Abstract
Background
Over the last 15 years, there has been a change in clinical practice for the detection of recurrence in all patients with papillary thyroid cancer (PTC). In the past, recurrence was detected by clinical examination supplemented with fine-needle aspiration cytology; however, routine neck ultrasonography (US) and measurements of serum thyroglobulin were introduced for follow-up in 2000 and are now used widely for recurrence surveillance. The aim of this study was to describe the effectiveness of this changing trend in the use of routine surveillance ultrasonography for the detection of recurrence in low-risk PTC at a single institution.
Methods
Patients undergoing total thyroidectomy for PTC between January 2000 and December 2010 were identified from an institutional database. Of these, 752 (43.1%) were categorized as low risk by the risk stratification of the American Thyroid Association and included for analysis. The number of US examinations per patient per year of follow-up was then determined. The number of recurrences and deaths from disease was recorded similarly.
Results
The median age was 48 years (range, 16–83) and the median follow-up was 34 months (range, 1–148). Between 2003 and 2012, the number of US examinations per patient-year of follow-up increased by 5.3-fold. Over the same time period, 3 structural recurrences (clinically evident neck masses or nodes) were detected with no disease-related deaths.
Conclusion
At our institution, the annual rate of neck US examination increased by 5.3-fold per low-risk PTC patients between 2003 and 2012. Despite this increase, only 3 structural recurrences were detected. The routine use of neck US for surveillance of low-risk PTC patients requires review.
Over the last 15 years, there has been a progressive change in the methods employed to detect locoregional recurrence in patients with papillary thyroid cancer (PTC). Detection of locoregional recurrence in the past was by clinical examination supplemented with fine-needle aspiration cytology (FNAC). Now, neck ultrasonography (US) and postoperative measurements of thyroglobulin (Tg) are the mainstay of postoperative surveillance for recurrence.
In the 2006 edition of the management guidelines of the American Thyroid Association (ATA), surveillance US of the neck was recommended every 6 months in the first year and then annually for ≥3–5 years, depending on the patient risk for recurrent disease.1 This recommendation was based on evidence that suggested that neck US was more sensitive than clinical examination and serum Tg levels alone.2–5 In the updated 2009 edition, the recommended frequency and duration of surveillance neck US was relaxed to “1 or 2” US examinations in the first postoperative year and then periodically, depending on patient risk.6 The change in the recommended frequency of surveillance was in part due to the high rate of unwarranted FNAC of minute, subclinical disease as a result of increased neck imaging. In the current 2015 ATA guidelines, recommendations concerning surveillance neck US recommendations remain unchanged with much of surveillance management based on clinician discretion.
The focus of this study was to analyze the impact of the frequency of surveillance neck US in ATA low-risk patients over the past decade and to correlate surveillance practices with detection of recurrence over the same time period. With the rapid increase in low-risk PTC over the past 15 years, optimizing follow-up management in this low-risk category will have substantial clinical and economic benefits.
METHODS
After approval by our institutional review board, we identified 1,746 consecutive patients with differentiated thyroid cancer operated on within our institution between 2000 and 2010 from an established institutional database. Patient, tumor, and treatment characteristics were reviewed to categorize patients based on ATA risk stratification (Table I).6 Patients with nonpapillary histology or ATA intermediate-/high-risk categories were excluded from analysis. Patients with another cancer diagnosis at any time and those treated outside of the head and neck surgical service were also excluded, because this may influence choice of postoperative imaging modality and frequency. After this approach, 752 ATA low-risk PTC patients were available for analysis.
Table I.
American Thyroid Association 2009 risk categories for papillary thyroid cancer
| Low risk | Intermediate risk | High risk |
|---|---|---|
| No local or distant metastases | Microscopic perithyroidal invasion | Macroscopic tumor invasion |
| All macroscopic disease resected | Cervical lymph node metastases or 131I uptake outside thyroid bed on posttreatment scan, if done | Gross residual disease |
| No locoregional invasion | Aggressive histology | Distant metastases |
| No aggressive histology | ||
| No vascular invasion | ||
| No 131I uptake outside thyroid bed on post treatment scan, if done |
Follow-up duration was calculated from the date of thyroidectomy to the date last seen by a physician within the thyroid cancer multidisciplinary team at Memorial Sloan Kettering Cancer Center (MSKCC). All follow-up surveillance data were captured between 2003 and 2012. In 2003, we instituted a policy of annual postoperative US surveillance. We therefore elected to analyze the outcomes related to US after the initiation of our US surveillance program in 2003. The annual surveillance US rate relative to outcomes are therefore analyzed for 2003 through to 2012.
The number of thyroid cancer patients under surveillance and the number of surveillance US was recorded for each calendar year with adjustments for a partial year of follow-up (ie, 6 months of follow-up surveillance for the calendar year equates to 0.5 patients). Similarly, the number of neck US performed per calendar year was also recorded. The total number of neck US performed was divided by the total number of patients under surveillance to calculate the average number of surveillance US per patient for each calendar year between 2003 and 2012. All US examinations included in this study were performed within the MSKCC radiology department by head and neck sonographers, not the head and neck surgeons.
Over the past 5 years, we have progressively used preoperative US examinations to assess the lymph nodes in the central and lateral compartment. We published our data recently on our institution’s use of preoperative neck US.7 Assessment of the central compartment for all patients is by intraoperative palpation of the lymph nodes in the central compartment at the time of thyroidectomy. Prophylactic neck dissections are not performed in patients with a clinically N0 neck. Assessment of the lateral neck is performed by palpation at preoperative clinical visit or by preoperative US examination.
Surveillance practices have evolved at our center over recent years. For low risk patients, routine radioactive iodine scans are no longer performed. There is increasing preference for routine, nonstimulated, serum Tg levels and high-resolution US of the thyroid bed and cervical lymph nodes. Current surveillance practices at our institution are shown in Table II.8
Table II.
Postoperative surveillance strategy based on American Thyroid Association risk stratification of papillary thyroid cancer
| 6 months | 12 months | 18 months | 24 months | |
|---|---|---|---|---|
| Thyroglobulin | All | All | All | All |
| Ultrasound neck | — | All | — | All |
| Diagnostic RAI scan | — | — | Intermediate/High risk | — |
| CT/MRI | — | High risk | — | High risk |
| PET scan | — | High risk | — | High risk |
All, Low, intermediate, and high risk; CT, computed tomography; RIA, radioactive iodine.
Locoregional recurrence outcomes were determined by clinical examination supplemented with US or other imaging and FNAC confirmation. Only patients with a recurrence with structural abnormality (enlarged lymph node or mass) confirmed by cytology were considered to have recurrence. Only patients with nodules in the thyroid bed or lateral neck of >1 cm were considered for FNAC. Patients with biochemical recurrence, defined by increased serum levels of Tg, without cytologic or histopathologic confirmation, were not considered sufficient endpoints of disease outcome.
RESULTS
The median age was 48 years (range, 16–83), and the median follow-up was 34 months (range, 1–148). Fig 1 shows the progressive increase in surgical volume in low-risk PTC patients over time. Low-risk PTC increased from 15 patients in 2000 to 142 patients in 2010, corresponding to a 9.5-fold increase in annual incidence over a decade.
Fig 1.
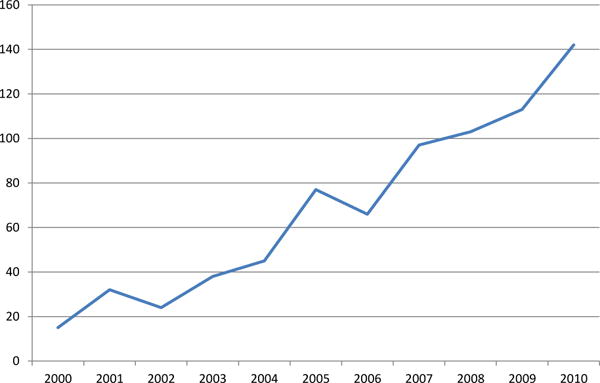
Number of patients with low-risk papillary thyroid cancer requiring operative management by year.
The number of patients with low-risk PTC under surveillance, corresponding number of surveillance US performed, and the recurrence events by calendar year are shown are Table III; 1,115 surveillance neck US were performed on this cohort of low-risk patients between 2003 and 2012. In 2003, 11 neck US were performed for 69 patients under surveillance, or 0.16 US per patient. By 2012, there were 247 US performed for 290 patients being followed, or 0.85 per patient. These changes represent a 5.3-fold increase in the number of surveillance US examinations per patient (Fig 2). Over this time period, adjuvant radioactive iodine therapy for patients with low-risk PTC decreased over the 10-year period from 40% in 2000 to 8.5% in 2010 (P < .001; Supplementary Table, available at http://dx.doi.org/10.1016/j.surg.2015.11.018).
Table III.
Number of patients with low-risk papillary thyroid cancer under surveillance and corresponding number of surveillance US performed by calendar year
| ATA Low-risk | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| No of patients followed | 69.4 | 102.4 | 138.8 | 179.7 | 225.8 | 282.0 | 347.3 | 413.8 | 416.9* | 290.2* |
| No of surveillance US | 11 | 17 | 27 | 31 | 68 | 104 | 142 | 210 | 258* | 247* |
| No of US per patient | 0.16 | 0.17 | 0.19 | 0.17 | 0.30 | 0.37 | 0.41 | 0.51 | 0.62 | 0.85 |
| Recurrence | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
Only includes patients and US for patients with papillary thyroid cancer between 2000 and 2010.
ATA, American Thyroid Association; US, ultrasonography.
Fig 2.
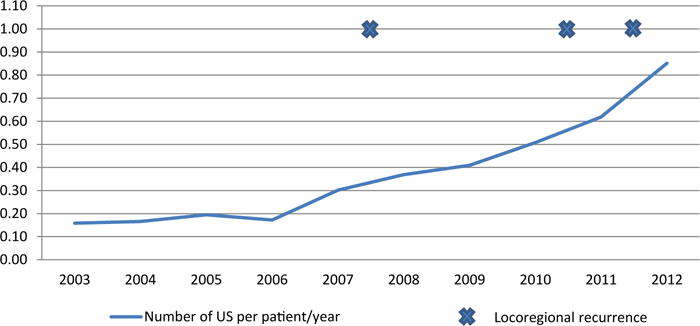
Number of locoregional structural recurrences and number of surveillance neck ultrasonographies (US) per patient per year of follow-up.
Over the same time period, 3 structural recurrences (0.4%) were detected. All 3 recurrences were detected with routine neck surveillance US. Two patients were found to have thyroid bed nodules confirmed on FNAC and were treated with therapeutic radioactive iodine dose. One patient had an enlarged lateral neck lymph node and was treated with lateral neck dissection. All 3 patients had no evidence of disease at last follow-up, and there were no disease-related deaths in the cohort.
DISCUSSION
Over the last 15 years, there has been a change in the management of thyroid cancer with increased emphasis on a systematic care plan and institution of management guidelines.1 Traditionally, locoregional recurrences were detected by clinical palpation of the neck supplemented with FNAC when necessary. High-resolution neck US and serum measurements of Tg are now recommended and used in routine surveillance.6 In this study, we analyzed the impact of this change in the use of surveillance US examinations in patients with low-risk PTC at our institution and correlated these changes with locoregional recurrences over the same time period.
The average number of surveillance US examinations performed on each patient increased from 0.16 to 0.85 during the study period, corresponding with a >5-fold increase over the decade. During this still short follow-up period for PTC, only 3 locoregional recurrences (0.4%) were detected, and there were no disease-specific death events. These data question what is appropriate follow-up of low-risk PTC. Is routine performance of follow-up US always necessary or cost effective in patients with low-risk PTC? If so, how frequently should this be done and for what duration? More controversially, is surveillance of patients with low-risk PTC necessary at all, given the extremely low rates of recurrence? Although we cannot resolve all these issues based on a single institutions experience and still somewhat limited follow-up, the data herein should promote a discussion regarding the clinical and economic aspects of care in patients with low-risk PTC.
Historically, surveillance of PTC patients after surgery was life-long secondary to the belief that recurrence was common and estimated to be as high as 30%. These outcomes reported by Mazzaferri et al,9,10 however, were based on patients treated 30–40 years ago. More recent literature suggests that recurrence rates are substantially less.11–14 Patients with low-risk PTC have an excellent recurrence-free survival of in excess of 97%.11,12 Durante et al12 followed 312 consecutive patients with T1aN0M0 classic PTC and with a median follow-up of 6.7 years; there were no structural disease recurrences and no disease-specific deaths. The authors demonstrated that, in a well-selected cohort of patients with very low-risk PTC, outcomes are excellent irrespective of surveillance. Our data along with the recent literature demonstrates clearly that recurrence rates for patients with low-risk PTC are very low. Given this fact, it seems that follow-up imaging should be tailored to the risk of recurrence.
The ATA now categorizes patients with PTC into low, intermediate, and high risk for recurrence and recognizes that follow-up imaging should be tailored to this risk. The ATA guidelines recommend currently neck US 6–12 months postoperatively and then periodically, depending on the risk of recurrent disease and Tg status.6 Survey results from thyroid cancer clinicians in the United States by Haymart et al,15 however, demonstrated wide variation in all aspects of long-term management of patients with thyroid cancer. Of the 534 endocrine and nuclear medicine physicians surveyed, 45.4% would routinely follow American Joint Committee on Cancer stage I patients with PTC with an undetectable serum Tg level with surveillance US examinations.15 Limited research, the ready availability of portable US machines, and the clinician concern over missing disease recurrence are likely reasons for the increased use of surveillance US over the past decade.
The rationale for surveillance US in PTC is based on the fact that neck US is more sensitive than clinical examination or other modalities of disease follow-up.2,3 Neck US can detect cervical nodal disease as small as 2–3 mm in diameter. Such small nodal recurrence is often difficult to find even intraoperatively in scar tissue and likely has no relevance in the overall oncologic outcome. The use of neck US would be justified if the development of clinically meaningful recurrence was a frequent event; however, the recurrence events in our series have remained remarkably stable with a total of only 3 (0.4%) recurrence events.
Neck US is viewed generally as a safe, low-cost imaging modality with greater sensitivity than clinical examination alone. One may argue that there is no harm associated with routine US surveillance. Also it is important to acknowledge that results of US are known to be highly user dependent and frequently cannot distinguish thyroid bed recurrences from benign nodules.16,17 In the postoperative setting, indeterminate or false-positive readings on US examination can lead to unnecessary FNAC and additional investigations. Such investigations are associated with additional cost and complications and can lead to unnecessary anxiety for the physician and the patient.18 Any iatrogenic harm associated with low-risk PTC is difficult to justify, when recurrence rates in ATA low-risk patients are only 3%, and disease-specific mortality is zero. The incidence of PTC is increasing in the United States and worldwide, and the majority of increases are owing to low-risk, and often subclinical, disease.19–21 Although physicians generally view US as a low-cost imaging modality, from a societal perspective, the cumulative cost of routine US surveillance in all patients with low-risk PTC is substantial and will only continue to increase if not addressed.
It is important to note that our study has several limitations. Owing to its retrospective nature, our study is susceptible to all the limitations associated with such studies. First, many patients are followed ultimately by their local endocrinologist; it is not possible to know how many of these patients also underwent additional surveillance US. Our study therefore, underestimates the true rate of increase in US examinations. Similarly, it may also underestimate the “true” risk of recurrent disease in patients who were followed elsewhere and did not return to our institution when recurrence developed. Second, we report a very low rate of structural recurrence. One may argue that due to the surgical expertise of our high-volume center and the inherent bias of a tertiary referral center, these findings are not generalizable. It is important to emphasize that surgical and medical expertise has remained unchanged at our institution over the study period, yet the rate of surveillance US has increased with no corresponding change in structural recurrence. Nevertheless, we would recommend that similar studies should be repeated at other institutions to establish the generalizability of our findings.
In conclusion, we report a 5.3-fold increase in the use of surveillance neck US for patients with low-risk PTC over a decade. This increase in US surveillance has not resulted in increased detection of structural recurrence events nor improved the already excellent outcomes of this low-risk cohort. The results presented in this study question the benefit and justification of routine and frequent surveillance US in patients with low-risk PTC. We acknowledge that patients with an increased Tg level in the absence of structural abnormality should continue to be followed with serial US; however, the routine use of US in patients with undetectable Tg level seems difficult to justify. In the context of the increasing incidence of low-risk PTC in the United States and worldwide, the clinical and economic implications of routine US surveillance will become more substantial with time if follow-up US use is not rationalized.
Supplementary Material
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.surg.2015.11.018.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 2.Kouvaraki M, Shapiro S, Fornage B, Edeiken-Monro B, Sherman S, Vassilopoulou-Sellin R, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–54. doi: 10.1016/s0039-6060(03)00424-0. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 3.Torlontano M, Crocetti U, Augello G, D’Aloiso L, Bonfitto N, Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–3. doi: 10.1210/jc.2005-1185. [DOI] [PubMed] [Google Scholar]
- 4.Pacini F, Molinaro E, Castagna M, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–73. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 5.Bachelot A, Cailleux A, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–11. doi: 10.1089/105072502760258686. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Palmer F, Thomas D, Shaha A, Shah J, Patel S, et al. Preoperative neck ultrasound in clinical node negative differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99:3686–93. doi: 10.1210/jc.2014-1681. [DOI] [PubMed] [Google Scholar]
- 8.Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43:401–21. doi: 10.1016/j.ecl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–63. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–9. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durante C, Attard M, Torlontano M, Ronga G, Monzani F, Costante G, et al. Identification and optimal postsurgical follow-up of patients with very low-risk papillary thyroid microcarcinomas. J Clin Endocrinol Metab. 2010;95:4882–8. doi: 10.1210/jc.2010-0762. [DOI] [PubMed] [Google Scholar]
- 13.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98:636–42. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Prognosis of patients with papillary thyroid carcinoma showing postoperative recurrence to the central neck. World J Surg. 2011;35:767–72. doi: 10.1007/s00268-010-0924-3. [DOI] [PubMed] [Google Scholar]
- 15.Haymart MR, Banerjee M, Yang D, Stewart AK, Sisson JC, Koenig RJ, et al. Variation in the management of thyroid cancer. J Clin Endocrinol Metab. 2013;98:2001–8. doi: 10.1210/jc.2012-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondeau G, Fish S, Hann L, Fagin J, Tuttle R. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011;21:845–53. doi: 10.1089/thy.2011.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin J, Han B, Ko E, Kang S. Sonographic findings in the surgical bed after thyroidectomy: comparison of recurrent tumors and nonrecurrent lesions. J Ultrasound Med. 2007;26:1359–66. doi: 10.7863/jum.2007.26.10.1359. [DOI] [PubMed] [Google Scholar]
- 18.Robenshtok E, Fish S, Bach A, Dominguez J, Shaha A, Tuttle R. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012;97:2706–13. doi: 10.1210/jc.2012-1553. [DOI] [PubMed] [Google Scholar]
- 19.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451–7. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Davies L, Welch H. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–6. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


