066 ± 0.128 |
0.61 |
| HDL-C (mg/dl) | 0.003 ± 0.009 |
0.77 | 0.010 ± 0.007 |
0.20 | 0.017 ± 0.012 |
0.16 | 0.436 ± 0.315 |
0.17 |
| Triglycerides (mg/dl) |
0.003 ± 0.002 |
0.17 | 0.003 ± 0.002 |
0.10 | 0.005 ± 0.003 |
0.08 | 0.123 ± 0.078 |
0.12 |
| Body-mass index (kg/m2) |
−0.034 ± 0.027 |
0.21 | −0.011 ± 0.020 |
0.57 | −0.104 ± 0.035 |
0.004 | −1.089 ± 0.894 |
0.23 |
| Education (years) | −0.050 ± 0.054 |
0.35 | −0.033 ± 0.042 |
0.44 | −0.048 ± 0.067 |
0.48 | −2.216 ± 0.139 |
0.24 |
| Lp(a) (mg/dl) | 0.011 ± 0.004 |
0.009 | 0.008 ± 0.003 |
0.02 | 0.018 ± 0.006 |
0.003 | 0.359 ± 0.139 |
0.01 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein; Lp(a) = lipoprotein(a)
Of those participants with coronary plaque, 43.6% had 3 vessel and/or left main artery involvement and 17.3% had at least one stenosis ≥50%. Median Lp(a) levels were significantly higher in African Americans with extensive plaque (3 vessel and/or left main disease) compared to all others; 32.5 [13.9,70.8] mg/dl versus 19.9 [14.2,39.4] mg/dl, Wilcoxon p=0.04. Similar results were found for severe plaque (at least one stenosis ≥50%) compared to all others; 49.3 [17.4,77.8] mg/dl versus 20.6 [14.1,40.4] mg/dl, Wilcoxon p=0.01. In the fully adjusted model, continuous Lp(a) level was significantly associated with the number of coronary vessels affected (p=0.05), the presence of three vessel and/or left main disease (p=0.04) and at least one stenosis >50% in severity (p=0.006). The maximum likelihood best fit for both models was Lp(a)>40 mg/dl (Tables 3 and 4). Subjects with Lp(a)> 40 mg/dl were approximately 3-fold more likely to have three vessel and/or left main plaque and 4-fold more likely to have at least one stenosis >50% in severity.
Table 3.
Logistic regression predicting three vessel and/or left main plaque (N=269)
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Age (years) | 1.13 | 1.08-1.19 | <0.0001 |
| Men | 2.79 | 1.21-6.59 | 0.02 |
| Hypertension | 2.03 | 0.86-5.08 | 0.11 |
| Diabetes mellitus | 1.31 | 0.48-3.46 | 0.58 |
| Current smoker | 2.92 | 1.17-7.50 | 0.02 |
| LDL cholesterol (per 10 mg/dl) | 1.08 | 0.98-1.20 | 0.13 |
| HDL cholesterol (per 10 mg/dl) | 0.94 | 0.72-1.22 | 0.64 |
| Triglycerides (per 10 mg/dl) | 1.03 | 0.97-1.04 | 0.30 |
| Body-mass index (kg/m2) | 0.95 | 0.07-13.27 | 0.97 |
| Education (years) | 1.11 | 0.11-10.63 | 0.93 |
| Lp(a) <40 (mg/dl)* | 3.09 | 1.41-6.89 | 0.005 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein; Lp(a) = lipoprotein(a)
Maximum best fit
Table 4.
Logistic regression predicting at least 1 stenosis >50% in severity (N=269)
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Age (years) | 1.18 | 1.08-1.31 | <0.0001 |
| Men | 1.58 | 0.43-5.97 | 0.49 |
| Hypertension | 2.41 | 0.61-12.30 | 0.22 |
| Diabetes mellitus | 6.63 | 1.78-28.00 | 0.005 |
| Current smoker | 1.90 | 0.39-8.74 | 0.41 |
| LDL cholesterol (per 10 mg/dl) | 1.20 | 1.05-1.38 | 0.01 |
| HDL cholesterol (per 10 mg/dl) | 0.74 | 0.48-1.14 | 0.18 |
| Triglycerides (per 10 mg/dl) | 1.05 | 0.96-1.14 | 0.31 |
| Body-mass index (kg/m2) | 0.96 | 0.86-1.07 | 0.49 |
| Education (years) | 1.05 | 0.83-1.32 | 0.69 |
| Lp(a) >40 (mg/dl)* | 4.08 | 1.24-14.51 | 0.02 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein; Lp(a) = lipoprotein(a)
Maximum best fit
Discussion
To our knowledge this is the first study to show that higher plasma Lp(a) levels in African Americans from families with early-onset CAD are strongly related to subclinical total, noncalcified, and calcified coronary plaque volumes, total length of affected coronary vessels, the presence of three vessel and/or left main disease, and severe stenoses, independent of traditional risk factors, including other lipid and lipoprotein levels. Older age and higher Lp(a) were the only factors to be consistently associated with every plaque phenotype studied. The Multi-Ethnic Study of Atherosclerosis (MESA) recently reported a strong independent association of Lp(a) with incident clinically manifest CAD in Americans from different ancestral backgrounds but race was a key determinant of the Lp(a) level that associated with risk.9 Our current findings suggest that Lp(a) may be of particular importance in African Americans during even the earliest stages of atherosclerosis prior to thrombosis-mediated clinical manifestations, especially given the association with noncalcified plaque volumes, an antecedent to plaque rupture and acute CAD events. 10, 11
Importantly we examined coronary plaque phenotypes at a preclinical stage in African Americans. Prior studies have demonstrated associations of Lp(a) with plaque phenotypes in patients of Japanese and European ancestry with clinical CAD. Momiyama et al found that Lp(a) level was associated with the number of stenotic coronary vessels in a stepwise fashion in patients undergoing coronary angiography with suspected or known CAD,12 consistent with prior studies of angiographically defined stenosis severity.13, 14 Lp(a) has been also been associated with plaque progression by angiography15 and serial IVUS studies,16 and more recently with necrotic core progression in non-culprit coronary lesions in statin-treated patients with angina pectoris.17 In one of few studies using CTA to examine the relationship of Lp(a) with plaque morphology, Hikita et al found higher Lp(a) was associated with the number of total and noncalcified plaques in patients with acute myocardial infarction.18 Our findings that Lp(a) level is strongly associated with multiple preclinical plaque phenotypes in African Americans indicate a potentially important mechanistic role for Lp(a) in atherogenesis in persons of African ancestry.
Plasma Lp(a) level is known to be highly heritable, is primarily determined by the copy number variation at the LPA locus on chromosome 6,19 and is typically stable within individuals over time but highly heterogeneous across individuals, populations, and ancestry.20 Results from genetic studies, including those examining Mendelian randomization, have suggested a causal relationship of Lp(a) with CAD.21-23 Helgadottir et al found that apo(a) genetic sequence variants were associated with coronary atherosclerotic burden.24 Two recent studies also reported that two single nucleotide polymorphisms (SNPs) in the LPA gene accounted for 36% of the variance in Lp(a) concentrations in those of European ancestry21 but only one SNP in African Americans accounted for only 5% of the variance,25 supporting the potential importance of ancestry and genetic admixture in Lp(a) level.
The exact mechanisms by which Lp(a) contributes to atherogenesis are not fully elucidated. Although the homology of Lp(a) to plasminogen is thought to be a mechanism of Lp(a) to promote thrombotic events,26 there are several lines of evidence implicating Lp(a) in atherosclerosis. Lp(a) is a primary carrier of oxidized phospholipids which associate with angiographically defined CAD.27 Lp(a) is thought to accumulate in the vessel wall, stimulate endothelial cell permeability, promote cholesterol accumulation in macrophages, and induce secretion of proinflammatory cytokines.28 Unfortunately, there is a paucity of data showing that lowering Lp(a) level decreases CAD risk, primarily because of few available interventions to lower Lp(a), and moreover, the concomitant changes that occur in other lipids and lipoproteins using medical therapy. For example, niacin is considered the best pharmacological treatment for elevated Lp(a) capable of lowering levels 20-40% but also increases HDL cholesterol and decreases LDL cholesterol and triglycerides.29 Trials of novel medical therapies to lower Lp(a) are currently ongoing and a recent study reported that chronic lipoprotein apheresis decreases major cardiovascular events in people with Lp(a) hyperlipoproteinemia.30 However the evidence to treat or not to treat high Lp(a) is only beginning to emerge.
Acknowledgments
Funding Sources: This work was supported by grants from the National Heart, Lung, and Blood Institute (Grants RC1HL099747, K23HL094747, R01HL59684, and R01HL071025), The Johns Hopkins General Clinical Research Center (Grant M01-RR000052 from the National Center for Research Resources, National Institutes of Health), and the National Institute of Nursing Research (Grant R01NR08153).
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kral BG, Becker LC, Vaidya D, Yanek LR, Qayyum R, Zimmerman SL, Dey D, Berman DS, Moy TF, Fishman EK, Becker DM. Noncalcified coronary plaque volumes in healthy people with a family history of early onset coronary artery disease. Circulation Cardiovasc Imag. 2014;7:446–453. doi: 10.1161/CIRCIMAGING.113.000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46:1762–1772. [PubMed] [Google Scholar]
- 3.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 4.Zimmerman SL, Kral BG, Fishman EK. Diagnostic quality of dual-source coronary CT examinations performed without heart rate control: importance of obesity and heart rate on image quality. J Comput Assist Tomogr. 2014;38:949–955. doi: 10.1097/RCT.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpern EJ, Halpern DJ. Diagnosis of coronary stenosis with CT angiography comparison of automated computer diagnosis with expert readings. Acad Radiol. 2011;18:324–333. doi: 10.1016/j.acra.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 6.McCollough CH, Ulzheimer S, Halliburton SS, Shanneik K, White RD, Kalender WA. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007;243:527–538. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 7.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257:516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 8.Dey D, Cheng VY, Slomka PJ, Nakazato R, Ramesh A, Gurudevan S, Germano G, Berman DS. Automated 3-dimensional quantification of noncalcified and calcified coronary plaque from coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3:372–382. doi: 10.1016/j.jcct.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Schuijf JD, Beck T, Burgstahler C, Jukema JW, Dirksen MS, de Roos A, van der Wall EE, Schroeder S, Wijns W, Bax JJ. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care. 2007;9:48–53. doi: 10.1080/17482940601052648. [DOI] [PubMed] [Google Scholar]
- 12.Momiyama Y, Ohmori R, Fayad ZA, Tanaka N, Kato R, Taniguchi H, Nagata M, Ohsuzu F. Associations between serum lipoprotein(a) levels and the severity of coronary and aortic atherosclerosis. Atherosclerosis. 2012;222:241–244. doi: 10.1016/j.atherosclerosis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Budde T, Fechtrup C, Bosenberg E, Vielhauer C, Enbergs A, Schulte H, Assmann G, Breithardt G. Plasma Lp(a) levels correlate with number, severity, and length-extension of coronary lesions in male patients undergoing coronary arteriography for clinically suspected coronary atherosclerosis. Arterioscler Thromb. 1994;14:1730–1736. doi: 10.1161/01.atv.14.11.1730. [DOI] [PubMed] [Google Scholar]
- 14.Wang XL, Tam C, McCredie RM, Wilcken DE. Determinants of severity of coronary artery disease in Australian men and women. Circulation. 1994;89:1974–1981. doi: 10.1161/01.cir.89.5.1974. [DOI] [PubMed] [Google Scholar]
- 15.Terres W, Tatsis E, Pfalzer B, Beil FU, Beisiegel U, Hamm CW. Rapid angiographic progression of coronary artery disease in patients with elevated lipoprotein(a) Circulation. 1995;91:948–950. doi: 10.1161/01.cir.91.4.948. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann M, von Birgelen C, Mintz GS, Stoel MG, Eggebrecht H, Wieneke H, Fahy M, Neumann T, van der Palen J, Louwerenburg HW, Verhorst PM, Erbel R. Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. J Am Coll Cardiol. 2006;48:446–452. doi: 10.1016/j.jacc.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I. Lipoprotein(a) is associated with necrotic core progression of non-culprit coronary lesions in statin-treated patients with angina pectoris. Lipids Health Dis. 2014;13:59. doi: 10.1186/1476-511X-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hikita H, Shigeta T, Kojima K, Oosaka Y, Hishikari K, Kawaguchi N, Nakashima E, Sugiyama T, Akiyama D, Kamiishi T, Kimura S, Takahashi Y, Kuwahara T, Sato A, Takahashi A, Isobe M. Lipoprotein(a) is an important factor to determine coronary artery plaque morphology in patients with acute myocardial infarction. Coronary Artery Dis. 2013;24:381–385. doi: 10.1097/MCA.0b013e3283622329. [DOI] [PubMed] [Google Scholar]
- 19.Utermann G. The mysteries of lipoprotein(a) Science. 1989;246:904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 20.Sandholzer C, Hallman DM, Saha N, Sigurdsson G, Lackner C, Csaszar A, Boerwinkle E, Utermann G. Effects of the apolipoprotein(a) size polymorphism on the lipoprotein(a) concentration in 7 ethnic groups. Human Genet. 1991;86:607–614. doi: 10.1007/BF00201550. [DOI] [PubMed] [Google Scholar]
- 21.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 22.Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der Harst P, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 23.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 24.Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, Jones GT, van Rij AM, Eapen DJ, Baas AF, Tregouet DA, Morange PE, Emmerich J, Lindblad B, Gottsater A, Kiemeny LA, Lindholt JS, Sakalihasan N, Ferrell RE, Carey DJ, Elmore JR, Tsao PS, Grarup N, Jorgensen T, Witte DR, Hansen T, Pedersen O, Pola R, Gaetani E, Magnadottir HB, Wijmenga C, Tromp G, Ronkainen A, Ruigrok YM, Blankensteijn JD, Mueller T, Wells PS, Corral J, Soria JM, Souto JC, Peden JF, Jalilzadeh S, Mayosi BM, Keavney B, Strawbridge RJ, Sabater-Lleal M, Gertow K, Baldassarre D, Nyyssonen K, Rauramaa R, Smit AJ, Mannarino E, Giral P, Tremoli E, de Faire U, Humphries SE, Hamsten A, Haraldsdottir V, Olafsson I, Magnusson MK, Samani NJ, Levey AI, Markus HS, Kostulas K, Dichgans M, Berger K, Kuhlenbaumer G, Ringelstein EB, Stoll M, Seedorf U, Rothwell PM, Powell JT, Kuivaniemi H, Onundarson PT, Valdimarsson E, Matthiasson SE, Gudbjartsson DF, Thorgeirsson G, Quyyumi AA, Watkins H, Farrall M, Thorsteinsdottir U, Stefansson K. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. doi: 10.1016/j.jacc.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 25.Deo RC, Wilson JG, Xing C, Lawson K, Kao WH, Reich D, Tandon A, Akylbekova E, Patterson N, Mosley TH, Jr., Boerwinkle E, Taylor HA., Jr. Single-nucleotide polymorphisms in LPA explain most of the ancestry-specific variation in Lp(a) levels in African Americans. PloS One. 2011;6:e14581. doi: 10.1371/journal.pone.0014581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deb A, Caplice NM. Lipoprotein(a): new insights into mechanisms of atherogenesis and thrombosis. Clin Cariol. 2004;27:258–264. doi: 10.1002/clc.4960270503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 28.Kiechl S, Willeit J. The mysteries of lipoprotein(a) and cardiovascular disease revisited. J Am Coll Cardiol. 2010;55:2168–2170. doi: 10.1016/j.jacc.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 29.Seed M, O'Connor B, Perombelon N, O'Donnell M, Reaveley D, Knight BL. The effect of nicotinic acid and acipimox on lipoprotein(a) concentration and turnover. Atherosclerosis. 1993;101:61–68. doi: 10.1016/0021-9150(93)90102-z. [DOI] [PubMed] [Google Scholar]
- 30.Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128:2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]