Abstract
Carney Complex (CNC) is a multiple neoplasia syndrome that is caused mostly by PRKAR1A mutations. Cardiac myxomas are the leading cause of mortality in CNC patients who, in addition, often develop growth hormone (GH) excess. We studied patients with CNC who were observed for over a period of 20 years (1995–2015) for the development of both GH excess and cardiac myxomas. GH secretion was evaluated by standard testing; dedicated cardiovascular imaging was used to detect cardiac abnormalities. Four excised cardiac myxomas were tested for expression of insulin-like growth factor-1 (IGF-1). A total of 99 CNC patients (97 with a PRKAR1A mutation) were included in the study with a mean age of 25.8 ± 16.6 years at presentation. Over an observed follow-up mean of 25.8 years, 60% of patients with GH excess (n=46) developed a cardiac myxoma compared to only 36% of those without GH excess (n=54) (p=0.016). Patients with GH excess were also overall more likely to have a tumor versus those with normal GH secretion (OR=2.78, 95% CI: 1.23–6.29; p=0.014). IGF-1 mRNA and protein were higher in CNC myxomas than in normal heart tissue. We conclude that the development of cardiac myxomas in CNC may be associated with increased GH secretion, in a manner analogous to the association between fibrous dysplasia and GH excess in McCune Albright syndrome, a condition similar to CNC. We speculate that treatment of GH excess in patients with CNC may reduce the likelihood of cardiac myxoma formation and/or recurrence of this tumor.
Keywords: tumor, acromegaly, growth hormone, insulin-like growth factor-I (IGF-I), myxoma
Introduction
Carney Complex (CNC) is a multiple neoplasia syndrome that affects several organs, including the endocrine glands, heart, and skin, and is caused mostly by mutations in the PRKAR1A gene, which codes for the regulatory sub-unit type 1α of the cyclic AMP-dependent protein kinase A.(Stratakis, et al. 2001) The most common endocrine tumors in CNC are primary pigmented nodular adrenocortical disease (PPNAD) and growth hormone (GH) and prolactin–producing pituitary adenomas. Antecedent somatotrope hyperplasia without overt adenoma is a common finding, whereas frank acromegaly is seen in only about 15% of the patients.(Pack, et al. 2000; Stratakis, et al. 1996; Watson, et al. 2000) Thus, most patients with CNC exhibit modest GH excess due to dysregulation of GH secretion starting in the second decade of life, but they only rarely progress to acromegaly.(Pack et al. 2000; Stergiopoulos, et al. 2004)
In the heart, CNC is associated with the development of myxomas. These tumors are of particular concern due to the potential for thrombus development, embolization, as well as hemodynamically significant mechanical obstruction. Of additional concern is the recurrent nature of the cardiac myxomas. The operative risk of repeat cardiothoracic surgery increases as patients undergo additional surgeries with the potential for the formation of chest wall adhesions and a modified anatomy complicating cardiac access.(DeLeon, et al. 1986; Roselli 2011; Russell, et al. 1998) Cardiac myxomas are the leading cause of disease-associated death among patients with CNC, as well as a source of significant morbidity; serial imaging of the heart is currently recommended at least annually for the early detection of primary and recurrent tumors.(Stratakis et al. 2001)
A number of studies have suggested that both GH and insulin-like growth factor 1 (IGF-1), such as in patients with acromegaly, may play a role in increasing the risk for certain cancers.(Baris, et al. 2002; Jenkins, et al. 1999; Jenkins and Besser 2001; Orme, et al. 1998; Ron, et al. 1991) An analysis of almost 700 patients who underwent prospective colonoscopic screening gave an overall prevalence of colon cancer at 3.7% among patients with acromegaly (relative risk 13.4 compared with a rate of 0.5% among control subjects).(Jenkins and Besser 2001) Epidemiological studies demonstrated an association between serum IGF-1 levels and risk of colorectal cancer.(Ma, et al. 2000; Manousos, et al. 1999; Renehan, et al. 2004) In the two largest series, comprising more than 400 patients with acromegaly, almost identical prevalence for colonic adenoma and colonic carcinoma were recorded: 23–24% of patients having a colonic adenoma and 4.3–4.5% having cancer.(Jenkins, et al. 1997; Terzolo, et al. 2005) A large retrospective cohort study has shown a 2.5-fold increase in mortality from colon cancer in acromegaly versus the general population of the United Kingdom.(Orme et al. 1998) Also, in patients who received GH therapy in childhood for various GH deficiency states, there has been the suggestion for an increased risk for certain tumors.(Giovannucci and Pollak 2002; Raman, et al. 2015) Other studies reported increased height at a young age as a risk factor for development of malignancy at a later date.(Gunnell, et al. 2001; Vatten and Kvinnsland 1990) While these findings have raised speculation as to the possible association between GH and an increased risk for certain tumors, there have been no data to prove a cause and effect relationship.(Jenkins, et al. 2006)
At the National Institutes of Health (NIH) Clinical Research Center (CRC), a cohort of patients with CNC has been followed since 1995.(Stratakis et al. 1996; Stratakis et al. 2001) Serial cardiac imaging has been performed over the last 20 years for a subset of these patients; these patients have had frequent screening of their GH. We undertook the present study to investigate the possible association between increases in GH secretion and the development of heart tumors in patients with CNC. The data show a link between the two components of the complex; this has significant implications for the care of patients with CNC, and in addition, for the field of GH research.
Materials and methods
Clinical Studies
The research protocol was approved by the National Institute of Child Health and Human Development (NICHD) Institutional Review Board. All participants gave written informed consent for clinical trial NCT00001452. Patients were enrolled and screened in this natural history study from 1995 to the current time. Detailed family and medical histories were obtained, and the patients underwent extensive laboratory and imaging evaluations. Data from 269 patients evaluated at the NIH Clinical center for potential CNC were retrospectively reviewed. Only patients who fulfilled the criteria for the diagnosis of CNC1 and had investigations of their GH secretion and heart imaging at the NIH CRC were included in the current study.
Echocardiography was performed in all patients, and cardiovascular magnetic resonance imaging (MRI) in a subset, as part of the patients’ initial assessment and routine follow-up. In all patients, GH and IGF-1 levels were measured. All patients underwent screening in order to detect possible GH excess. This was achieved by measuring insulin-like growth factor-1 (IGF-1), GH levels after oral glucose tolerance test (OGTT) (n=74) and 24-hour GH levels (n=22). In a subset of patients, GH response to thyrotropin-releasing hormone (TRH) test was measured (n=13). For the evaluation of IGF-1, assay-specific age and gender matched normal range levels were considered. The assay for GH changed over the duration of this study as follows: From 1995- Jan 1999 double antibody radioimmunoassay technique, from Jan 1999 –July 2012 Chemiluminescence immunoassay on Immulite 2000 analyzer, from July 2012- present Chemiluminescence immunoassay on Siemens Immulite 2000 XPI. A mean GH level of 2.5 μg/L and above (Ho and Weissberger 1994; Tzanela 2006) indicated normal GH secretion. Regarding GH levels after OGTT and GH response to TRH test, nadir GH level above 1 μg/L and a two-fold increase of GH levels was used as an indication for GH excess, respectively.(Chin, et al. 2013; Kageyama, et al. 2005; Tzanela 2006)
Tumor Studies
IGF-1 mRNA and protein expression were tested in samples of cardiac myxomas. Four intraoperative specimens of myxomas from patients with CNC were frozen in liquid nitrogen after excision. The tumors were defrosted and lysed using a BulletBlender homogenizer (Next Advance, Averill Park, NY) in the appropriate buffer (below).
RNA was isolated with the RNEasy kit (Qiagen, Venlo, Netherlands) including on-column DNAse I treatment. 500 ng of RNA were reverse transcribed using Superscript III (Life Technologies, Carlsbad, CA) and cDNA levels were quantified in triplicate by real-time quantitative PCR (RT-qPCR) using TaqMan gene expression assays for IGF-1 along with GAPDH on a Viia Real-Time OCR system (all from Life Technologies) according to manufacturer‘s instructions. Normal human heart RNA pooled from 3 donors was obtained from Clontech (Mountain View, CA).
To study protein expression, myxomas were lysed in T-PER buffer (Thermo Scientific, Waltham, MA) containing protease inhibitors (Complete, Roche, Basel, Switzerland). Protein concentrations were determined by BCA protein assay (Thermo Scientific). Normal human heart protein medley was obtained from Clontech. 10 μg of lysates were subjected to SDS-PAGE and Western Blotting. Blots were probed using α-IGF1 (insulin-like growth factor 1) antibody (Novus, Littleton, CO) followed by α-GAPDH (glyceraldehyde 3-phosphate dehydrogenase) antibody (Santa Cruz Biotechnology, Dallas, TX) and HRP-conjugated secondary antibodies (Jackson Immuno Research, West Grove, PA). Visualization was achieved by enhanced chemiluminescence on a gel imager (Bio-Rad, Hercules, CA).
Statistical methods
Data were described by frequency distributions and percents or by mean ± standard deviation. Categorical data were compared by Fisher’s exact tests or by the Jonckheere-Terpstra test for doubly-ordered data. Logistic regression modeling tested the relation between GH excess and cardiac myxomas or their recurrence, adjusting for covariates as appropriate. Time to penetrance [development of event (first cardiac myxoma)] was estimated using the Kaplan-Meier survival function and is described as median [95% Confidence Intervals (CI)]; differences in survival distributions between GH excess groups were compared using the log-rank test. P-values less than 0.05 and odds ratios (OR) with 95 % CI exclusive of 1.0 were considered statistically significant. Data analysis was carried out by SAS v. 9.2 (SAS Institute, Inc, Cary, NC) or stata v. 11.2 (StataCorp, College Station, TX).
Role of the Funding Source
The study was funded by intramural research support of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Clinical and genetic epidemiology
Ninety-nine patients fulfilled criteria for entry into study: 42 (42.4%) were male. The group was predominantly white (82.8%), with a mean age of 25.8 ± 16.6 years at initial evaluation at the NIH. Almost all (n = 97) patients had a PRKAR1A mutation. Table 1 provides a summary of findings in these patients.
Table 1.
Summary of clinical findings in the 99 study patients with Carney Complex (CNC) and cardiac myxomas.
| Manifestations of CNC | Number patients (%) |
|---|---|
| Spotty skin pigmentation (lentiginosis) | 84 (84.8%) |
| Cardiac myxoma | 47 (47.5%) |
| Cutaneous or mucosal myxoma | 25 (25.3%) |
| Breast myxomatosis | 15 (15.2%) |
| PPNAD* | 61 (61.6%) |
| Acromegaly | 18 (18.2%) |
| LCCST** or calcification on testicular U/S*** | 27 (27.3%) |
| Thyroid carcinoma or multiple hypoechoic nodules | 36 (36.4%) |
| Psammomatous melanotic schwannoma | 11 (11.1%) |
| Blue nevus | 42 (42.4%) |
| Breast ductal adenoma | 2 (2.0%) |
| Osteochondromyxoma | 2 (2.0%) |
Primary pigmented nodular adrenocortical disease
Large cell calcifying Sertoli cell tumor
U/S = ultrasound
GH excess and cardiac myxomas
Forty-six (46.5%) of the 99 patients had GH excess by laboratory assessment. Thirty patients had elevated nadir GH levels after OGTT, and 27 presented with increased IGF-1 levels, matched for age and gender. In patients that were not evaluated recently for GH excess, 10 presented with elevated GH levels after TRH stimulation and eight had increased 24-hour GH levels. Twenty-one (45.7%) had GH excess in two or more tests. (Table 2).
Table 2.
Growth hormone (GH) evaluation in patients with CNC
| Evaluation test for GH excess | Patients with GH excess (n=46)* |
|---|---|
| Elevated GH levels after OGTT test | 30 (65.3%) |
| Elevated IGF-1 levels | 27 (58.7%) |
| Elevated GH levels after TRH test | 10 (21.7%) |
| Elevated 24-hour GH levels | 8 (17.4%) |
21 patients had GH excess in two or more evaluation tests
Acromegaly was present in 18 (18.2%) patients; 13 (72.2%) had a cardiac myxoma (p = 0.0349). More females (29 of 57, 57.6%) had myxoma than males (18 of 42, 42.4%), but the difference was not statistically significant (p = 0.5417). Almost half of all patients (n = 47, 47.5%) had a cardiac myxoma at some point within their lifetime. At their first evaluation, 33 patients (33.3%) already had history of cardiac myxoma; 24 (24.2% of total patients or 51.1% of those with myxomas) had recurrence of tumor.
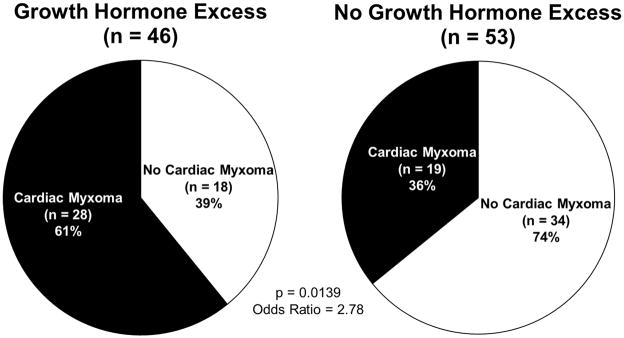
Figure 1 illustrates the distribution of patients with and without GH excess and with and without cardiac myxoma. The majority (n=28, 60.9%) of those patients with a history of GH excess had a cardiac myxoma during their lifetime, whereas only a third (n=19, 35.9%) of those without GH excess had cardiac myxomas. (p = 0.0160). Thus, GH excess was a statistically significant predictor of cardiac myxoma (OR = 2.78, 95% CI: 1.23–6.29; p = 0.0139). Controlling for age at the time of the GH test did not affect the increased odds of occurrence of myxoma (OR = 2.72, 95% CI: 1.17–6.33, p = 0.0202). Although patients with GH excess also tended to have more recurrent myxomas (30.4% vs. 18.9%) the difference was not statistically significant (p = 0.2405). However, when we evaluated the absolute number of myxomas occurring within the period of observation, 23 of 47 patients (48.9%) had a single myxoma, 12 (25.5%) had two myxomas, 9 (19.2%) had three myxomas, one (2.1%) had four myxomas, and two (4.3%) patients had greater than five myxomas. Indeed, patients with the highest number of cardiac myxomas were those with GH excess (p = 0.0154).
Figure 1.
Cardiac myxoma and growth hormome (GH) excess in 99 patients with Carney Complex (CNC): 60.9% (n = 28 of 46) of the patients with GH excess had a cardiac myxoma, compared to 35.9% (n = 19 of 53) of those who did not have GH excess (p = 0.0160). Patients with a history of GH excess were more likely to develop a cardiac mass versus those without GH excess during their lifetime (OR = 2.78, 95% CI: 1.23–6.29, p = 0.0139).
Age, cardiac myxomas, and GH excess
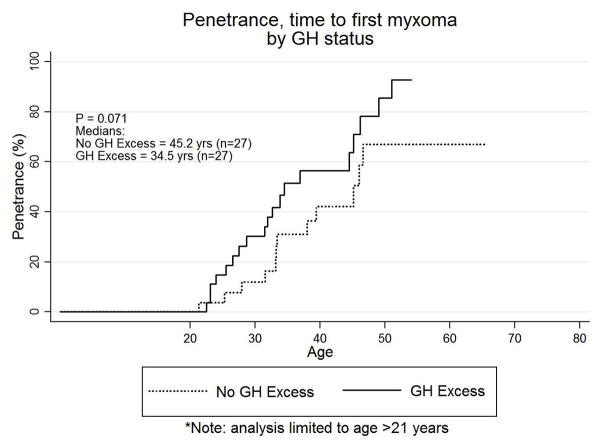
The median time to detection of a patient’s initial cardiac myxoma was 34.5 years (95% CI: 31.5–45.2 years). Over the observed duration of 65.3 years of follow-up (mean 25.8 years), the Kaplan-Meier survivor function estimated an up to 25% chance of having a cardiac mass by age 25.3 years, a 50% chance by 34.5 years, and a 75% chance by 46.6 years. In those patients with GH excess, the median time to penetrance was 32.6 years (95% CI: 26.5–44.5). The median time to penetrance in those without GH excess was 39.4 years (95% CI: 33.1–46.6). While there may be a modest earlier manifestation in those with GH excess, the finding was not statistically significant (p = 0.1061). The median time to presentation with a myxoma was also compared between patients with CNC and GH excess before and after the age of 21 years: those with GH excess had a median time to first myxoma of 34.5 years (95% CI: 28.7–46.2) compared to 45.2 years [95% CI: 33.4-(undefined upper bound)] in those without GH excess (p =0.0709) (Figure 2).
Figure 2.
Kaplan-Meier Curve of penetrance (time to first myxoma) grouped by presence or absence of growth hormone (GH) excess, restricted to those age 21 or older at the time of GH testing. Those with GH excess had a median time to first myxoma of 34.5 years (95% CI: 28.7–46.2) compared to 45.2 years [95% CI: 33.4-(undefined upper bound)] in those without GH excess (p =0.0709).
GH signaling in CNC
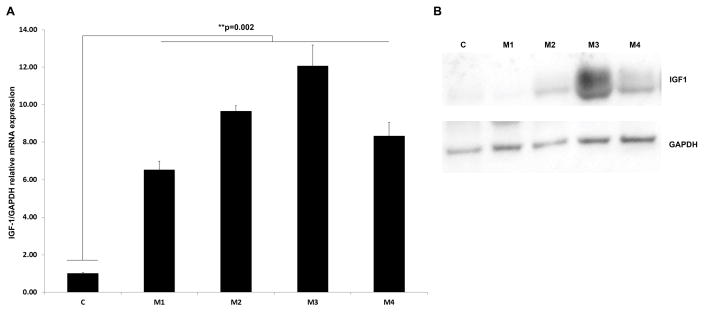
In vitro analysis of IGF-1/GAPDH relative mRNA expression in 4 cardiac myxomas from separate patients was performed. IGF-1 expression was found to be up-regulated in myxomas at the mRNA and protein levels, when compared to pooled normal heart tissue (3 donors) (p = 0.002) (Figure 3).
Figure 3.
IGF-1 mRNA and protein expression in cardiac myxomas. Samples were four different CNC associated cardiac myxomas (M1–M4) and normal control heart (C). A. IGF-1 mRNA expression levels were determined by RT-qPCR performed in triplicate. Error bars represent the S.E.M. and show that IGF-1 expression is clearly up-regulated in myxomas on the mRNA (p = 0.002). B. Western blot showing the protein expression of IGF-1.
Treatment for acromegaly and myxomas
Of the 46 patients with GH excess, nine (19.6%) underwent transsphenoidal surgery for removal of pituitary adenoma, one (2.2%) received radiation therapy, and five (10.9%) had medical therapy (somatostatin analogues or dopamine agonists). There were 10 patients (21.7%) that had to have a combination of these treatments because of aggressive acromegaly that did not respond to previous therapeutic attempts; of these, eight had a cardiac myxoma, a difference that was also significant (p = 0.0438); two had recurrent myxoma.
Discussion
This study represents the first report of an association between excess GH and increased risk of cardiac myxomas in patients with CNC. The differences between CNC patients with GH excess and those without GH excess were striking: 60.9% of patients with GH excess had a cardiac myxoma versus 35.9% of the patients without GH excess. Certainly, not all cardiac myxomas in CNC patients can be attributed to GH excess, as evidenced by the number of patients with cardiac myxoma in the absence of elevated GH levels. However, GH excess appears to be the only significant risk factor for the development of the cardiac myxoma in patients with CNC.
This finding has significant clinical implications for a potential therapeutic target to decrease myxoma occurrence, i.e., those patients with CNC and excess GH may need earlier and/or more aggressive treatment of hypersomatotropinemia. It should be noted, however, that although our data confirm that CNC patients with frank acromegaly who required treatment for their condition had a higher number of cardiac tumor recurrences, only one patient received treatment for acromegaly preceding myxoma development. Thus, we do not know if treatment will alter the likelihood of developing myxomas. Interestingly, the recent paper by Chesnokova and colleagues found that using pegvisomant to block colon cell GH receptors in vitro and in patients with acromegaly increased p53 expression, thus suggesting that blocking GH signaling may yield tumor-protective effects. (Chesnokova, et al. 2016)
The present study is also the first to report the lifetime prevalence of cardiac myxoma in CNC patients with a 25% risk of developing the tumor by the age of 25 and an increasing risk of developing cardiac myxoma over time. What causes the myxomas remains unclear. Almost all (97 out of 99) of our patients had a PRKAR1A mutation which is a slightly higher percentage than previously reported.(Bertherat, et al. 2009; Horvath, et al. 2010) PRKAR1A is a tumor suppressor gene whose inactivation causes tumors in mice (Kirschner, et al. 2005) and abnormal growth and proliferation of human cells in vitro.(Nadella, et al. 2008; Nesterova, et al. 2008) In addition, inactivation of the gene in murine cardiac tissue leads to myxomatous lesions.(Yin, et al. 2008)
Gender does not appear to play a role in CNC-related myxomas.(Carney 1985) Although more than 75% of sporadic cardiac myxomas occur in females(Bjessmo and Ivert 1997; Zheng, et al. 2013), the present study, in accordance with older data in CNC(Carney 1985), did not show a higher prevalence of myxomas among female patients with CNC; likewise, GH elevation did not appear to have a different effect in male versus female patients with CNC (data not shown).
Our study does not suggest that GH is a factor in the first molecular events that are needed for the formation of cardiac myxoma, but it does implicate GH and/or IGF-1 in the facilitation of new tumor formation or growth. In the cardiac myxomas we studied after surgical removal, IGF-1 was upregulated compared to normal heart tissue (Figure 3). GH and/or IGF-1 can promote neoplastic growth of various cell types. However, there is controversy regarding the effect of exogenous GH treatment.(Raman et al. 2015; Renehan et al. 2004) Perhaps the best example of increased endogenous GH and/or IGF1 associated with growth of a neoplasm in the context of a multiple neoplasia disorder, is that of fibrous dysplasia in patients with McCune-Albright syndrome (MAS) and hypersomatotropinemia.(Boyce, et al. 2013) Also, bone sarcoma in MAS may be associated with excess GH and/or IGF-1.(Salenave, et al. 2014)
It is unclear whether our findings have implications for the sporadic cardiac myxoma. In autopsy studies, myxoma is the most common primary cardiac tumor found, accounting for one-half of all post-mortem benign cardiac tumors.(McAllister Jr and Fenoglio Jr 1978) It would be interesting to study the GH and/or IGF-1 levels of patients with sporadic myxomas, as well as possible co-occurrence with other tumors, especially those of organs that have been linked to GH and/or IGF-1.
In conclusion, excess GH has a significantly increased risk of cardiac myxoma in patients with CNC. GH excess may be playing an augmentative role in the growth of cardiac myxoma; however, other additional factors likely contribute as 36% of patients without GH excess were found to have cardiac myxoma. This association poses the important clinical question of whether or not early identification and treatment of GH excess in patients with CNC will reduce morbidity and mortality from cardiac myxomas. The time course of the development of cardiac myxoma in CNC underscores the need for affected patients to undergo cardiac surveillance imaging, starting at a young age and continuing indefinitely.
Acknowledgments
Funding Source: intramural research support of the Eunice Kennedy Shriver National Institute of Child Health and Human Development
We thank all of the Carney Complex patients and their families who participated in this study. We also thank Megan Monroe, PhD, for allowing us to use her EventFlow Visualization software in our initial data analysis.
Footnotes
Disclosure Summary: The authors have no conflicts of interest related to the manuscript to report. Dr. Schernthaner-Reiter reports personal fees from Boehringer Ingelheim, outside the submitted work. Unrelated to the submitted work, Dr. Arai has a U.S. Government Cooperative Research and Development Agreement (CRADA) with Siemens. Dr. Stratakis also holds patents not directly related to the submission.
Clinical Trial Registration: Defining the Genetic Basis for the Development of Primary Pigmented Nodular Adrenocortical Disease and the Carney Complex, Registration number: NCT00001452 URL: https://clinicaltrials.gov/ct2/show/NCT00001452
Author Contributions:
Drs. Stratakis and Lodish had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Study Concept and design: Arai, Lodish, Stratakis, Carney.
Acquisition, Analysis, or interpretation of data: Bandettini, Karageorgiadis, Lodish, Sinaii, Schernthaner-Reiter, Gourgari, Papadakis, Lyssikatos.
Drafting of the manuscript: Bandettini, Karageorgiadis, Sinaii, Lodish, Stratakis, Schernthaner-Reiter.
Critical revision of the manuscript for important intellectual content: Bandettini, Karageorgiadis, Sinaii, Rosing, Sachdev, Schernthaner-Reiter, Gourgari, Papadakis, Keil, Lyssikatos, Carney, Arai, Lodish, Stratakis.
Statistical analysis: Sinaii.
Administrative, technical, or material support: Sachdev, Schernthaner-Reiter, Gourgari, Papadakis, Keil, Lyssikatos.
Study supervision: Arai, Stratakis, Rosing.
References
- Baris D, Gridley G, Ron E, Weiderpass E, Mellemkjaer L, Ekbom A, Olsen JH, Baron JA, Fraumeni JF., Jr Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control. 2002;13:395–400. doi: 10.1023/a:1015713732717. [DOI] [PubMed] [Google Scholar]
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjessmo S, Ivert T. Cardiac myxoma: 40 years’ experience in 63 patients. Ann Thorac Surg. 1997;63:697–700. doi: 10.1016/s0003-4975(96)01368-9. [DOI] [PubMed] [Google Scholar]
- Boyce AM, Glover M, Kelly MH, Brillante BA, Butman JA, Fitzgibbon EJ, Brewer CC, Zalewski CK, Cutler Peck CM, Kim HJ, et al. Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab. 2013;98:E126–134. doi: 10.1210/jc.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA. Differences between nonfamilial and familial cardiac myxoma. Am J Surg Pathol. 1985;9:53–55. doi: 10.1097/00000478-198501000-00009. [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, et al. Growth hormone is permissive for neoplastic colon growth. Proc Natl Acad Sci U S A. 2016;113:E3250–3259. doi: 10.1073/pnas.1600561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, Kim SW. Investigation of responsiveness to thyrotropin-releasing hormone in growth hormone-producing pituitary adenomas. Int J Endocrinol. 2013;2013:159858. doi: 10.1155/2013/159858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon SY, LoCicero J, 3rd, Ilbawi MN, Idriss FS. Repeat median sternotomy in pediatrics: experience in 164 consecutive cases. Ann Thorac Surg. 1986;41:184–188. doi: 10.1016/s0003-4975(10)62665-3. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak M. Risk of cancer after growth-hormone treatment. Lancet. 2002;360:268–269. doi: 10.1016/S0140-6736(02)09561-2. [DOI] [PubMed] [Google Scholar]
- Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–342. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- Ho KY, Weissberger AJ. Characterization of 24-hour growth hormone secretion in acromegaly: implications for diagnosis and therapy. Clin Endocrinol (Oxf) 1994;41:75–83. doi: 10.1111/j.1365-2265.1994.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libe R, Remmers E, Rene-Corail F, Faucz FR, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PJ, Besser GM, Fairclough PD. Colorectal neoplasia in acromegaly. Gut. 1999;44:585–587. doi: 10.1136/gut.44.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001;86:2935–2941. doi: 10.1210/jcem.86.7.7634. [DOI] [PubMed] [Google Scholar]
- Jenkins PJ, Fairclough PD, Richards T, Lowe DG, Monson J, Grossman A, Wass JA, Besser M. Acromegaly, colonic polyps and carcinoma. Clin Endocrinol (Oxf) 1997;47:17–22. doi: 10.1046/j.1365-2265.1997.1911029.x. [DOI] [PubMed] [Google Scholar]
- Jenkins PJ, Mukherjee A, Shalet SM. Does growth hormone cause cancer? Clin Endocrinol (Oxf) 2006;64:115–121. doi: 10.1111/j.1365-2265.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Moriyama T, Sakihara S, Takayasu S, Nigawara T, Suda T. Usefulness of the thyrotropin-releasing hormone test in pre-clinical acromegaly. Tohoku J Exp Med. 2005;206:291–297. doi: 10.1620/tjem.206.291. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- Ma J, Pollak M, Giovannucci E, Chan JM, Tao Y, Hennekens C, Stampfer MJ. A prospective study of plasma levels of insulin-like growth factor I (IGF-I) and IGF-binding protein-3, and colorectal cancer risk among men. Growth Horm IGF Res. 2000;10(Suppl A):S28–29. doi: 10.1016/s1096-6374(00)90013-3. [DOI] [PubMed] [Google Scholar]
- Manousos O, Souglakos J, Bosetti C, Tzonou A, Chatzidakis V, Trichopoulos D, Adami HO, Mantzoros C. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83:15–17. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- McAllister HA, Jr, Fenoglio JJ., Jr . Tumors of the cardiovascular system. Atlas of tumor pathology, 2nd series. Washington, D.C: Armed Forces Institute of Pathology; 1978. Fascicle 15. [Google Scholar]
- Nadella KS, Jones GN, Trimboli A, Stratakis CA, Leone G, Kirschner LS. Targeted deletion of Prkar1a reveals a role for protein kinase A in mesenchymal-to-epithelial transition. Cancer Res. 2008;68:2671–2677. doi: 10.1158/0008-5472.CAN-07-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterova M, Bossis I, Wen F, Horvath A, Matyakhina L, Stratakis CA. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type Allele and other protein kinase A subunits. J Clin Endocrinol Metab. 2008;93:565–571. doi: 10.1210/jc.2007-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex) J Clin Endocrinol Metab. 2000;85:3860–3865. doi: 10.1210/jcem.85.10.6875. [DOI] [PubMed] [Google Scholar]
- Raman S, Grimberg A, Waguespack SG, Miller BS, Sklar CA, Meacham LR, Patterson BC. Risk of Neoplasia in Pediatric Patients Receiving Growth Hormone Therapy--A Report From the Pediatric Endocrine Society Drug and Therapeutics Committee. J Clin Endocrinol Metab. 2015;100:2192–2203. doi: 10.1210/jc.2015-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF., Jr Acromegaly and gastrointestinal cancer. Cancer. 1991;68:1673–1677. doi: 10.1002/1097-0142(19911015)68:8<1673::aid-cncr2820680802>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Roselli EE. Reoperative cardiac surgery: challenges and outcomes. Tex Heart Inst J. 2011;38:669–671. [PMC free article] [PubMed] [Google Scholar]
- Russell JL, LeBlanc JG, Sett SS, Potts JE. Risks of repeat sternotomy in pediatric cardiac operations. Ann Thorac Surg. 1998;66:1575–1578. doi: 10.1016/s0003-4975(98)00829-7. [DOI] [PubMed] [Google Scholar]
- Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos SG, Abu-Asab MS, Tsokos M, Stratakis CA. Pituitary pathology in Carney complex patients. Pituitary. 2004;7:73–82. doi: 10.1007/s11102-005-5348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- Terzolo M, Reimondo G, Gasperi M, Cozzi R, Pivonello R, Vitale G, Scillitani A, Attanasio R, Cecconi E, Daffara F, et al. Colonoscopic screening and follow-up in patients with acromegaly: a multicenter study in Italy. J Clin Endocrinol Metab. 2005;90:84–90. doi: 10.1210/jc.2004-0240. [DOI] [PubMed] [Google Scholar]
- Tzanela M. Dynamic tests and basal values for defining active acromegaly. Neuroendocrinology. 2006;83:200–204. doi: 10.1159/000095528. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Kvinnsland S. Body height and risk of breast cancer. A prospective study of 23,831 Norwegian women. Br J Cancer. 1990;61:881–885. doi: 10.1038/bjc.1990.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, Carney JA, Oldfield EH. Neurosurgical implications of Carney complex. J Neurosurg. 2000;92:413–418. doi: 10.3171/jns.2000.92.3.0413. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jones GN, Towns WH, 2nd, Zhang X, Abel ED, Binkley PF, Jarjoura D, Kirschner LS. Heart-specific ablation of Prkar1a causes failure of heart development and myxomagenesis. Circulation. 2008;117:1414–1422. doi: 10.1161/CIRCULATIONAHA.107.759233. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Geng XG, Wang HC, Yan Y, Wang HY. Clinical and histopathological analysis of 66 cases with cardiac myxoma. Asian Pac J Cancer Prev. 2013;14:1743–1746. doi: 10.7314/apjcp.2013.14.3.1743. [DOI] [PubMed] [Google Scholar]