Abstract
In a nerve terminal, synaptic vesicle docking and release are restricted to an active zone. The active zone is a protein scaffold that is attached to the presynaptic plasma membrane and opposed to postsynaptic receptors. Here, we generated conditional knockout mice removing the active zone proteins RIM and ELKS, which additionally led to loss of Munc13, Bassoon, Piccolo, and RIM-BP, indicating disassembly of the active zone. We observed a near complete lack of synaptic vesicle docking and a strong reduction in vesicular release probability and the speed of exocytosis, but total vesicle numbers, SNARE protein levels, and postsynaptic densities remained unaffected. Despite loss of the priming proteins Munc13 and RIM and of docked vesicles, a pool of releasable vesicles remained. Thus, the active zone is necessary for synaptic vesicle docking and to enhance release probability, but releasable vesicles can be localized distant from the presynaptic plasma membrane.
Introduction
Ca2+-triggered fusion of synaptic vesicles is mediated by soluble NSF-attachment protein receptors (SNAREs) and is restricted to release sites called active zones (Couteaux and Pecot-Dechavassine, 1970; Südhof, 2012). The active zone is a highly organized structure that docks synaptic vesicles close to release machinery and presynaptic Ca2+ channels (Fig. 1A). This establishes the tight spatial organization required for fast synaptic vesicle fusion upon Ca2+ entry and it provides molecular machinery to set and regulate synaptic strength (Kaeser and Regehr, 2014). Functionally, synaptic strength is determined by two parameters that are controlled at the active zone. First, only a subset of vesicles can be released upon arrival of an action potential. This pool of vesicles is generated through a priming reaction and is called the readily releasable pool (RRP). Second, an action potential releases RRP vesicles with a certain probability, called vesicular release probability (P). Synaptic strength, the amount of release from a given synapse, is proportional to the product of RRP and P (Zucker and Regehr, 2002).
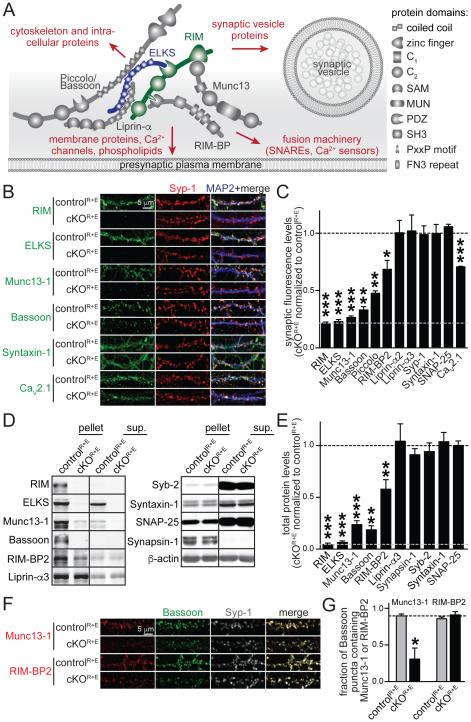
Figure 1. Genetic removal of RIM and ELKS leads to disruption of the active zone.
A. Schematic of the protein complex at the active zone and its connections to other important presynaptic protein families (marked in red). SAM: sterile alpha motif, MUN: Munc13 homology domain, PDZ: PSD-95/Dlg1/ZO-1 homology domain, SH3: Src homology 3 domain, PxxP: proline rich motif, FN3: fibronectin 3 repeat.
B, C. Sample images (B) and quantitation (C) of protein levels at RIM and ELKS knockout (cKOR+E) and control (controlR+E) synapses using confocal microscopy. The synaptic vesicle marker Synaptophysin-1 (Syp-1) was used to define the region of interest (ROI). The black dotted line indicates control levels and the grey dotted line non- specific staining as assessed for RIM. Example images for RIM-BP2, Piccolo, Liprin-α2, Liprin-α3, SNAP-25, and quantitation of puncta number and size are in Fig. S1 (controlR+E n = 3 independent cultures, cKOR+E n = 3, 10 images per culture).
D, E. Quantitative Western blotting for presynaptic proteins using fluorescent secondary antibodies. Some cultures were fractionated into pellet and supernatant (sup.) using Triton X-100 solubilization and ultracentrifugation. Quantitation (E) of total protein levels in cKOR+E neurons normalized to protein levels in controlR+E neurons are shown. Black and grey dotted as in C. For detailed analysis of protein solubility and protein levels in each fraction see Table S1B (controlR+E n = 6 independent cultures, cKOR+E n = 6, except for Bassoon where n = 3 for both conditions, Syb-2: synaptobrevin/VAMP-2).
F, G. Sample images (F) and quantitation (G) of the fraction of Bassoon puncta containing Munc13-1 or RIM-BP2. The fraction of Bassoon pixels and the fraction of Syp-1 puncta containing Munc13-1 or RIM-BP2 are in Fig. S1 (controlR+E n = 6 independent cultures, cKOR+E n = 6, 10 images per culture).
All data are means ± SEM; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by Student's t test. All numerical data are in Table S1.
The active zone matrix consists of multi-domain proteins that control RRP and P, and their localization is restricted to the presynaptic plasma membrane area opposed to the postsynaptic density (PSD). These proteins include RIM, ELKS (also known as ERC/CAST/Rab6IP2), Munc13, Bassoon/Piccolo, RIM-BP, and Liprin-α (Schoch and Gundelfinger, 2006; Südhof, 2012). Many additional proteins including SNAREs, ion channels, receptors, cytoskeletal proteins and adhesion molecules are also present (Boyken et al., 2013; Morciano et al., 2009; Muller et al., 2010) but are not restricted to the active zone matrix. Removing individual proteins of the active zone matrix has effects on release that vary in extent, and there are differences between synapses in vertebrates, C.elegans, and D. melanogaster in the reliance on specific active zone proteins (Acuna et al., 2015; Kaeser et al., 2011; Kittel et al., 2006; Koushika et al., 2001; Liu et al., 2014, 2011; Müller et al., 2012). At vertebrate synapses, release strongly depends on synaptic vesicle priming activities of Munc13s such that loss of Munc13 leads to loss of all fusion competent vesicles (Augustin et al., 1999; Varoqueaux et al., 2002). RIMs contribute to priming through anchoring and activation of Munc13 (Andrews-Zwilling et al., 2006; Betz et al., 2001; Deng et al., 2011; Lu et al., 2006), and they tether primed vesicles to presynaptic Ca2+ channels to enhance release probability (Han et al., 2011; Kaeser et al., 2011, 2012). ELKS (Held et al., 2016; Kaeser et al., 2009; Liu et al., 2014), Bassoon/Piccolo (Davydova et al., 2014; Hallermann et al., 2010), and RIM-BPs (Acuna et al., 2015) are also present, but knockouts for these proteins show milder impairments in release. The domain structure and the in vitro interactions of RIM, ELKS and Bassoon/Piccolo further predicted strong scaffolding roles (Schoch and Gundelfinger, 2006; Südhof, 2012), but except for a partial loss of Munc13 in RIM knockout mice (Schoch et al., 2002) or of RIM-BP in Bassoon knockout mice (Davydova et al., 2014), the active zone protein complex was intact in knockout mice for individual protein families (Davydova et al., 2014; Deng et al., 2011; Held et al., 2016; Liu et al., 2014). Together with the notion that synaptic vesicle docking and fusion are spatially restricted to the active zone, these studies led to the hypothesis that the active zone is required to translate the incoming action potential into neurotransmitter release (Schoch and Gundelfinger, 2006; Südhof, 2012).
Synaptic vesicle tethering and docking are thought to precede fusion and have been studied using various methods and definitions. Docking is often defined as synaptic vesicles that are attached to the plasma membrane in electron microscopic images of glutaraldehyde fixed tissue such that the electron densities of the vesicle membrane and target membrane merge (Acuna et al., 2015; Augustin et al., 1999; Bronk et al., 2007; Han et al., 2011; Kaeser et al., 2011). Using glutaraldehyde fixation only RIMs participate in synaptic vesicle docking without affecting total numbers of synaptic vesicles in a nerve terminal (Augustin et al., 1999; Bacaj et al., 2015; Bronk et al., 2007; Kaeser et al., 2011). Recently, the use of high pressure freezing and tomography have allowed for distinction of tight vesicle docking with a resolution of a few nanometers. This has led to the discovery that SNARE proteins and Munc13 contribute to the tight attachment of synaptic vesicles to the presynaptic plasma membrane. Their genetic removal leads to a loss of tightly docked vesicles when observed in high pressure frozen tissue (Imig et al., 2014; Siksou et al., 2009), but these phenotypes are too subtle to be uncovered using glutaraldehyde fixation (Augustin et al., 1999; Bronk et al., 2007).
Overall, there is a good correlation between the number of docked vesicles observed with either fixation method, the size of the active zone, and the size of the RRP, which suggested, along with the observation that docked vesicles fuse upon stimulation, that docked vesicles are the RRP (Holderith et al., 2012; Imig et al., 2014; Rosenmund and Stevens, 1996; Schikorski and Stevens, 2001; Watanabe et al., 2013). Here, we measure the RRP as vesicles that are released by the application of hypertonic sucrose, and we assume that the same vesicles are accessible to action potentials although differences may exist (Rosenmund and Stevens, 1996; Schikorski and Stevens, 2001; Thanawala and Regehr, 2016; Zucker and Regehr, 2002). We use the term ‘fusion competent’ for vesicles in the RRP and vesicles that are released through spontaneous miniature events (Augustin et al., 1999).
To date, no knockout mutation has led to a strong structural disruption of the vertebrate active zone matrix. We set out to generate such a mutation with the goal to test whether the active zone is necessary for the structural assembly of synapses, whether it is required for fusion, and how it participates in setting RRP size and P. We produced conditional mouse mutants to simultaneously remove all active zone isoforms of RIM and ELKS in cultured hippocampal neurons. This led to loss of Munc13, Bassoon, Piccolo, RIM-BP, and CaV2.1 Ca2+ channels. The overall synaptic assembly including the postsynaptic densities, the synaptic vesicle cluster, and the levels of SNARE proteins remained unaffected. However, we observed a near complete loss of vesicle docking and release probability was strongly decreased. Surprisingly, a pool of fusion competent vesicles, released as spontaneous miniature events or during stimulation with action potential trains or hypertonic sucrose, persisted upon strong disruption of the active zone and vesicle docking.
Results
Genetic disruption of the presynaptic active zone
We generated mice to simultaneously and conditionally remove all presynaptic RIM and ELKS proteins. We targeted RIM and ELKS proteins because they are expressed at all synapses and they interact with all major active zone proteins (Fig. 1A). We crossed conditional knockout mice for presynaptic RIM proteins (Kaeser et al., 2008, 2011), encoded by the genes Rims1 and Rims2, to conditional knockout mice for both genes encoding ELKS proteins (Kaeser et al., 2009; Liu et al., 2014), Erc1 and Erc2, to generate quadruple conditional knockout mice (Fig. S1A). All analyses were done in primary hippocampal neurons from these mice or the double conditional knockout mice for either RIM or ELKS proteins. Lentivirus expressing cre recombinase or an inactive mutant of cre in neurons (Liu et al., 2014) was supplied at 5 days in vitro (DIV) to generate knockout neurons (cKOR+E) or control neurons (controlR+E). In cKOR+E neurons, we remove RIM and ELKS proteins as assessed by confocal microscopy (Figs. 1B, C, S1B, C, G) and quantitative Western blotting using fluorescent secondary antibodies (Figs. 1D, E, numerical values including mean ± SEM, p values, and number of repeats for all experiments can be found in supplemental tables for each figure). In immunostaining, 22% of RIM and 23% of ELKS signal remained upon knockout of these proteins (grey dotted line, Fig. 1C) despite much stronger reductions in Western blotting (Fig. 1E), establishing that this signal is non-specific (see Fig. S1D for background from secondary antibodies only). Genetic removal of RIM and ELKS led to a very strong reduction of interacting active zone proteins. Munc13-1 was eliminated from synaptic puncta in an extent similar to RIM and ELKS, and synaptic Bassoon and Piccolo were reduced nearly as strongly (Figs. 1B, C), whereas the total protein levels of Munc13-1 and Bassoon were reduced by ~80% (Figs. 1D, E). Synaptic and total RIM-BP2 was reduced by 31% and 42% respectively (Figs. 1C-E, S1B) and synaptic CaV2.1 Ca2+ channel levels were reduced by 29% (Figs. 1B, C). Because synaptic Munc13-1 levels (Fig. 1C) were more strongly reduced than total Munc13-1 levels (Fig. 1E), we tested whether the remaining Munc13-1 was clustered at synapses. The Munc13-1 protein that was left in the cKOR+E neurons was insoluble as measured in a fractionation experiment of the cultured neurons (Fig. 1D, and Table S1B). Furthermore, when we evaluated whether the remaining Munc13 clusters co-localized with the remaining Bassoon (Fig. 1G, S1F) or with synaptic vesicles (Figs. S1E), we found that many Bassoon puncta and synapses did not contain Munc13-1, whereas the remaining RIM-BP2 co-localized well with the same markers (Figs. 1G and S1E, S1F).
This is the most extensive genetic disruption of the vertebrate active zone protein complex to date with a near complete loss of most of the vital components. Somewhat surprisingly, synapse number and size were unchanged (Fig. S1C), and the levels and localization of SNARE proteins and synaptic vesicle markers (Syntaxin-1, SNAP-25 and Synaptobrevin-2/VAMP-2, and Synaptophysin-1) were not affected (Figs. 1B-E, S1B). At invertebrate synapses, Liprin-α controls presynaptic assembly upstream of ELKS and RIM (Dai et al., 2006; Kaufmann et al., 2002; Patel et al., 2006; Zhen and Jin, 1999). Vertebrates express Liprin-α proteins from four genes (Zürner and Schoch, 2009). Although it is not well understood which Liprin-α isoforms localize to the active zone, and post- and extra-synaptic localization has also been observed, Liprin-α2 and Liprin-α3 are likely the prominent synaptic Liprin-α isoforms (Spangler et al., 2011; Wyszynski et al., 2002; Zürner et al., 2011). In cKOR+E neurons, Liprin-α2 and Liprin-α3 localization (Fig. 1C), and Liprin-α3 levels (Fig. 1E) and biochemical solubility (Table S1B) were unaffected. Notably, Liprin-α2 and Liprin-α3 antibodies reveal relatively widespread labeling (Fig. S1B), compatible with additional roles for Liprin-α outside active zones (Miller et al., 2005). In summary, simultaneous deletion of RIM and ELKS reveals strong, redundant, and active zone specific scaffolding functions for these proteins that were not detected when a single protein family was deleted.
Loss of synaptic vesicle docking but normal postsynaptic assembly upon active zone disruption
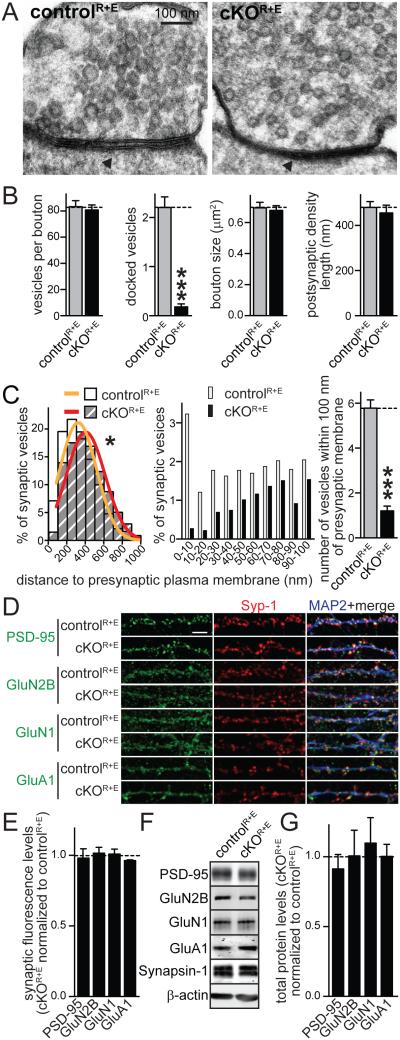
To characterize effects on presynaptic and postsynaptic structure, we fixed cultures by high-pressure freezing and analyzed them using transmission electron microscopy. In agreement with the immunostainings, cKOR+E synapses had normal bouton size and synaptic vesicle numbers (Figs. 2A, B). At cKOR+E synapses, we observed a massive, 92% reduction of docked vesicles (Fig. 2B). We repeated this analysis in glutaraldehyde fixed tissue (Figs. S2A-C) and saw a similarly robust 85% reduction of docked vesicles. This effect was much stronger than loss of RIMs alone, as assessed with glutaraldehyde fixed tissue (Kaeser et al., 2011). Furthermore, in cKOR+E neurons we observe a 79% loss of vesicles within 100 nm of the target membrane (which we refer to as tethered vesicles) using high pressure frozen tissue (Fig. 2C) and a similar reduction using glutaraldehyde fixed tissue (Fig. S2C). Knockout mutations for Munc13 and the SNAREs, SNAP-25 and Syntaxin-1 do not have a reduced number of docked vesicles using glutaraldehyde fixed tissue nor a reduction in tethered vesicles, but only the use of high-pressure freezing and/or tomography reveals their functions in tight vesicle docking (Augustin et al., 1999; Bronk et al., 2007; Imig et al., 2014; Siksou et al., 2009; de Wit et al., 2006). Thus, because the cKOR+E docking deficit is easily detected in glutaraldehyde fixed tissue and extends to distances up to 100 nm away from the target membrane, we conclude that these neurons have a very strong deficit in docking and tethering synaptic vesicles to the presynaptic plasma membrane, establishing a requirement for the active zone for these processes.
Figure 2. Disruption of the active zone leads to loss of synaptic vesicle docking but PSDs appear normal.
A, B. Sample images (A) and quantification (B) of synaptic morphology of high pressure frozen neurons analyzed by electron microscopy of cKOR+E and controlR+E synapses. For an identical analysis using glutaraldehyde fixed tissue, see Fig. S2 (controlR+E n = 50 synapses, cKOR+E n = 50).
C. Distribution of synaptic vesicles relative to the presynaptic plasma membrane area opposed to the PSD. Vesicle distribution is shown in 100 nm bins (left) in cKOR+E and controlR+E synapses. Gaussian fits were used to model the vesicle distribution. The two genotypes were significantly different (* p < 0.05) and could not be fit with a single distribution, requiring individual fits. Distribution of synaptic vesicles within the first 100 nm in 10 nm bins and the number of tethered vesicles (defined as vesicles within 100 nm of the presynaptic plasma membrane) are shown in the middle and on the right, respectively (controlR+E n = 50 synapses, cKOR+E n = 50).
D, E. Sample images (D) and quantification (E) of PSD protein synaptic fluorescence levels at cKOR+E and controlR+E synapses using confocal microscopy as described in Figs. 1B, C. The black dotted line indicates control levels (controlR+E n = 3 independent cultures, cKOR+E n = 3, 10 images per culture).
F, G. Quantitative Western blotting for PSD proteins using fluorescent secondary antibodies. Sample images (F) and quantification (G) of total postsynaptic protein levels in cKOR+E neurons normalized to protein levels in controlR+E neurons (black dotted line) are shown (controlR+E n = 3 independent cultures, cKOR+E n = 3).
All data are means ± SEM; ***p ≤ 0.001 as determined by Student's t test (B) or *p < 0.05 by extra sum of squares F test (C). All numerical data are in Table S2.
Because the active zone protein complex interacts through trans-synaptic protein complexes with the PSD (Südhof, 2012), it is possible that a strong disruption of the active zone affects the integrity of the PSD. In cKOR+E neurons, the length of the postsynaptic density was not affected (Figs. 2B, S2B), and levels and localization of PSD-95, N-methyl-D-aspartate (NMDA) receptors, and α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid (AMPA) receptors were not changed (Figs. 2D-G). Thus, we conclude that structural effects of RIM and ELKS deletion are largely restricted to the active zone.
Active zone disruption leads to strong reductions in release probability and presynaptic Ca2+influx
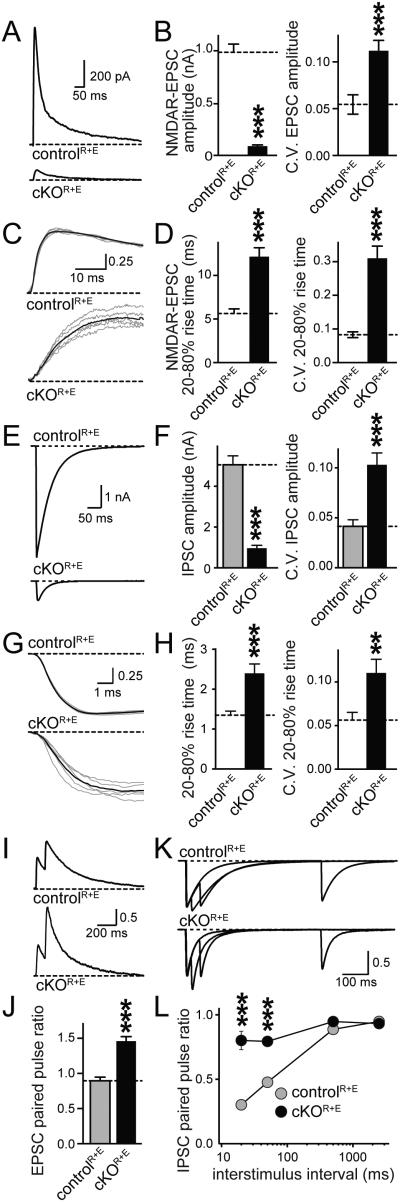
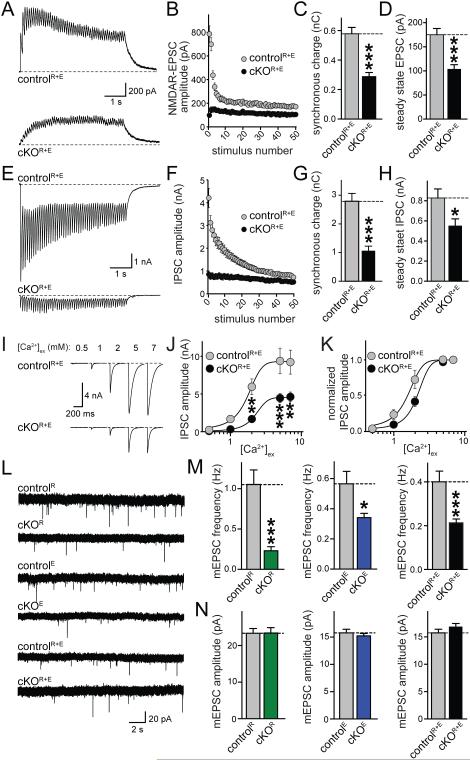
Given that individual active zone proteins such as RIM and Munc13 are essential for generating an RRP and for the Ca2+-mediated release of this pool (Augustin et al., 1999; Deng et al., 2011; Han et al., 2011; Kaeser et al., 2011; Varoqueaux et al., 2002), we hypothesized that massive disruption of the active zone in cKOR+E synapses leads to loss of neurotransmitter release. We monitored synaptic transmission electrophysiologically in cultured neurons and found that single action potential evoked excitatory or inhibitory postsynaptic currents (EPSCs or IPSCs, respectively) were strongly reduced (by 90% and 81%, respectively) but not abolished (Figs. 3A, B, E, F). The rise time of the synaptic response was slowed (Figs. 3C, D, G, H), and the variability in amplitude and rise time was strongly increased, suggesting increased asynchrony in cKOR+E synapses.
Figure 3.
Single action potential evoked synaptic transmission and release probability are strongly decreased upon disruption of the active zone
A, B. NMDAR-EPSCs were evoked by a focal stimulation electrode. Example traces (A) and quantitation of EPSC amplitudes (B) and their coefficient of variation (C.V.) in cKOR+E and controlR+E neurons are shown (controlR+E n = 24 cells/4 independent cultures, cKOR+E n = 26/4).
C, D. Sample traces (C) and quantitation (D) of EPSC rise times and their C.V. (n as in B). Individual sweeps are shown in grey and the average of all sweeps is shown in black. Traces are normalized to the average response.
E-H. Same analysis as in A-D for IPSCs (controlR+E n = 19/3, cKOR+E n = 19/3).
I,J. Analysis of NMDAR-EPSC paired pulse ratios (PPRs) in cKOR+E and controlR+E neurons. Sample traces (I, traces normalized to the first response) and quantitation (J) of the PPR at 100 ms interstimulus interval (controlR+E n = 23/4 independent cultures, cKOR+E n= 26/4).
K, L. Scaled sample traces (K, traces normalized to the first response) and summary data (L) of IPSC PPRs at variable interstimulus intervals (controlR+E n = 19/3, cKOR+E n = 19/3).
All data are means ± SEM; **p ≤ 0.01, ***p ≤ 0.001 as determined by Student's t test in A-H, or by two-way ANOVA in L (genotype, interstimulus interval, and interaction p ≤ 0.001, p values of post-hoc Holm-Sidak tests are shown). All numerical data are in Table S3.
We next stimulated the synapses with pairs of stimuli at closely spaced time intervals and calculated the paired-pulse ratios (PPRs). PPRs are inversely correlated with initial vesicular release probability P (Zucker and Regehr, 2002) and can be used as a relative measure of P when comparing genotypes. Consistent with a strong reduction in P, PPR was strongly increased at short interstimulus intervals at excitatory (Figs. 3I, J) and inhibitory synapses (Figs. 3K, L). This is reminiscent of the decrease in release probability observed in RIM knockout synapses (Kaeser et al., 2008, 2011; Schoch et al., 2002), which is caused by reduced tethering of presynaptic Ca2+ channels (Kaeser et al., 2011).
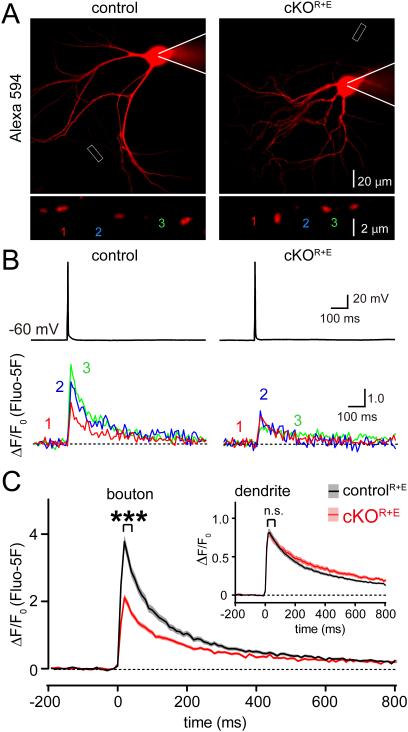
To test whether Ca2+ influx is reduced upon disruption of the active zone, we imaged single action potential evoked presynaptic Ca2+ transients (Figs. 4A-C). Briefly, individual neurons were patched and filled with an Alexa 594 dye to identify the axon and the presynaptic boutons, and with the Ca2+ indicator Fluo-5F that increases fluorescence upon Ca2+ binding (Fig. 4A). After dye filling, a brief somatic current injection was used to induce a single action potential, and Ca2+ transients were recorded in individual boutons and secondary dendrites (Fig. 4B). We found a 44% reduction in the peak amplitude of the Ca2+ influx in boutons, but dendritic Ca2+ transients remained unaffected (Fig. 4C). These data match well with the observation of a loss of CaV2.1 Ca2+ channels (Fig. 1C), with the strong reduction in vesicular release probability (Figs. 3I-L), and with the previously described roles for RIM and ELKS proteins in enhancing presynaptic Ca2+ influx in hippocampal neurons (Kaeser et al., 2011; Liu et al., 2014).
Figure 4. Impaired Ca2+ influx upon active zone disruption.
A. Sample images of cKOR+E and controlR+E neurons filled via patch pipette with Fluo-5F and Alexa 594 (red, top) and enlarged view of boutons (bottom) analyzed in B.
B. Somatic action potentials (top) and presynaptic Ca2+ transients imaged via Fluo-5F fluorescence (bottom) of the color coded boutons shown in A.
C. Summary plots of single action potential-induced Ca2+ transients in boutons, inset: same plot for dendrites. Data are shown as mean (line) ± SEM (shaded area). ***p < 0.001 for Ca2+ transients during the first 60 ms after the action potential as assessed by two-way ANOVA for genotype and time; interaction n.s. (boutons: controlR+E n = 202 boutons/16 cells/3 independent cultures, cKOR+E n = 157/13/3; dendrites: controlR+E n = 148 dendrites/16 cells/3 independent cultures, cKOR+E n = 100/13/3). All numerical data are in Table S4.
Persistence of release upon active zone disruption
We next stimulated the cKOR+E neurons with short action potential trains (50 stimuli at 10 Hz). Surprisingly, we detected a strong buildup of release at excitatory cKOR+E synapses starting with the second action potential, and the increase was sustained throughout the action potential train (Figs. 5A, B, S3A). Similarly, vesicles were released quite efficiently throughout an action potential train at inhibitory synapses (Figs. 5E, F, S3C). When we quantified the synchronous charge component throughout the train, we observed a reduction of 50% at excitatory cKOR+E synapses (Fig. 5C) and of 62% at inhibitory synapses (Fig. 5G). The total charge, the tonic component during the train, and delayed charge starting 100 ms after stimulation ended were affected to a similar extent (Figs. S3B, D). The steady state amplitude at the end of the train was reduced by 41% and 33%, for EPSCs and IPSCs, respectively (Figs. 5D, H). Thus, despite loss of docked vesicles, disruption of the active zone and a strong impairment of single action potential induced release, release persisted during trains of action potentials. This finding is consistent with a strong reduction in P, but suggests that an increase in P due to Ca2+ buildup during the stimulus train releases synaptic vesicles quite efficiently. This is reminiscent of a similar electrophysiological phenotype upon deletion of RIM-BP in D. melanogaster (Liu et al., 2011).
Figure 5. Release during action potential trains and mini release are sustained upon disruption of the active zone.
A-D. Sample traces (A) and quantitation of amplitudes (B), synchronous charge (C), and steady state EPSC amplitude (D, average of the last ten EPSCs) of NMDAR-EPSCs evoked by stimulation trains (10 Hz, 50 stimuli) in cKOR+E and controlR+E neurons (controlR+E n = 17/3, cKOR+E n = 18/3).
E-H. Analysis as in A-D but for IPSCs evoked by stimulation trains (10 Hz, 50 stimuli, controlR+E n = 19/3, cKOR+E n = 19/3).
I-K. Example traces (I) of action-potential evoked IPSCs at [Ca2+]ex of 0.5, 1, 2, 5, and 7 mM in cKOR+E and controlR+E neurons. Absolute IPSC amplitudes (J) and amplitudes normalized to the response at 7 mM [Ca2+]ex (K) are shown (controlR+E n = 8/3, cKOR+E n = 8/3).
L-N. Recordings of mEPSCs in synapses lacking RIM (cKOR), ELKS (cKOE), or both (cKOR+E). In each experiment, control neurons are identical to the respective cKO neurons except that the cre lentivirus is inactive in the control neurons. Sample traces (L) and quantitative analysis of mEPSC frequencies (M) and amplitudes (N) are shown (controlR n = 20/3, cKOR n = 21/3; controlE n = 25/3, cKOE n = 24/3; controlR+E n = 32/5, cKOR+E n = 31/5). For expanded mEPSC traces and mIPSC data, see Fig. S3.
All data are means ± SEM; * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 as determined by Student's t test (A-H, L-N) or two-way ANOVA in J (genotype: ***p < 0.001; [Ca2+]ex: ***p < 0.001, interaction: *p ≤ 0.05; p values of post-hoc Holm-Sidak tests are shown in J). For analyses of release components during trains, see Figs. S3A-D. All numerical data are in Table S5.
We next varied the extracellular Ca2+ concentration ([Ca2+]ex) and measured the IPSC amplitude. Remarkably, despite the reduction of presynaptic Ca2+ channels and Ca2+ influx, increasing [Ca2+]ex from 2 mM to 5 mM strongly enhanced the IPSC in cKOR+E neurons by a factor of 2.7 to 4.5 nA (Figs. 5I, J). In both conditions, release saturated above 5 mM [Ca2+]ex, likely because Ca2+ influx itself saturates at 5 mM (Ariel and Ryan, 2010). When we expressed these data normalized to the largest response (Fig. 5K), we observed a right-shift in the dependence of release on [Ca2+]ex (EC50 values as obtained through fitting each cell: controlR+E EC50 = 1.55 ± 0.238, cKOR+E EC50 = 2.33 ± 0.197, *, p < 0.05), confirming a reduction in P. Thus, the active zone per se is not required for fusion of synaptic vesicles, and when P is increased, for example during action potential trains or by increasing [Ca2+]ex, vesicles can be quite efficiently released. Because release is proportional to the product of RRP size and P, these data suggest that RRP vesicles remain in cKOR+E neurons, despite the loss of RIM, Munc13, and vesicle docking.
We next assessed the frequency and amplitude of miniature excitatory and inhibitory PSCs in the presence of tetrodotoxin (mEPSCs, mIPSCs, Figs. 5L-N, S3E-I) in cKOR+E synapses, and compared these data with synapses that either lack only RIM (cKOR) or ELKS (cKOE). Simultaneous removal of RIM and ELKS led to surprisingly mild, 47% and 49% reductions in mEPSC (Fig. 5M) and mIPSC (Fig. S3H) frequencies, respectively. The effect on spontaneous release after disruption of the active zone in cKOR+E synapses was comparable to the deletion of ELKS alone, and was milder than the reduction upon loss of RIM (78%, Figs. 5M, S3F) or Munc13 (Augustin et al., 1999; Varoqueaux et al., 2002) alone. Consistent with the normal architecture of the PSD (Figs. 2B, S2B), mini amplitudes were not affected. Direct comparison of the miniature frequencies in the three lines confirmed a statistically significantly stronger reduction of mini frequency in the synapses that lack only RIM compared to the synapses in which the entire active zone is disrupted (Fig. S3F).
Uniform disruption of synaptic composition and release in cKOR+Eneurons
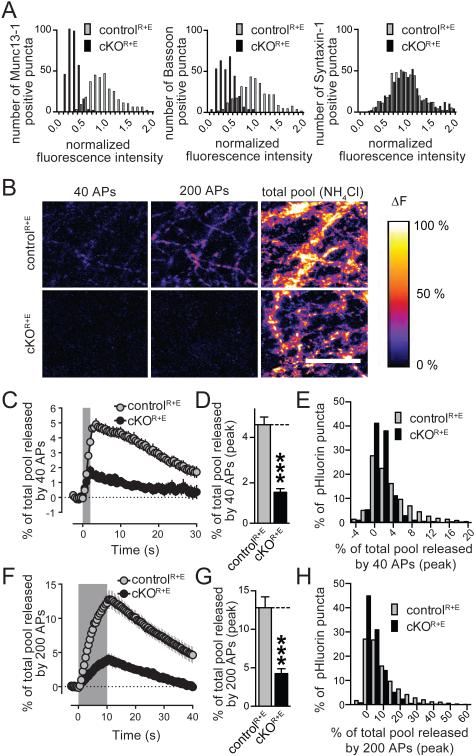
Thus far, our data reveal a strong reduction in release probability at cKOR+E synapses and show that mini release and release in response to stimulus trains is more mildly impaired than one would predict from the strong structural disruption of the active zone. This suggests that fusion competent vesicles are present despite loss of Munc13, RIM, and vesicle docking. An alternative explanation is that only a subset of synapses is affected in cKOR+E neurons, and that a population of near normal synapses confounds our analysis. Such heterogeneity could be due to the presence of different neuronal subtypes in our cultures and could arise at the molecular or functional level. We first excluded that the heterogeneity is derived from a population of cells that does not express cre recombinase. Consistent with the analysis of protein levels (Figs. 1E), all cells expressed cre (Figs. S4A, B). We then tested whether the active zone components of synapses showed a distribution consistent with heterogeneity. We plotted the data presented in Figs. 1B, 1C and S1B, G as a frequency distribution of the fluorescence intensity. Peaks for Munc13, Bassoon (Fig. 6A), Piccolo, and RIM-BP (Fig. S4C) shifted uniformly to lower intensities. These data argue against strong molecular heterogeneity upon active zone disruption.
Figure 6. Uniform disruption of active zone composition and function in cKOR+E neurons.
A. Histograms of the distribution of fluorescence intensity levels in cKOR+E and controlR+E synapses (normalized to the average fluorescence in control). Data are from the experiments shown in Figs. 1B, C. For histograms of RIM, ELKS, Piccolo, RIM-BP2, Liprin-α3, and PSD-95 see Fig. S4C.
B. Pseudocolored images of SypHy expressing cultures stimulated with 40 and 200 action potentials (APs), and dequenched with NH4Cl. Images represent peak fluorescence change.
C-E. Quantification of fluorescence changes in cKOR+E and controlR+E neurons stimulated with 40 APs, including the time course of the mean fluorescence change in active synapses as a percentage of the fluorescence increase upon NH4Cl application (C), the peak response (D) of active synapses, and frequency distribution of the % response in active synapses at the end of the stimulus train (E; controlR+E n = 3493 NH4Cl responsive synapses/ 2486 active synapses/ 9 coverslips/3 independent cultures, cKOR+E n = 2192/1272/9/3, the number of coverslips is used as a basis for statistics).
F-H. Quantification as in C-D but for neurons stimulated with 200 APs (controlR+E n = 3493/2640/9/3, cKOR+E n = 2291/1405/9/3).
All data are means ± SEM; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by Student's t test. All numerical data are in Table S6.
We next turned to presynaptic imaging in cultures expressing SypHy (Granseth et al., 2006), a version of synaptophysin coupled to an intravesicular pHluorin tag. In brief, neuronal cultures were infected with two independent lentiviruses expressing SypHy (for imaging exocytosis) and SV2-TdTomato (to identify synapse-rich areas in the cultures) at DIV3 in addition to the cre and control lentiviruses (which were supplied at DIV5). At DIV15-18, a synapse dense area was chosen based on SV2-TdTomato expression, and the neurons were stimulated with a focal stimulation electrode for 40 or 200 action potentials at 20 Hz. In this experiment, exocytosis is identified as an increase in fluorescence due to unquenching of the pHluorin when it is exposed to the neutral extracellular pH. For the analysis, only puncta that showed at least a 2-fold increase in fluorescence upon application of NH4Cl (which uniformly raised the intravesiclular pH to unquench pHluorin fluorescence) were included. We first determined the fraction of synapses responsive to electrical stimulation at both frequencies, and observed a small but significant decrease in active synapses in the cKOR+E neurons (Fig. S4F). We next characterized release at active synapses in both genotypes and found a 67% and 68% decrease in the peak response at the end of stimulation at 40 or 200 action potentials, respectively (Figs. 6B-H). When we plotted a frequency histogram of the % of the total pool released at the end of the stimulus train for all active synapses, there was a prominent increase in synapses with smaller pHluorin fluorescence changes in cKOR+E synapses. This experiment supports that active synapses in cKOR+E neurons have impaired release and establishes that the secretory deficit cannot be explained by inactive synapses only. Furthermore, it establishes that vesicle recycling, which contributes strongly to the response to 200 action potentials, is reduced in the cKOR+E synapses. These experiments exclude that a large fraction of synapses has normal active zones and operates at essentially normal levels.
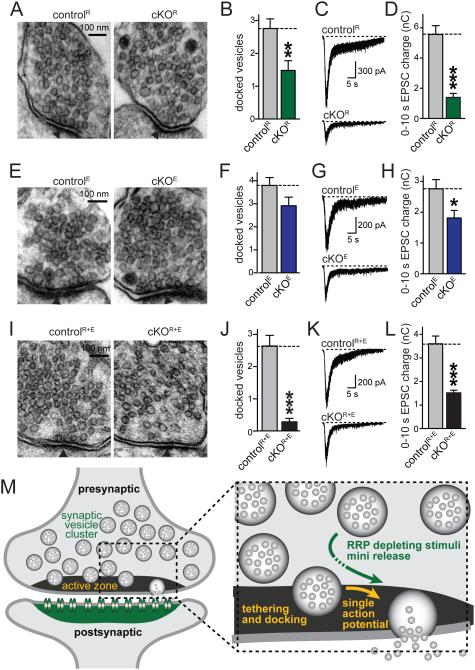
Comparison of docking and RRP size upon active zone disruption
Our data thus far suggest that there may be a sizable RRP left in cKOR+E synapses even though such a pool is thought to be reflected in docked vesicles at hippocampal synapses (Imig et al., 2014; Rosenmund and Stevens, 1996; Schikorski and Stevens, 2001). We measured the RRP at excitatory synapses using the application of 500 mM hypertonic sucrose and compared the RRP with vesicle docking and distribution at cKOR, cKOE, and cKOR+E synapses (Fig. 7). RIM deletion resulted in a 46% reduction in docked synaptic vesicles paralleled by a 75% reduction in the RRP, but the distribution of vesicles within a nerve terminal was normal (Figs. 7A-D, S5A, B). Removal of ELKS alone did not result in a detectable effect on vesicle docking or vesicle distribution (albeit there was a non-significant trend towards a small reduction in docked vesicles), but induced a 34% reduction in RRP at excitatory synapses (Figs.7E-H, S5C, D). At cKOR+E synapses the RRP was more mildly affected (Figs. I-L) than one would predict from the effects observed in cKOR or cKOE synapses, from the loss of docking, and from the massive reduction in RIM, Munc13, and other active zone proteins (Fig. 1). 42% of RRP vesicles remained despite the strong reduction in vesicle docking (92% in high pressure frozen tissue, Fig. 2B; 89% in glutaraldehyde fixed tissue, Fig. 7J). Direct comparison of the three genotypes revealed a significantly stronger loss of docking in cKOR+E synapses compared to cKOR synapses. Conversely, RRP was more strongly reduced in cKOR synapses than in cKOR+E or cKOE synapses (Figs. S5G, H). These data suggest that at least some RRP vesicles can be recruited over distance and do not have to be stably docked at the active zone before the application of hypertonic stimulus.
Figure 7. Persistence of a readily releasable pool upon loss of synaptic vesicle tethering and docking.
A-D. Sample images (A) and quantitative analyses of synaptic vesicle docking (B) in glutaraldehyde fixed neuronal cultures, and sample traces (C) and quantitation (D) of RRP of RIM deficient cKOR and corresponding controlR synapses. Focal application of hypertonic sucrose for 10 s was used to deplete the RRP (D).
E-H. Analyses as outlined in A-D, but of ELKS deficient cKOE and controlE synapses.
I-L. Analyses as outlined in A-D, but of RIM/ELKS deficient cKOR+E and controlR+E synapses.
For analyses of vesicle numbers, bouton size, PSD length, and vesicle distribution, see Figs. S5A-F. All data are means ± SEM; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by Student's t test (analysis of vesicle docking and tethering: controlR n = 25 synapses, cKOR n = 25; controlE n = 25, cKOE n = 25; controlR+E n = 25, cKOR+E n = 25; analysis of RRP: controlR n = 20 cells/3 independent cultures, cKOR n = 20/3; controlE n = 17/3, cKOE n = 17/3; controlR+E n = 20/3, cKOR+E n = 20/3). All numerical data are in Table S7.
M. Schematic of synaptic architecture and function upon disruption of the active zone. Structures and processes that are strongly disrupted upon RIM and ELKS deletion are labeled in yellow (active zone, docking, single action potential mediated release). Synaptic structures and functions that remain at least partially intact are labeled in green (the synaptic vesicle cluster, the postsynaptic density containing neurotransmitter receptors, mini release, and release in response RRP depleting stimuli such as action potential trains or hypertonic sucrose). Our experiments indicate that at least some vesicles can be recruited from vesicle pools distant from the presynaptic plasma membrane for release, and that these vesicles may be released immediately or undergo a transient docking state (dotted arrow) that is initiated after the onset of stimulation.
Discussion
We here establish a conditional mouse mutant that strongly and specifically disrupts the active zone matrix and synaptic vesicle docking in cultured hippocampal neurons (Figs. 1 and 2). We find that disruption of the active zone results in a strong impairment of vesicular release probability, but surprisingly >40% of RRP vesicles remained (Figs. 3 to 7).
Redundant scaffolding functions of RIM and ELKS
The multi-domain structure of RIM and ELKS and their extensive biochemical binding activities with other active zone proteins suggested that they operate as scaffolds (Ohtsuka et al., 2002; Schoch et al., 2002; Takao-Rikitsu et al., 2004; Wang et al., 2002). However, loss of function approaches thus far provided mixed support for this hypothesis. Knockout mutants for ELKS1 and/or ELKS2 showed no changes in active zone composition at hippocampal synapses (Held et al., 2016; Liu et al., 2014), except for a small increase in the biochemical solubility of RIM (Kaeser et al., 2009). Similarly, RIM1 and/or RIM2 knockout mice revealed isolated changes in the clustering and levels of Munc13-1 (Deng et al., 2011; Schoch et al., 2002). Beyond these changes in individual active zone proteins however, the active zone protein complex was intact. Here, we reveal a strong, redundant scaffolding role for RIM and ELKS: simultaneous removal leads to disruption of the active zone with a loss of three out of four additional active zone protein families. Our morphological and functional analyses further strongly support redundant scaffolding roles for RIM and ELKS that are similar at excitatory and inhibitory synapses. Thus, we establish an important presynaptic clustering role for RIM and ELKS that is shared across synapses and that tethers Piccolo, Bassoon, Munc13-1, and RIM-BP2. Because these proteins cannot be anchored and maintained at normal levels at mutant synapses, RIM and ELKS are necessary and thus upstream for their tethering to the active zone.
Interestingly, levels and localization of Liprin-α2 and Liprin-α3, the two Liprin-α isoforms that are strongly expressed in brain and are thought to be localized at the active zone (Spangler et al., 2011; Zürner and Schoch, 2009; Zürner et al., 2011), are not affected in our mutants. This suggests that Liprin-α2/3 are either upstream in active zone assembly and can be tethered independent of RIM and ELKS, or that Liprin-α2/3 are not part of the same protein complex. Genetic experiments have firmly established presynaptic roles for Liprin-α/syd-2 in synapse assembly in C. elegans and D. melanogaster (Kaufmann et al., 2002; Zhen and Jin, 1999). Although the localization of individual vertebrate Liprin-α proteins has not been conclusively solved and the available data support pre-, post-, or extra-synaptic localization of Liprin-α proteins (Spangler et al., 2011; Wyszynski et al., 2002; Zürner et al., 2011), a recent study employed knockdowns for Liprin-α2 and supported presynaptic scaffolding functions (Spangler et al., 2013). In vitro binding of Liprin-α1 through 4 to RIM and/or ELKS (Ko et al., 2003; Schoch et al., 2002) provide further support for a presynaptic scaffolding role. One possible explanation for these and our data is that Liprin-α is upstream of RIM and ELKS in vertebrate active zone assembly. Importantly, invertebrate Liprin-α/syd-2 mutant synapses also have reduced vesicle numbers in the nerve terminal (Kaufmann et al., 2002; Patel et al., 2006; Zhen and Jin, 1999) suggesting that the active zone may recruit vesicles to a presynaptic nerve terminal. Our experiments at vertebrate synapses reveal that synaptic vesicle numbers are unchanged upon active zone disassembly, establishing that the active zone protein complex downstream of Liprin-α is not required for recruitment of vesicles to the nerve terminal. Our data are consistent with additional roles for Liprin-α outside of the active zone, for example in trafficking of vesicles or active zone material, as has been shown for D. melanogaster Liprin-α (Miller et al., 2005). Further genetic experiments will be necessary to dissect the roles of Liprin-α in the vertebrate brain.
Recent studies support that synaptic and network activity contribute to active zone protein turnover (Lazarevic et al., 2011; Sugie et al., 2015; Weyhersmüller et al., 2011). It is thus possible that loss of synaptic activity in the cultured neurons contributes to the strong active zone disruption that we observe upon RIM/ELKS deletion. However, reduced activity is unlikely to play an important causative role for active zone disruption in our experiments because knockouts for only RIM (Deng et al., 2011) or Munc13 (Varoqueaux et al., 2002), which have similar or more severe reductions in activity, do not lead to strong active zone disruption. In the long-term, it will be important to test causes and effects of the active zone disruption we describe here in a system that allows manipulation and characterization of a specific synapse in a defined circuit to better understand how cell-type specificity and activity contribute to the phenotypes.
The role of the active zone in synaptic vesicle docking
Because synaptic vesicles are only docked at the active zone (Couteaux and Pecot- Dechavassine, 1970; Imig et al., 2014; Siksou et al., 2009; Südhof, 2012), it has been proposed that the active zone provides the molecular mechanism for docking of synaptic vesicles to the target membrane. Consistent with this hypothesis, RIM1/2 double knockout synapses have an approximately 50% reduction in the number of docked vesicles in cultured hippocampal neurons in glutaraldehyde fixed tissue (Kaeser et al., 2011). Importantly, using the same method, no other presynaptic protein, including Munc13 (Augustin et al., 1999; Varoqueaux et al., 2002), synaptobrevin-2 (Deak et al., 2004), SNAP-25 (Bronk et al., 2007), or CAPS (Jockusch et al., 2007) have a role in synaptic vesicle docking. Compellingly, disruption of the active zone in the cKOR+E neurons leads to a near complete loss of vesicle docking in glutaraldehyde fixed tissue (Figs. S2B, 7J). Recent experiments have used high-pressure freezing and tomography, which improved the resolution in the analysis of docking to two nanometers. Using this method, it has been found that loss of function mutations for Munc13, CAPS, and the SNAREs syntaxin-1, synaptobrevin-2, and SNAP-25 have a strong reduction in synaptic vesicles within 0-5 nm of the target membrane, but normal or increased vesicle numbers in bins at 10 and 20 nm away from the active zone (Imig et al., 2014; Siksou et al., 2009). The reduction of docked vesicles at cKOR+E synapses is apparent with both fixation methods. With high-pressure freezing, the 92% reduction at cKOR+E synapses (Fig. 2A-C) is similar to Munc13 null mutants, which have an 96% reduction in docked vesicles when using the same method combined with electron tomography (Table 1 in (Imig et al., 2014)). Furthermore, unlike Munc13 deficient synapses, cKOR+E synapses fail to accumulate vesicles 10-20 nm away from the target membrane, but show a 75% reduction in numbers of tethered vesicles within 100 nm of the target membrane. Thus, we conclude that the loss of docking and tethering of synaptic vesicles in the cKOR+E mutant is stronger than in previous mutants because the loss of docking is readily detected in glutaraldehyde fixed tissue and there is a shift of the entire vesicle cluster away from the target membrane that has not been seen in other mutants. We conclude that the active zone is required for synaptic vesicle docking and tethering.
The relationship between synaptic vesicle docking, priming, and release
The strong decrease in single action potential mediated release (Fig. 3) corresponds well with the loss of vesicle docking (Fig. 2) and is consistent with the hypothesis that single action potentials release docked vesicles (Rosenmund and Stevens, 1996). Release from a single synapse is proportional to the RRP size and P (Zucker and Regehr, 2002). Our analysis revealed a strong reduction in P upon disruption of the active zone (Figs. 3I-L, 4, 5I-K). This observation is supported by a strong increase in PPRs, a right-shift in the [Ca2+]ex dependence of release, an increase in C.V. of the PSC amplitude, and a loss of presynaptic CaV2.1 Ca2+ channels and Ca2+ influx. Surprisingly, manipulations that enhanced P (increasing [Ca2+]ex and action potential trains) or bypassed the need for Ca2+ (stimulation with hypertonic sucrose) demonstrated that a significant pool of vesicles is available for release in cKOR+E synapses.
Many studies have defined the RRP as vesicles that are either released during the transient response to hypertonic sucrose (Augustin et al., 1999; Deng et al., 2011; Rosenmund and Stevens, 1996; Varoqueaux et al., 2002) or during brief trains of action potentials (Schikorski and Stevens, 2001) and RRP size estimates determined by these methods are well correlated with the number of morphologically docked vesicles. Using hypertonic sucrose in cKOR+E neurons, 42% of the RRP remained (Fig. 7L), which is significantly larger than the RRP left after deletion of RIM (Fig. S5H) or Munc13 (Augustin et al., 1999; Varoqueaux et al., 2002) despite the more severe docking deficit at cKOR+E synapses. This challenges the notion that the priming functions of these proteins are identical to their functions in vesicle docking. Furthermore, spontaneous miniature release (Figs. 5L-N, S3G-I) and release during action potential trains (Figs. 5A-H, 6C-H) are also more mildly reduced than expected. Due to the strong reduction in P at cKOR+E synapses, it was not possible to measure RRP size using high frequency action potential stimulation (Thanawala and Regehr, 2016). Nevertheless these data reveal that fusion competent vesicles can be recruited over distance and do not require a persistently docked state. RRP vesicles may therefore be stored away from the presynaptic plasma membrane, at least in the absence of an active zone, as has been proposed based on experiments that labeled RRP vesicles after recycling (Rizzoli and Betz, 2004).
It is possible that hypertonic sucrose stimulation leads to a transient increase in vesicle docking that is not captured in our electron-microscopic images. This may also be the case for some of our other manipulations, for example high [Ca2+]ex or prolonged stimulus trains. The small amount of remaining Munc13 in the cKOR+E neurons may rapidly add vesicles to the RRP, but this hypothesis implies that Munc13 dependent priming in the cKOR+E neurons occurs upstream or simultaneous to contact with the target membrane and is transient, since there are no stably docked vesicles. The relatively mild reduction in mini frequency supports that docking in cKOR+E neurons is transient and not a stable state of a primed vesicle. Alternatively, massive disruption of the active zone may bypass the need for Munc13 to prime vesicles before fusion.
A long-standing question has been whether the partial assembly of SNARE complexes is required for synaptic vesicle docking and priming (Jahn and Fasshauer, 2012; Sudhof and Rothman, 2009). Our experiments reveal that SNARE proteins are present in the nerve terminal upon disruption of the active zone, and that synaptic vesicle fusion, which is mediated by SNARE proteins, is not abolished. However, SNARE proteins are not sufficient to drive docking at synapses in the absence of an active zone, suggesting that not all fusion competent vesicles require stable preassembly of SNARE complexes. Finally, the molecular mechanisms that underlie docking upstream of SNARE complex assembly are poorly understood. With a gene mutation that disrupts docking, but leaves synaptogenesis and presynaptic vesicle clustering intact, analysis of the minimal protein interactions between synaptic vesicles and release sites required for docking will now be possible.
Experimental Procedures
The quadruple homozygote floxed mice for RIM1αβ, RIM2αβγ, ELKS1α and ELKS2α were generated by crossing single conditional knockout mice (Kaeser et al., 2008, 2009, 2011; Liu et al., 2014). All experiments were performed in cultured hippocampal neurons infected at DIV 5 with lentiviruses expressing cre recombinase or an inactive mutant of cre under a synapsin promoter, and experiments were performed at DIV 15-19. Biochemical, confocal, electron microscopic and electrophysiological analyses were performed as described (Deng et al., 2011; Kaeser et al., 2008, 2011). Quantitative Western blotting was performed using fluorescently tagged secondary antibodies. Electrophysiological recordings were done in whole cell patch clamp configuration, and action potentials were triggered by a focal stimulation electrode. For pHluorin imaging, the neurons were infected with lentiviruses expressing SypHy and SV2-TdTomato at DIV 3 in addition to the cre and control lentiviruses supplied at DIV 5. Experiments were performed and analyzed by an experimenter blind to the genotype and significance was determined using Student’s t-tests unless otherwise noted. Detailed descriptions of the methods are provided in the supplemental materials.
Supplementary Material
Highlights.
Deletion of RIM and ELKS leads to loss of presynaptic Munc13, Bassoon and RIM-BP.
This active zone disruption results in loss of synaptic vesicle docking.
Synaptic vesicle clustering and the PSD remain intact after active zone disruption.
Fusion competent vesicles persist upon disruption of the active zone and docking.
Acknowledgments
We thank J. Wang and L. Bickford for technical support, Drs. N. Brose, S. Schoch and T. Südhof for antibodies, Drs. M. Verhage and J. Broeke for providing a Matlab template to analyze electron microscopic images, Dr. A. de Jong for writing a Matlab template to analyze pixel-to-pixel correlation, and Drs. W. Regehr, Z. Pang and A. de Jong for comments on the manuscript. This work was supported by a National Science Foundation graduate research fellowship (DGE1144152 to S.H. W.), grants from the NIH (F31NS089077 to R.G.H. and R01NS083898 to P.S.K.), the Nancy Lurie Marks Foundation, the Brain Research Foundation, the Harvard Brain Initiative and the Lefler Foundation. We also acknowledge the Neurobiology Imaging Facility (supported by a P30 Core Center Grant NS072030) and the Electron Microscopy Facility at Harvard Medical School.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
SHW, RGH, and PSK designed research, performed experiments, and wrote the manuscript. MYW, CL, and AK designed and performed experiments and provided feedback on the manuscript. PSK supervised research.
References
- Acuna C, Liu X, Gonzalez A, Südhof TC. RIM-BPs Mediate Tight Coupling of Action Potentials to Ca2+-Triggered Neurotransmitter Release. Neuron. 2015;87:1234–1247. doi: 10.1016/j.neuron.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J. Biol. Chem. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- Ariel P, Ryan TA. Optical mapping of release properties in synapses. Front. Neural Circuits. 2010;4:1–10. doi: 10.3389/fncir.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Wu D, Burré J, Malenka RC, Liu X, Südhof TC. Synaptotagmin-1 and -7 Are Redundantly Essential for Maintaining the Capacity of the Readily-Releasable Pool of Synaptic Vesicles. PLOS Biol. 2015;13:e1002267. doi: 10.1371/journal.pbio.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Boyken J, Grønborg M, Riedel D, Urlaub H, Jahn R, Chua JJ. Molecular Profiling of Synaptic Vesicle Docking Sites Reveals Novel Proteins but Few Differences between Glutamatergic and GABAergic Synapses. Neuron. 2013;78:285–297. doi: 10.1016/j.neuron.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Bronk P, Deák F, Wilson MC, Liu X, Südhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J. Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- Couteaux R, Pecot-Dechavassine M. [Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction] C R Acad Sci Hebd Seances Acad Sci D. 1970;271:2346–2349. [PubMed] [Google Scholar]
- Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, Jin Y. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 2006;9:1479–1487. doi: 10.1038/nn1808. [DOI] [PubMed] [Google Scholar]
- Davydova D, Marini C, King C, Klueva J, Bischof F, Romorini S, Montenegro-Venegas C, Heine M, Schneider R, Schröder MS, et al. Bassoon specifically controls presynaptic P/Q-type Ca(2+) channels via RIM-binding protein. Neuron. 2014;82:181–194. doi: 10.1016/j.neuron.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Sudhof TC. RIM Proteins Activate Vesicle Priming by Reversing Autoinhibitory Homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmuller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM Determines Ca(2+) Channel Density and Vesicle Docking at the Presynaptic Active Zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held RG, Liu C, Kaeser PS. ELKS controls the pool of readily releasable vesicles at excitatory synapses through its N-terminal coiled-coil domains. Elife. 2016;5:e14862. doi: 10.7554/eLife.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat. Neurosci. 2012;15:988–997. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig C, Min SW, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee JS, Brose N, Cooper BH. The Morphological and Molecular Nature of Synaptic Vesicle Priming at Presynaptic Active Zones. Neuron. 2014;84:416–431. doi: 10.1016/j.neuron.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch WJ, Speidel D, Sigler A, Sørensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014;76:333–363. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Chiu CQ, Deng L, Castillo PE, Sudhof TC. RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J. Neurosci. 2008;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Chavez AE, Liu X, Castillo PE, Sudhof TC. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64:227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Fan M, Sudhof TC. RIM genes differentially contribute to organizing presynaptic release sites. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11830–11835. doi: 10.1073/pnas.1209318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. (80-. ). [DOI] [PubMed] [Google Scholar]
- Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-alpha family of multidomain proteins. J. Biol. Chem. 2003;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. A post-docking role for active zone protein Rim. Nat. Neurosci. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Schone C, Heine M, Gundelfinger ED, Fejtova A. Extensive remodeling of the presynaptic cytomatrix upon homeostatic adaptation to network activity silencing. J. Neurosci. 2011;31:10189–10200. doi: 10.1523/JNEUROSCI.2088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Bickford LS, Held RG, Nyitrai H, Sudhof TC, Kaeser PS. The Active Zone Protein Family ELKS Supports Ca2+ Influx at Nerve Terminals of Inhibitory Hippocampal Neurons. J. Neurosci. 2014;34:12289–12303. doi: 10.1523/JNEUROSCI.0999-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KSY, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, et al. RIM-Binding Protein, a Central Part of the Active Zone, Is Essential for Neurotransmitter Release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. (80-. ). [DOI] [PubMed] [Google Scholar]
- Lu J, Machius M, Dulubova I, Dai H, Sudhof TC, Tomchick DR, Rizo J. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch. PLoS Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van Vactor D. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr. Biol. 2005;15:684–689. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Morciano M, Beckhaus T, Karas M, Zimmermann H, Volknandt W. The proteome of the presynaptic active zone: From docked synaptic vesicles to adhesion molecules and maxi-channels. J. Neurochem. 2009;108:662–675. doi: 10.1111/j.1471-4159.2008.05824.x. [DOI] [PubMed] [Google Scholar]
- Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Liu KSY, Sigrist SJ, Davis GW. RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool. J. Neurosci. 2012;32:16574–16585. doi: 10.1523/JNEUROSCI.0981-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H, et al. CAST: A novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and Munc13-1. J. Cell Biol. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, Marty S. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur. J. Neurosci. 2009;30:49–56. doi: 10.1111/j.1460-9568.2009.06811.x. [DOI] [PubMed] [Google Scholar]
- Spangler SA, Jaarsma D, De Graaff E, Wulf PS, Akhmanova A, Hoogenraad CC. Differential expression of liprin-alpha family proteins in the brain suggests functional diversification. J. Comp. Neurol. 2011;519:3040–3060. doi: 10.1002/cne.22665. [DOI] [PubMed] [Google Scholar]
- Spangler SA, Schmitz SK, Kevenaar JT, de Graaff E, de Wit H, Demmers J, Toonen RF, Hoogenraad CC. Liprin-alpha2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. J. Cell Biol. 2013;201:915–928. doi: 10.1083/jcb.201301011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. (80-. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The Presynaptic Active Zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie A, Hakeda-Suzuki S, Suzuki E, Silies M, Shimozono M, Möhl C, Suzuki T, Tavosanis G. Molecular Remodeling of the Presynaptic Active Zone of Drosophila Photoreceptors via Activity-Dependent Feedback. Neuron. 2015:1–15. doi: 10.1016/j.neuron.2015.03.046. [DOI] [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J. Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawala MS, Regehr WG. Determining Synaptic Parameters Using High-Frequency Activation. J. Neurosci. Methods. 2016 doi: 10.1016/j.jneumeth.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Sudhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl-Kielczynski B, Rosenmund C, Jorgensen EM. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 2013;504:242–247. doi: 10.1038/nature12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J. Neurosci. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Cornelisse LN, Toonen RFG, Verhage M. Docking of secretory vesicles is syntaxin dependent. PLoS One. 2006;1:e126. doi: 10.1371/journal.pone.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- Zürner M, Schoch S. The mouse and human Liprin-alpha family of scaffolding proteins: genomic organization, expression profiling and regulation by alternative splicing. Genomics. 2009;93:243–253. doi: 10.1016/j.ygeno.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Zürner M, Mittelstaedt T, tom Dieck S, Becker A, Schoch S. Analyses of the spatiotemporal expression and subcellular localization of liprin-α proteins. J. Comp. Neurol. 2011;519:3019–3039. doi: 10.1002/cne.22664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.