INTRODUCTION
The estimated body mass index (BMI) in the United States has increased drastically over the past several decades with estimates from 2008 suggesting that greater than 60% of adult females and 70% of adult males are overweight (BMI>25 kg/m2)1. In the United States, BMI > 25 kg/m2 is considered overweight, BMI > 30 kg/m2 is considered obese2. Higher BMI has been associated with worse overall survival and disease-adjusted life years due to comorbidities such as cardiovascular disease and diabetes. There is an increased risk of a number of cancers in patients having a high BMI including esophageal, breast, colon, gallbladder, uterine and pancreatic cancer. Elevated BMI has also been associated with worsened survival in breast, pancreatic and colon cancers3,4. However, pretreatment BMI > 25 kg/m2 has been shown to be a positive prognostic factor for disease-specific and overall survival in patients with head and neck cancer5–10 and specifically those treated with chemoradiation6,9,10. Different hypotheses have been proposed for the mechanism of this association with most positing that patients with higher body mass index have a greater reserve to withstand the demands of radiation therapy and its associated short- and long-term side effects10. Other hypotheses include concomitant illness causing weight loss or HPV status (and its known better prognosis) trending toward a higher BMI11.
Despite these studies being adjusted via multivariable analysis, most are not stratified by disease subsite, and only one previous study has been stratified by human papilloma virus (HPV) status, which limits the interpretability of results. One previous study has shown a lack of association in HPV+ patients with survival; however, this study was limited by its inclusion of oral, pharyngeal and laryngeal subsites in the analysis12. In review of the literature, a minority of previous studies have evaluated outcomes by disease subsite and, at odds with other analyses of head and neck cancer, the two which have examined disease subsite found obesity to be a negative prognostic factor for disease specific survival in squamous cell carcinoma of the oral cavity and of no effect in the oropharynx7,13.
Given that more advanced tumors or those involving the larynx or hypopharynx impair swallowing function, and therefore may lead to weight loss and a lower BMI at presentation, it is important to focus on as narrow of a subgroup as possible to delineate causality from association. Further, in an era of increasing HPV incidence (and better prognosis), patients should be stratified into separate groups by HPV status when examining oropharyngeal squamous cell carcinoma14. It is easy to imagine a scenario in which HPV+ oropharyngeal squamous cell carcinoma (OPSCC) patients have a higher BMI and better prognosis than HPV− OPSCC patients, potentially leading to a Type II error. In a separate analysis not reported here we found a significant difference in BMI between our HPV+ patients and HPV− OPSCC patients (29.6 kg/m2 and 23.9 kg/m2) (data unpublished). Recent studies have also suggested improved long-term dysphagia in HPV+ patients as compared to HPV− patients after intensity-modulated radiation therapy further suggesting that these groups are different and should be stratified as such15. Therefore, our primary objective in this study is to determine whether pretreatment body mass index > 25 kg/m2 is of prognostic significance for overall survival in patients with HPV positive OPSCC. Our secondary objective in this study is to determine whether disease-specific mortality is associated with pretreatment body mass index >25 kg/m2.
METHODS
This retrospective review was approved by the University of Pittsburgh Institutional Review Board. Patients were identified through a review of our head and neck SPORE database with inclusion criteria consisting of patients with HPV+ OPSCC treated either surgically or non-surgically at the University of Pittsburgh Medical Center between 8/1/2006 – 8/31/2014. Tumors were considered to be HPV-positive if immunostaining of their tumor was positive for either HPV or p16. All patients had HPV testing by either p16 immunohistochemistry (IHC) or HPV in situ hybridization. IHC for p16 (G175–405; BD Pharmingen, San Diego, California) as a surrogate marker for HPB was performed as per the manufacturer’s protocol. Cases were considered positive if >80% of tumor cells showed diffuse strong cytoplasmic and nuclear positivity staining. HPV detection was performed by in situ hybridization using probes targeting a wide spectrum of HPV strains (Y1404; Dako, Carpinteria, California). Cases with punctate nuclear signal were considered positive. The majority of patients had either of the HPV testing modalities as part of the initial diagnostic evaluation. All methods of treatment for HPV+ OPSCC (surgical, chemoradiation, radiation) were included. Patients were excluded for unknown BMI status prior to the initiation of treatment, unknown HPV status, and for less than one year of follow-up. Medical records were queried for pertinent clinical characteristics and outcomes. BMI data was obtained from the medical record, as recorded within one month of the initiation of treatment. Demographic and clinical characteristics including gender, age, race, T-stage, N-stage, alcohol use at diagnosis, smoking history, radiation dose, and primary treatment (either surgery or radiation/chemoradiation) were summarized and tested for association with body mass index (BMI), using Chi-square tests or Fisher’s exact test for categorical variables, a t-test for age, and the Wilcoxon-Mann-Whitney test for the ordinal stage variables. Patients were stratified by BMI status (>/< 25 kg/m2).
Survival curves were generated using the Kaplan-Meier survival method. Hazard ratios for overall survival and disease specific survival were calculated for BMI status in multivariate Cox proportional hazards models. Other factors adjusted for in the models included gender, age, race, T-stage (specifically T4), N-stage (specifically N2c/N3), alcohol history, and tobacco use history. Factors were selected for inclusion in the multivariate models if they were considered clinically significant or demonstrated univariate associations with both survival and BMI status. Results were based on 2-tailed tests and were considered significant when p<0.05. Analyses were performed in SAS 9.4 (SAS Institute, Cary NC) and in R version 3.1.1.
RESULTS
Based on our initial search criteria of OPSCC with at least 1 year of follow-up 579 patients were initially identified. 139 patients were excluded for unknown HPV status and 78 were excluded for HPV negative status. 62 patients were excluded for unknown BMI status at the time of diagnosis leaving 300 patients that met our inclusion criteria (Table I). The mean age at time of diagnosis was 57 years with the predominance of these patients being male (253/300, 84.3%). 250/296 (84.5%) of the patients were T1/T2 at presentation but most (155/297, 52.2%) had advanced N-stage at presentation (N2b or greater). The most common oropharyngeal subsite was the tonsil. Most patients (198/297, 66.7%) were current or former tobacco users. In this cohort, most were treated primarily with a combination of radiation +/− chemotherapy (182/300, 60.7%) with the remainder undergoing primary surgical therapy. Of the surgical therapy group, 77/118 patients (65.3%) had some form of adjuvant radiation therapy > 5000 cGy.
Table 1.
Demographic and clinical characteristics
| All N=300 |
BMI <25 N=52 |
BMI ≥25 N=248 |
p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | 253 | 84 | 4 0 |
77 | 213 | 86 | 0.11 |
| Male | |||||||
| Female | 47 | 16 | 1 2 |
23 | 35 | 14 | |
| Age at diagnosis, years | 0.22 | ||||||
| mean (SD) | 57 (9) | 55 (10) | 57 (9) | ||||
| median (range) | 56 (30–87) | 56 (36–79) | 57 (30–87) | ||||
| Race | 0.27 | ||||||
| African American | 7 | 2 | 3 | 6 | 4 | 2 | |
| Caucasian | 272 | 91 | 4 7 |
90 | 225 | 91 | |
| Other | 1 | 0 | . | . | 1 | 0 | |
| NA | 20 | 7 | 2 | 4 | 18 | 7 | |
| T-Stage | 0.38 | ||||||
| 1 | 118 | 39 | 1 9 |
37 | 99 | 40 | |
| 2 | 132 | 44 | 2 2 |
42 | 110 | 44 | |
| 3 | 25 | 8 | 7 | 13 | 18 | 7 | |
| 4 | 21 | 7 | 4 | 8 | 17 | 7 | |
| NA | 4 | 1 | . | . | 4 | 2 | |
| N-Stage | 0.64 | ||||||
| 0 | 32 | 11 | 7 | 13 | 25 | 10 | |
| 1 | 58 | 19 | 1 1 |
21 | 47 | 19 | |
| 2a | 52 | 17 | 8 | 15 | 44 | 18 | |
| 2b | 114 | 38 | 1 7 |
33 | 97 | 39 | |
| 2c | 34 | 11 | 6 | 12 | 28 | 11 | |
| 3 | 7 | 2 | 2 | 4 | 5 | 2 | |
| NA | 3 | 1 | 1 | 2 | 2 | 1 | |
| Smoking History | 0.04 | ||||||
| Current Smoker | 88 | 29 | 2 3 |
44 | 65 | 26 | |
| Former Smoker | 110 | 37 | 1 4 |
27 | 96 | 39 | |
| Never Smoker | 99 | 33 | 1 5 |
29 | 84 | 34 | |
| Unknown | 3 | 1 | 0 | 0 | 3 | 1 | |
| Primary Treatment | 0.04 | ||||||
| XRT | 182 | 61 | 3 8 |
73 | 144 | 58 | |
| Surgery | 118 | 39 | 1 4 |
27 | 104 | 42 | |
| Alcohol History at Diagnosis | 0.74 | ||||||
| Yes | 192 | 64 | 3 5 |
67 | 157 | 63 | |
| No | 102 | 34 | 1 7 |
33 | 85 | 34 | |
| Unknown | 6 | 2 | 0 | 0 | 6 | 2 | |
| Local recurrece | 16 | 5 | 3 | 6 | 13 | 5 | 0.75 |
| Locoregional recurrence | 32 | 11 | 6 | 12 | 26 | 10 | 0.82 |
| Distant recurrence | 22 | 7 | 1 5 |
6 | 7 | 13 | 0.08 |
| Radiation Dose | 0.048 | ||||||
| N | 252 | 44 | 208 | ||||
| mean (SD) | 6710 (898) | 6890 (575) | 6672 (949) | ||||
| Follow-up among survivors | 0.30 | ||||||
| N | 242 | 35 | 207 | ||||
| mean (SD) | 42 months (20) | 45 months (22) | 42 months (19) | ||||
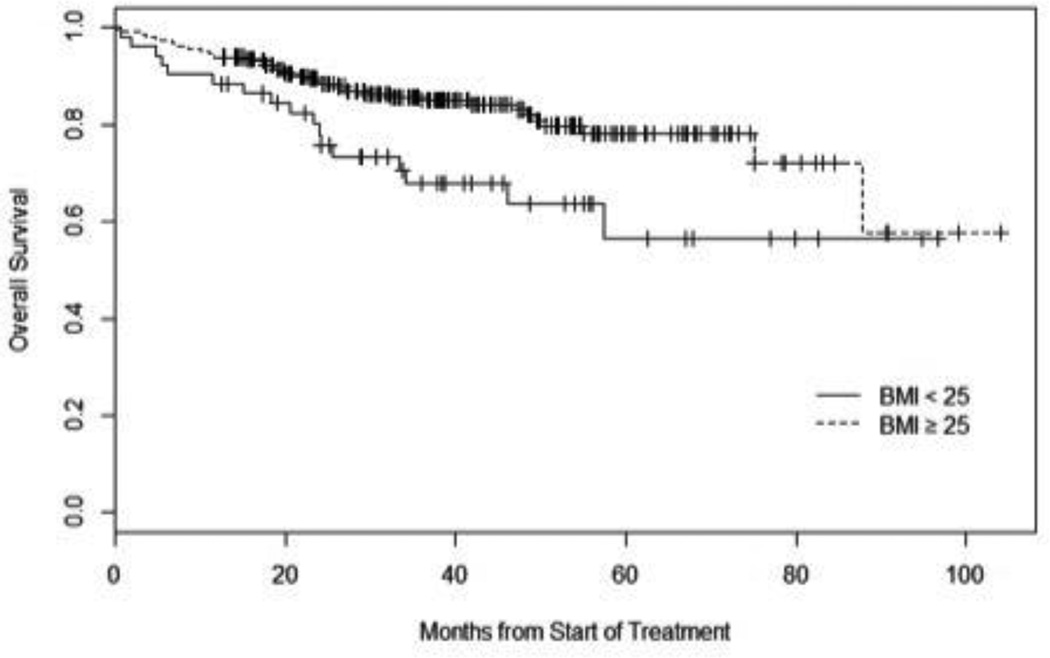
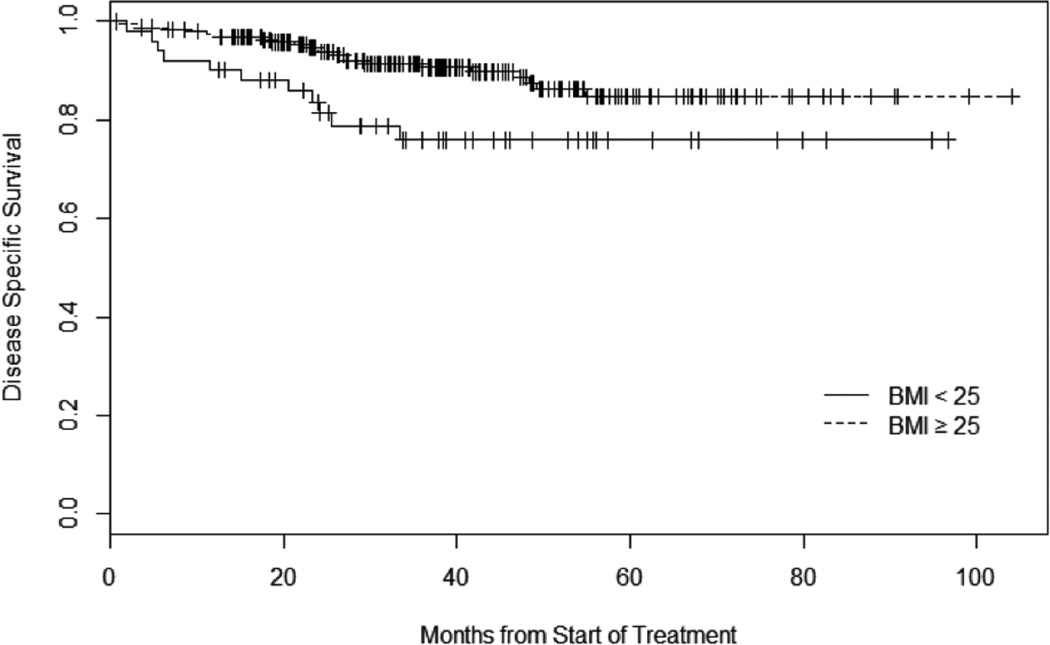
Of the 300 patients that met our inclusion criteria, 4 (1.3%) were underweight (BMI<=18.5 kg/m2), 48 (16.0%) were normal weight (18.5 kg/m2 < BMI < 25 kg/m2), 122 (40.7%) were overweight (25 kg/m2 < BMI <30kg/m2) and 126 (42.0%) were obese (BMI>30 kg/m2). The mean BMI at diagnosis was 29.6 kg/m2. When comparing patients stratified by BMI >/< 25 kg/m2, patients who were < 25kg/m2 were treated with higher doses of radiation (6890 cGy vs. 6672 cGy, p=0.048), were more likely to be current tobacco users (44% vs. 26%, p=0.04) and were more likely to be treated primarily with chemo/radiation (73% vs. 58%, p=0.04). There was no difference in proportion of T4 tumors (7.7% vs. 7.0%, p=0.853) or N2c/N3 nodal disease (15.7% vs. 13.4%, p=0.669) between the two groups. Otherwise, there were no differences in baseline characteristics between these two groups (Table I). There was no difference in local, regional or distant recurrence rates between the two groups although there was a trend toward more distant metastasis among patients with BMI < 25 kg/m2 (13.5% vs. 6.1%, p=0.08). In a univariate analysis, overall survival was significantly longer in the BMI >25 kg/m2 cohort with a hazard ratio (HR) = 0.49 (95% confidence interval (95% CI) 0.28 to 0.87), p=0.01 (Figure 1). Disease-specific survival was also significantly longer in the BMI > 25 kg/m2 cohort with a HR 0.43 (95% CI 0.21 to 0.89), p=0.02 (Figure 2). The association between BMI status and overall survival remained statistically significant in multivariate analysis (HR 0.54 (95% CI 0.30 to 0.98), p=0.04) (Table 2) however disease-specific survival did not reach statistical significance. Radiation dose, among subjects who received radiation therapy, was not associated with survival and therefore was not included in the final Cox proportional hazards model (p=0.18). Primary treatment (either surgery or chemo/radiation) was also not found to be associated with survival and was not included in the final Cox model (p=0.53).
Figure 1.
Kaplan Meier Curve of Overall Survival
HR for overall survival (95% CI) of univariate analysis for subjects with BMI ≥25 and compared with subjects with BMI < 25: 0.49 (0.28 to 0.87), p=0.01.
Figure 2.
Kaplan Meier Curve of Disease Specific Survival
HR for disease specific survival (95% CI) for subjects with BMI ≥25 and compared with subjects with BMI < 25: 0.43 (0.21 to 0.89), p=0.02.
Table 2.
Multivariate analysis of overall survival in Cox regression model
| Reference Category | HR | 95% CI | P-value | |
|---|---|---|---|---|
| BMI ≥ 25 | BMI < 25 | 0.54 | 0.30–0.98 | 0.04 |
| Age at diagnosis | -- | 1.06 | 1.03–1.10 | 0.0007 |
| T-stage | -- | 1.54 | 1.17–2.02 | 0.002 |
| N-stage | -- | 1.20 | 0.97–1.48 | 0.09 |
| Male | Female | 0.85 | 0.41–1.78 | 0.67 |
| African American | Caucasian | 1.97 | 0.66–5.94 | 0.23 |
| Former Smoker | Never smoker | 2.65 | 1.19–5.92 | 0.02 |
| Current Smoker | Never smoker | 3.28 | 1.41–7.60 | 0.006 |
| History of alcohol | No history of alcohol | 1.42 | 0.76–2.65 | 0.27 |
DISCUSSION
This study demonstrates that patient body mass index > 25 kg/m2 is an independent positive prognostic indicator for overall survival in HPV-positive oropharyngeal squamous cell carcinoma. While others have shown this characteristic to be prognostically important in head and neck cancer in general we believe it is necessary to stratify the various subsites of head and neck cancer, particularly given the known better prognosis in HPV-associated oropharyngeal squamous cell carcinoma.
While our groups at baseline had some dissimilarities in current tobacco use, primary treatment modality and radiation dose, these factors were either unrelated to survival (treatment modality and radiation dose) or were included within the multivariate models (tobacco use status).
Several previous studies have examined the association of BMI with survival in HNSCC using multivariate analysis7,9,10. In a population-based study of head and neck cancer patients, Gaudet found improved overall survival in self-reported overweight/obese patients (BMI>25 kg/m2) as compared with normal weight patients (22.5 – 25.0 kg/m2) in a multivariate analysis (HR 0.76)7. Lower weight patients (<22.5 kg/m2) had an increased cancer-related mortality in their analysis. When individual subsites were examined this association was present among non-oropharyngeal primaries but not among oropharyngeal primaries. However this analysis was limited by lack of reported HPV status. Similar statistically significant findings on multivariate analysis were reported in a prospective study using University of Michigan SPORE data evaluating nutritional parameters amongst head and neck cancer patients (BMI>25 kg/m2, HR 0.7). This study was not stratified by tumor subsite but 54% of its patients were of an oropharynx primary8. Again, these were not stratified by HPV status however given these patients were enrolled between 2003–2008 we would expect at least 72% HPV positivity16. Similar results using multivariate analysis have been found by Pai (BMI>25 kg/m2, HR 0.7), and McCrackan (BMI<25kg/m2, HR 3.63) however these were again not stratified by subsite nor did Pai’s multivariate analysis include tobacco status9,10. BMI >25kg/m2 has also been found to be associated with better disease-free survival on multivariate analysis8,10. However, in the only other work to look at a specific HNSCC subsite (oral cavity), Iyengar found a shorter disease-specific survival in obese patients (>30 kg/m2) with HNSCC and this was confirmed on multivariate analysis13. On subgroup analysis there was no such association among those who were merely overweight nor was overall survival associated with BMI status in either univariate or multivariate analysis.
In the present study of patients with HPV-positive OPSCC we find BMI > 25 kg/m2 to be positively and significantly associated with overall survival in both a univariate and multivariate analysis. BMI status is also associated with disease specific survival but not on multivariate analysis. This is an important addition to the literature given that well-known association of HPV status with improved overall and disease-specific survival14.
The reason for the association between improved survival and overweight/obese BMI status is unclear. In our analysis, there is no association with age or advanced stage implying a different explanation. The most likely explanation is that patients with greater BMI are able to withstand the inevitable weight loss and loss of functional pharyngeal musculature following either surgical treatment or radiotherapy. Several groups have posited that overweight and obese patients may have more reserve to allow them to better tolerate treatment10,17. Given that the mean weight loss during definitive intensity modulated radiation therapy to the oropharynx exceeds 20 pounds this is a plausible hypothesis18. Recently, Grossberg found that in patients undergoing definitive chemo/radiotherapy for head and neck cancer that pretreatment skeletal muscle depletion was predictive of overall survival but depletion during treatment was not19. However, this association was not found among oropharyngeal primaries when stratified by subsite. In addition, weight loss prior to treatment with definitive fractionated radiation for head and neck cancer has been associated with worse overall and recurrence free survival20. A secondary analysis of the ARTSCAN study (conventional versus accelerated fractionation for HNSCC) found that pretreatment BMI >25 kg/m2 is predictive of overall survival, however this analysis was not stratified by HPV status or disease subsite21. While weight loss prior to treatment in head and neck cancer patients is common it is less clear that this is true in HPV+ OPSCC and in our experience most patients with HPV+ OPSCC do not present with significant dysphagia or weight loss22. Given the retrospective nature of this research, accurate measure of pre-treatment weight loss is unachievable.
It may also be that rather than providing a buffer against the expected weight loss during treatment that neck fat specifically is actually protective against local radiation toxicity. Patients with high BMI (>25 kg/m2) before definitive chemoradiation for esophageal carcinoma have been shown to have fewer treatment related toxicities, and patients with higher BMI with HNSCC have lower long-term gastric tube rates after definitive chemoradiation for HNSCC10,23. However, BMI > 25 kg/m2 has also been associated in a randomized study with an increased risk of short-term grade 3 or grade 4 toxicity during radiation treatment and those with higher pretreatment weights have been shown to have more treatment-related weight loss24,25. Therefore, it is therefore unclear what effect, if any, body mass index has on local toxicity. A limitation of these studies is the lack of knowledge about the deposition of fat – whether it is peripherally deposited (i.e. greater neck circumference) which might be predictive of local toxicities or more visceral adiposity, which would not be expected to have such an effect.
To determine whether there indeed is an association with radiation, our cohort should be divided into those who received radiation versus those who did not. Unfortunately, the vast majority of our cohort (262/300, 87.3%) received at least 5000 cGy of radiotherapy and so we are unable to determine whether this association stands in patients who undergo only surgical therapy. Given Iyengar’s findings of no association between outcomes and BMI in the oral cavity, which we would expect to have been primarily treated with surgery, it is possible that that patients with higher BMI are better equipped to withstand the nearly inevitable dysphagia, dysgeusia and xerostomia associated with radiation therapy13. Further work is needed to evaluate this effect in surgery-only treated patients.
Others have suggested that BMI may be associated with unmeasured tobacco/alcohol status. While most studies examining BMI, including ours, have adjusted for tobacco status and alcohol use on multivariate analysis, it is certainly possible that unmeasured differences in consumption are related to survival outcomes. We did find a significant difference in current tobacco use between our two cohorts however this was controlled for in our multivariate analysis. There was no association between alcohol use and survival.
It may be that patients with a higher BMI have additional co-morbidities, such as hypertension or obstructive sleep apnea, and have established access to healthcare. However, it would be expected that patients with a higher BMI would present with an earlier stage of disease secondary to more frequent physician visits for comorbid diseases, which was not found in our study. This may be because of the natural tendency for HPV-positive oropharyngeal cancer to present with bulky neck disease.
There are other possibilities to explain the positive effect of BMI on survival including effects on the tumor microenvironment. Contrary to our findings, most evidence in the literature of other cancer sites suggests that adipose tissue promotes tumor growth through a variety of local and systemic effects including increased estrogen, insulin and IGF-1, TNF-a, and promotion of tumor-related pathways including the PI3K/Akt/mTOR and Ras/Raf/MAPK pathways which are known to be associated with HPV-positive oropharyngeal cancer26,27. Within the tumor microenvironment of breast cancers an obesity-inflammation-estrogen pathway has been proposed to explain tumor progression26. A similar pathway could explain the effect of estrogen on HPV-associated recurrent respiratory papillomatosis which has been shown to grow in response to estrogen metabolites28. Metabolic syndrome has been associated with an increased risk of persistent cervical HPV infection and an increased risk of HPV incident infection in a study conducted during cervical cancer screening. In addition, there is evidence that overweight patients with cervical cancer may have improved survival after chemoradiation29. These mechanisms would suggest that local fat may promote tumor growth rather than provide a survival advantage. Nevertheless, it is plausible that given the interplay between obesity and immune response that local therapy allows for increased immune response against the tumor or other local inflammatory/immune effects which could alter the tumor microenvironment. Evidence of this hypothesis is limited in the literature and further efforts should be made to characterize any such association.
While this manuscript may not alter patient management at this point, patients with lower BMI should be informed that they may be at risk for poorer survival and attention should be paid to their nutritional status throughout treatment. In addition, we view this study as idea-generating and would hope that the etiology of the associations with survival will be clarified in future works.
There are weaknesses in this analysis including its retrospective nature and our inability to control for some factors which are likely important including quantitative alcohol use and continued tobacco use during radiation therapy. Given our relatively small numbers we were also unable to further stratify into underweight/normal weight/overweight/obese. In addition, body mass index is not a perfect measure of body fat stores. Further, low body mass index may be a marker for other disease states not measured here or may be due to a frailty phenotype. These contributing factors should be investigated in further works and we would encourage use of multi-institutional pooling of data and/or use of data repositories such as the National Cancer Database when both HPV status and BMI data are available.
CONCLUSION
Body mass index >25 kg/m2 is independently associated with improved overall survival in HPV-positive squamous cell carcinoma of the oropharynx and is associated with disease-specific survival. Further work is needed to clarify the underlying etiology of this association.
Table 3.
Multivariate analysis of disease specific survival in Cox regression model
| Reference Category | HR | 95% CI | P-value | |
|---|---|---|---|---|
| BMI ≥ 25 | BMI < 25 | 0.58 | 0.28–1.21 | 0.15 |
| Age at diagnosis | -- | 1.01 | 0.97–1.06 | 0.62 |
| T-stage | -- | 2.05 | 1.45–2.89 | <0.0001 |
| N-stage | --- | 1.41 | 1.06–1.88 | 0.02 |
| Male | Female | 0.68 | 0.27–1.74 | 0.42 |
| African American | Caucasian | 4.16 | 1.12–15.43 | 0.03 |
| Former Smoker | Never smoker | 2.03 | 0.76–5.42 | 0.16 |
| Current Smoker | Never smoker | 2.38 | 0.89–6.39 | 0.08 |
| History of alcohol | No history of alcohol | 1.40 | 0.61–3.23 | 0.43 |
RESEARCH HIGHLIGHTS.
-
-
82% of HPV+ OPSCC patients have a body mass index greater than 25 kg/m2
-
-
Body mass index greater than 25 kg/m2 is associated with improved overall survival when controlling for other patient characteristics
-
-
Body mass index greater than 25 kg/m2 is associated with improved disease specific survival on univariate, but not multivariate, analysis
Acknowledgments
DISCLOSURES
Contract grant sponsor: This project was conducted using the University of Pittsburgh Cancer Institute Biostatistics Facility that is supported in part by award P30CA047904.
This works was supported in part by a Career Development Award from the Department of Veterans Affairs, BLR&D and a grant from the PNC Foundation (U.D.).
This work does not represent the views of the US Government nor the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared
REFERENCES
- 1.Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge AM, Zimmet PZ. The epidemiology of obesity. Baillieres Clin Endocrinol Metab. 1994;8(3):577–599. doi: 10.1016/s0950-351x(05)80287-3. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Leitzmann M, Freisling H, et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24(31):5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 6.Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Gaudet MM, Patel AV, Sun J, et al. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer Epidemiol Biomarkers Prev. 2012;21(3):497–503. doi: 10.1158/1055-9965.EPI-11-0935. [DOI] [PubMed] [Google Scholar]
- 8.Arthur AE, Peterson KE, Rozek LS, et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am J Clin Nutr. 2013;97(2):360–368. doi: 10.3945/ajcn.112.044859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai PC, Chuang CC, Tseng CK, et al. Impact of pretreatment body mass index on patients with head-and-neck cancer treated with radiation. Int J Radiat Oncol Biol Phys. 2012;83(1):e93–e100. doi: 10.1016/j.ijrobp.2011.11.071. [DOI] [PubMed] [Google Scholar]
- 10.McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope. 2008;118(7):1180–1185. doi: 10.1097/MLG.0b013e31816fca5c. [DOI] [PubMed] [Google Scholar]
- 11.Arthur AE, Duffy SA, Sanchez GI, et al. Higher micronutrient intake is associated with human papillomavirus-positive head and neck cancer: a case-only analysis. Nutr Cancer. 2011;63(5):734–742. doi: 10.1080/01635581.2011.570894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X, Nelson HH, Langevin SM, et al. Obesity and head and neck cancer risk and survival by human papillomavirus serology. Cancer Causes Control. 2015;26(1):111–119. doi: 10.1007/s10552-014-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120(7):983–991. doi: 10.1002/cncr.28532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 15.Naik M, Ward MC, Bledsoe TJ, et al. It is not just IMRT: Human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncol. 2015;51(8):800–804. doi: 10.1016/j.oraloncology.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen GP, Xu FH, He F, et al. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS One. 2012;7(5):e36515. doi: 10.1371/journal.pone.0036515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78(4):1026–1032. doi: 10.1016/j.ijrobp.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghadjar P, Hayoz S, Zimmermann F, et al. Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94) Radiat Oncol. 2015;10:21. doi: 10.1186/s13014-014-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottosson S, Soderstrom K, Kjellen E, Nilsson P, Zackrisson B, Laurell G. Weight and body mass index in relation to irradiated volume and to overall survival in patients with oropharyngeal cancer: a retrospective cohort study. Radiat Oncol. 2014;9:160. doi: 10.1186/1748-717X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009;17(11):1345–1351. doi: 10.1007/s00520-009-0684-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Myles B, Wei C, et al. Obesity and outcomes in patients treated with chemoradiotherapy for esophageal carcinoma. Dis Esophagus. 2014;27(2):168–175. doi: 10.1111/dote.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer F, Fortin A, Wang CS, Liu G, Bairati I. Predictors of severe acute and late toxicities in patients with localized head-and-neck cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82(4):1454–1462. doi: 10.1016/j.ijrobp.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Nourissat A, Bairati I, Samson E, et al. Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer. 2010;116(9):2275–2283. doi: 10.1002/cncr.25041. [DOI] [PubMed] [Google Scholar]
- 26.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 27.Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26(6):1216–1223. doi: 10.1093/annonc/mdv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepkovic DW, Bradlow HL. Estrogen hydroxylation--the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 29.Kizer NT, Thaker PH, Gao F, et al. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer. 2011;117(5):948–956. doi: 10.1002/cncr.25544. [DOI] [PMC free article] [PubMed] [Google Scholar]