Abstract
Objectives
Repair for ischemic mitral regurgitation with undersized annuloplasty is characterized by high recurrence rates. We sought to determine the value of pre-repair 3-dimensional echocardiography over 2-dimensional echocardiography in predicting recurrence at 6 months.
Methods
Intraoperative transesophageal 2-dimensional echocardiography and 3-dimensional echocardiography were performed in 50 patients undergoing undersized annuloplasty for ischemic mitral regurgitation. Two-dimensional echocardiography annular diameter and tethering parameters were measured in the apical 2- and 4-chamber views. A customized protocol was used to assess 3-dimensional annular geometry and regional leaflet tethering. Recurrence (grade ≥2) was assessed with 2-dimensional transthoracic echocardiography at 6 months.
Results
Preoperative 2- and 3-dimensional annular geometry were similar in all patients with ischemic mitral regurgitation. Preoperative 2- and 3-dimensional leaflet tethering were significantly higher in patients with recurrence (n = 13) when compared with patients without recurrence (n = 37). Multivariate logistic regression revealed preoperative 2-dimensional echocardiography posterior tethering angle as an independent predictor of recurrence with an optimal cutoff value of 32.0° (area under the curve, 0.81; 95% confidence interval, 0.68–0.95; P = .002) and preoperative 3-dimensional echocardiography P3 tethering angle as an independent predictor of recurrence with an optimal cutoff value of 29.9° (area under the curve, 0.92; 95% confidence interval, 0.84–1.00; P < .001). The predictive value of the 3-dimensional geometric multivariate model can be augmented by adding basal aneurysm/dyskinesis (area under the curve, 0.94; 95% confidence interval, 0.87–1.00; P < .001).
Conclusions
Preoperative 3-dimensional echocardiography P3 tethering angle is a stronger predictor of ischemic mitral regurgitation recurrence after annuloplasty than preoperative 2-dimensional echocardiography posterior tethering angle, which is highly influenced by viewing plane. In patients with a preoperative P3 tethering angle of 29.9° or larger (especially when combined with basal aneurysm/dyskinesis), chordal-sparing valve replacement should be strongly considered.
Graphical abstract
Ischemic mitral regurgitation (IMR) is common, and its presence adversely affects survival, with a strongly graded relationship between IMR severity and reduced survival. , Mitral valve repair with undersized ring annuloplasty has been the preferred treatment strategy for IMR3–5; however, the overall persistence and recurrence rate of moderate or severe IMR within 12 months of surgery consistently have been reported to affect approximately one third of the treated patients. Goldstein and colleagues9 recently showed that the recurrence rate of moderate or severe IMR may be as high as 58.8% after a 2-year follow-up period. IMR repair failure continues to be a significant clinical problem, because IMR recurrence predisposes to heart failure, atrial fibrillation, and repeat interventions and hospitalizations.7, 9
With 3-dimensional echocardiography (3DE) and advanced image modeling, we have recently shown that the degree of preoperative mitral leaflet tethering determines the risk of IMR recurrence after undersized ring annuloplasty.10 Current annuloplasty rings treat annular dilatation but do little to improve (and may potentiate) leaflet tethering.11, 12 This may explain the limited repair durability after annuloplasty (especially in patients with advanced tethering) and suggests that a patient-specific approach based on preoperative imaging is required to optimize postoperative results.
Because the results of 2-dimensional echocardiography (2DE) are highly dependent on viewing plane selection, studies reporting on preoperative 2DE predictors of IMR recurrence after annuloplasty show inconsistent, frequently nonreproducible, and sometimes conflicting results.6, 13–34 To improve patient selection and postoperative outcome, we sought to determine the incremental value of preoperative 3DE and advanced mitral valve modeling over 2DE in predicting recurrent IMR after mitral annuloplasty. We recently published the 3DE results of 50 patients with IMR,10 and to determine the incremental value of 3DE over 2DE, we performed additional 2DE analyses in this same group of 50 patients.
MATERIALS AND METHODS
This study was approved by the Institutional Review Boards of the University of Pennsylvania, University of Pittsburgh, and Beth Israel Deaconess Medical Center. Written informed consent was obtained from all patients.
Patients and Image Acquisition
Fifty patients with severe IMR underwent mitral valve repair with an undersized annuloplasty ring (2 sizes down) (Table 1). IMR was defined as mitral regurgitation (MR) occurring as a consequence of myocardial infarction or myocardial ischemia in the absence of any inherent structural damage to the leaflets, chordae, or papillary muscles. Ring type selection was at the discretion of the surgeon.
TABLE 1.
Preoperative and intraoperative patient characteristics
| Variable* | Normal (n = 21) | Nonrecurrent IMR (n = 37) | Recurrent IMR (n = 13) |
|---|---|---|---|
| Age, y | 66.1 ± 14.4 | 68.0 ± 9.0 | 62.5 ± 13.0 |
| Female | 8 (38) | 11 (30) | 6 (46) |
| Body mass index, kg/m2 | 32.2 ± 8.0 | 28.5 ± 4.4 | 29.4 ± 6.5 |
| Medical history | |||
| Hypertension | 11 (52) | 29 (78) | 9 (69) |
| Diabetes | 6 (29) | 18 (49) | 5 (38) |
| Renal insufficiency | 3 (14) | 8 (22) | 1 (8) |
| Atrial fibrillation | 2 (10) | 14 (38)‡ | 4 (31) |
| Stroke | 2 (10) | 4 (11) | 1 (8) |
| Previous PCI | 3 (14) | 14 (38) | 5 (38) |
| Previous CABG | 2 (10) | 6 (16) | 5 (38) |
| NYHA class, 1–4 scale | 2.4 ± 0.8 | 2.4 ±0.8 | 2.7 ± 0.8 |
| LVEF, % | 65.2 ± 10.1 | 38.2 ± 14.7‡ | 32.3 ± 12.5§ |
| IMR grade, 0–4 scale | 0.3 ± 0.5 | 3.2 ± 0.7‡ | 3.3 ± 0.8§ |
| Basal aneurysm/dyskinesis | 0 (0) | 1 (3) | 7 (54)†, § |
| Inferior wall motion abnormality | 0 (0) | 32 (86) | 10 (77) |
| LVEDD, cm | 4.7 ± 0.8 | 5.7 ± 0.8‡ | 6.0 ± 1.0§ |
| LVESD, cm | 3.2 ± 0.8 | 4.6 ± 0.8‡ | 5.1 ± 1.2§ |
| Annuloplasty ring | |||
| Profile 3D ring | - | 23 (62) | 12 (92) |
| CE Physio II ring (Edwards Lifesciences, Irvine, Calif) | - | 7 (19) | 1 (8) |
| CG Future band (Medtronic, Minneapolis, Minn) | - | 6 (16) | 0 (0) |
| St Jude tailor flexible ring (St Jude Medical Inc, St Paul, Minn) | - | 1 (3) | 0 (0) |
| Ring size, mm | - | 29.0 ±1.7 | 28.6 ± 1.3 |
| Concomitant procedures | |||
| CABG | 6 (29) | 25 (68)‡ | 8 (62) |
| Aortic valve replacement | 14 (67) | 4 (11)‡ | 0 (0)§ |
| Tricuspid valve repair | 0 (0) | 4 (11) | 2 (15) |
| Atrial maze | 0 (0) | 7 (19) | 0 (0) |
| Atrial septal defect closure | 1 (5) | 0 (0) | 0 (0) |
IMR, Ischemic mitral regurgitation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; 3D, 3-dimensional.
Data are presented as mean ± standard deviation or number (%).
P < .05 recurrent versus nonrecurrent.
P < .05 nonrecurrent versus normal.
P < .05 recurrent versus normal.
Transthoracic 2DE was performed preoperatively and 6 months after repair. Images were acquired through a transthoracic apical 4-chamber view. Severity of IMR was determined semiquantitatively with color Doppler by assessing the area of the regurgitant jet as a percentage of left atrial area in the apical 4-chamber view. The following grading scale was used: grade 0, no IMR; grade 1, less than 20%; grade 2, 20% to 40%; grade 3, 40% to 60%; and grade 4, more than 60%.35 Recurrent IMR 6 months after repair was defined as IMR grade 2 or greater.
Left ventricular (LV) wall motion abnormalities also were assessed on the preoperative echocardiograms (aneurysm, dyskinesis, akinesis, and hypokinesis). Criteria for inferior basal aneurysm were evidence of thinning and localized LV dilatation or distortion. Dyskinesis was defined as the presence of outward displacement of the LV wall during systole. Inferoposterior aneurysm and dyskinesis were combined in 1 variable.
Real-time 3-dimensional (3D) transesophageal echocardiography (TEE) was performed before mitral valve repair. Preoperative imaging data sets were acquired in the operating room after induction of general anesthesia and before sternotomy in all 50 patients with IMR and in 21 patients with normal mitral valves and normal LV function who required cardiac surgery for indications other than mitral valve disease. Images were acquired through a midesophageal view with a Philips ie33 (Philips Medical, Andover, Mass) ultrasound system equipped with a 2 to 7 MHz X7-2t TEE matrix transducer.
Two-Dimensional Geometric Analysis
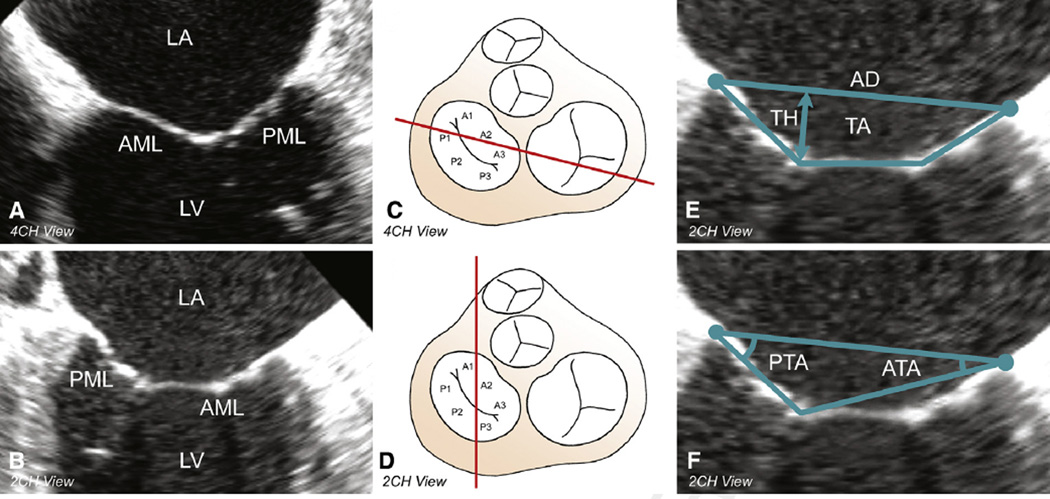
All analyses were performed in the 2- and 4-chamber views at midsystole (Figure 1, A-D). Determinations of annular diameter, tethering height, tethering area, anterior tethering angle, and posterior tethering angle (PTA) are shown in Figure 1, E and F.
Figure 1.
2DE geometric analysis of the mitral valve. A, Preoperative midsystolic 4-chamber view. B, Preoperative midsystolic 2-chamber view. C and D, Cross-section through the base of the heart with an atrial view of the mitral valve with its 6 segments. The red line indicates the cross-sectional transesophageal 2DE plane through the mitral valve (segments) in the 4-chamber (C) and 2-chamber (D) views. E, Determination of annular diameter, tethering height, and tethering area in the 2-chamber view. F, Determination of anterior tethering angle and PTA in the 2-chamber view. LA, Left atrium; AML, anterior mitral valve leaflet; PML, posterior mitral valve leaflet; LV, left ventricle; 4CH, 4-chamber; 2CH, 2-chamber; AD▪ ▪ ▪; TH▪ ▪ ▪; TA▪ ▪ ▪; PTA, posterior tethering angle; ATA, anterior tethering angle.
Three-Dimensional Geometric Analysis: Image Segmentation and Annular and Leaflet Modeling
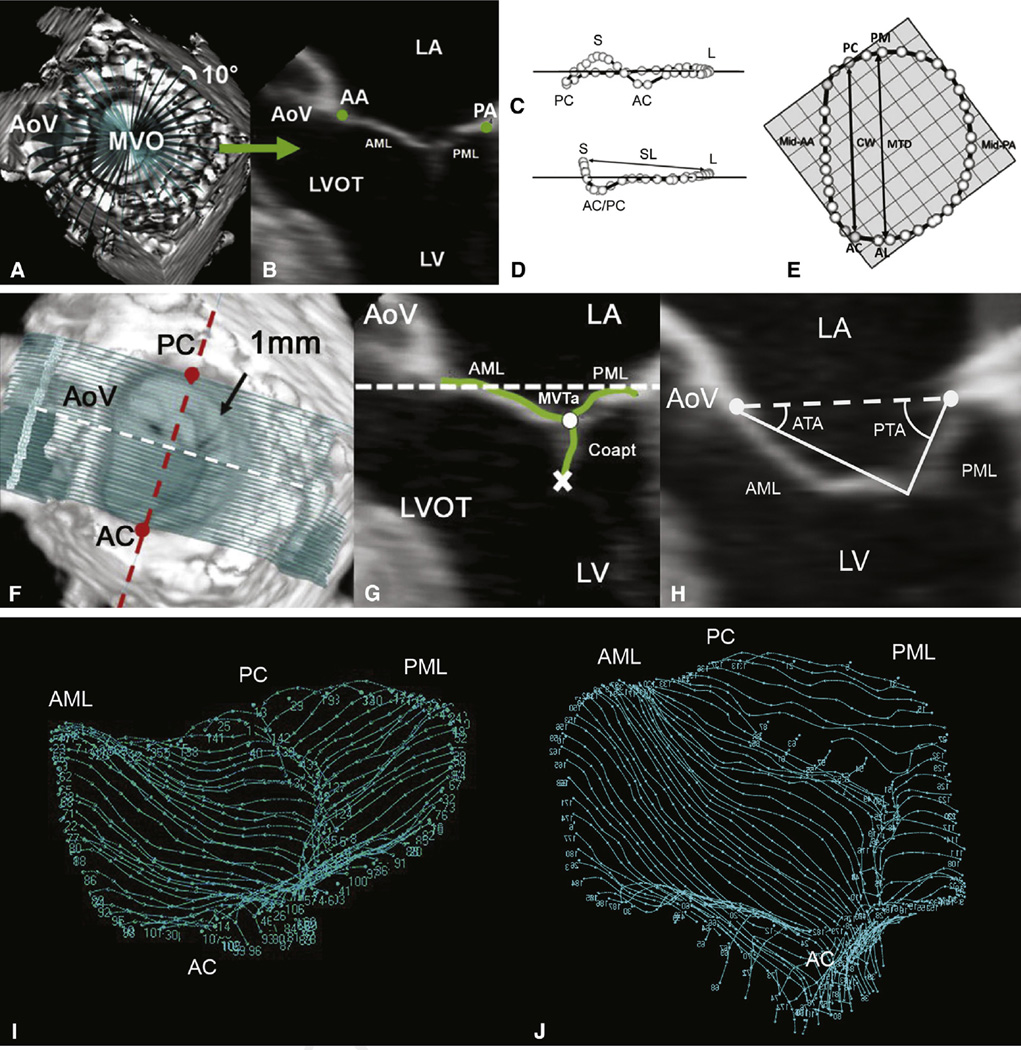
Each full-volume preoperative 3D TEE data set was exported to an Echo-View 5.4 (TomTec Imaging Systems, Munich, Germany) software workstation. All analyses were performed at midsystole. Techniques of annular segmentation and modeling and leaflet segmentation and modeling have been described.10 Briefly, the Cartesian (x, y, z) coordinates of each data point were exported from TomTec to Matlab software (The Math-works, Natick, Mass) to perform quantitative reconstruction of the valve. Determinations of septolateral diameter; intercommissural width; mitral transverse diameter; mitral annular area; mitral annular circumference; mitral valve tethering area, volume, and index; (segmental) anterior tethering angles; and (segmental) PTAs are shown or described in Figure 2.
Figure 2.
3D annular (A–E) and leaflet (F–H) segmentation technique and geometric analysis. A, 3DE volume containing the mitral valve with cross-sectional planes at 10-degree increments. B, Representative 2-dimensional cross-section with green dots representing the selected annular points. Oblique (C), intercommissural (D), and transvalvular annular (E), views of a single real-time 3D–derived mitral annular model with annular landmarks and the 36 annular data points (circles). The least-squares plane has been superimposed on the annulus in each view. The least-squares plane is depicted by a horizontal line in C and D and by the check boxes in E. Determinations of septolateral diameter, intercommissural width, and mitral transverse diameter are shown in D and E. Mitral annular area (the area enclosed by the 2-dimensional projection of an annular data set onto its corresponding least-squares plane) and mitral annular circumference also were determined. F, Template of transverse cross-sections every 1 mm along intercommissural axis. G, One of the 2-dimensional cross-sections represented by the white dashed line in F; the white and red dashed lines are both within least-squares annular plane. Determinations of mitral valve tethering (MVT) area, anterior tethering angle, and posterior tethering angle are shown in G and H. The atrial surface of the mitral valve leaflets and the coaptation zone is interactively marked (green curves), resulting in a 500- to 1000-point data set for each valve. MVT area was defined as the area enclosed by the mitral annular plane (white dashed line) and the mitral leaflets for a given point along the intercommissural axis. MVT area was calculated at known intervals (0.1 mm), Δc, along the intercommissural axis. MVT volume was calculated as the sum of the incremental regional volumes (MVT area × Δcn). MVT index (MVT volume divided by mitral annular area) also was calculated for each data set. ATA and PTA were computed at known intervals (0.1 mm) along the entire length of the intercommissural axis by measuring the angle formed by the anterior or posterior leaflet tangent relative to the mitral annular plane (H). Segmental (mean) tethering angles were determined by dividing the valve into equal thirds along the intercommissural axis to conform to the standard 6 anatomic leaflet segments (A1, A2, A3; P1, P2, P3) and by calculating the mean segmental tethering angle for each specific segment on the basis of computed tethering angles at 0.1-mm intervals (along the intercommissural axis). I, Normal mitral valve. J, Tethered mitral valve. LA, Left atrium; AA, anterior mitral annulus; PA, posterior mitral annulus; AoV, aortic valve; MVO, mitral valve orifice; AML, anterior mitral leaflet; PML, posterior mitral leaflet; LVOT, left ventricular outflow tract; LV, left ventricle; S, septal aspect of the annulus; L, lateral aspect of the annulus; PC, posterior commissure; AC, anterior commissure; SL, septolateral diameter; PM, posteromedial annulus; CW, commissural width; MTD, mitral transverse diameter; MVT(a), mitral valve tethering (area); Coapt, coaptation; ATA, anterior tethering angle; PTA, posterior tethering angle.
Systematic Review of the Literature
A systematic review of the literature on 2DE and 3DE predictors of IMR recurrence after annuloplasty was performed in January 2016. Separate Medline (PubMed), EMBASE, and Cochrane database queries were performed with the following text and keywords: “ischemic mitral regurgitation,” “repair,” and “recurrence.” All articles were considered irrespective of the journal in which they were published. Titles and abstracts were screened, and relevant articles were included. Articles not written in English were excluded. Articles were thoroughly checked to ensure that the cause of MR was ischemic. Articles had to be on preoperative echocardiographic predictors of IMR recurrence. Therefore, articles on applied preoperative cutoff values, on changes in pre- versus postrepair echocardiographic parameters, on postoperative echocardiographic predictors of IMR recurrence, or on predictors of LV (reverse) remodeling were excluded.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. Categoric variables were expressed as percentages. Comparisons between groups were performed using Pearson’s chi-square test or Fisher exact test (2-sided) as appropriate for categoric variables and the independent samples t test or Mann–Whitney U test (2-sided) as appropriate for continuous variables. Univariate echocardiographic variables with a P value less than .10 were included in the multivariate analyses. Age and gender were included in all multivariate models. Multivariate logistic regression analyses by means of a forward stepwise algorithm (cutoff for entry and removal 0.05) were performed to identify independent 2DE and 3DE geometric predictors of IMR recurrence. Odds ratios (ORs) were reported with 95% confidence intervals (CIs). Goodness-of-fit of the final logistic regression models was assessed with the Hosmer–Lemeshow statistic.
Receiver operating characteristic (ROC) curves were calculated for continuous independent predictors to single out the optimal cutoff value of predicting IMR recurrence. The statistical significance of difference of area under the curve (AUC) from the “no discrimination line” was evaluated by the Mann–Whitney U statistic.
All calculations were performed using commercially available statistical packages (IBM SPSS Statistics 21.0; IBM Corporation, Armonk, NY, and Stats Direct 2.8.0; StatsDirect Ltd, Cheshire, United Kingdom).
RESULTS
Patient Characteristics
A total of 13 patients (26%) experienced recurrent IMR 6 months after undersized annuloplasty (6 patients had grade 2 + MR, 4 patients had grade 3 + MR, and 3 patients had grade 4 + MR). On the basis of these findings, patients were divided into recurrent and nonrecurrent IMR groups. Preoperative and intraoperative patient characteristics are presented in Table 1. As a reference, data from 21 patients with normal mitral valves and normal LV function are included in Table 1. Preoperative degree of IMR, LV size, and LV ejection fraction were similar in the nonrecurrent and recurrent IMR groups. The basal aneurysm/dyskinesis rate was significantly higher in the recurrent IMR group compared with the nonrecurrent IMR group (54% vs 3%, P = .001).
Annular Geometry
Annular parameters are summarized in Table 2. Preoperative 2DE and 3DE annular parameters were similar for patients with and without recurrent IMR. All patients with IMR had significantly dilated annuli relative to patients with normal mitral valves.
TABLE 2.
Preoperative 2- and 3-dimensional echocardiography annular and leaflet tethering parameters
| Parameter* | Normal (n = 21) | Nonrecurrent IMR (n = 37) | Recurrent IMR (n = 13) |
|---|---|---|---|
| 2DE | |||
| 4-chamber view | |||
| Annular diameter, mm | 34.8 ± 2.6 | 39.4 ± 4.7‡ | 38.0 ± 3.9§ |
| Tethering height, mm | 5.4 ± 2.4 | 9.3 ± 3.1‡ | 9.8 ± 3.0§ |
| Tethering area, cm2 | 1.12 ± 0.38 | 2.03 ± 0.92‡ | 2.31 ± 0.94§ |
| Anterior tethering angle, ° | 22.2 ± 7.4 | 21.1 ± 6.4 | 25.8 ± 8.7 |
| PTA, ° | 29.6 ± 9.2 | 32.5 ±8.1 | 36.4 ± 9.0 |
| 2-chamber view | |||
| Annular diameter, mm | 38.0 ± 3.9 | 41.2 ± 5.0 | 38.6 ± 4.0 |
| Tethering height, mm | 6.9 ±1.5 | 7.2 ± 4.4 | 10.5 ± 3.9†, § |
| Tethering area, cm2 | 0.99 ± 0.51 | 1.62 ± 0.82 | 2.49 ±0.83†, § |
| Anterior tethering angle, ° | 16.2 ± 13.0 | 16.3 ± 7.6 | 22.4 ± 11.1† |
| PTA, ° | 24.8 ±11.9 | 26.0 ±11.1 | 38.0 ± 6.9†, § |
| 3DE | |||
| Septolateral diameter, mm | 28.7 ± 5.1 | 31.3 ± 3.7† | 31.3 ± 5.1 |
| Commissural width, mm | 31.4 ± 3.2 | 32.9 ± 5.0 | 32.4 ± 6.5 |
| Mitral transverse diameter, mm | 34.6 ± 3.9 | 37.4 ± 4.4‡ | 36.9 ± 4.8 |
| Mitral annular area, mm2 | 786 ± 155 | 943 ± 210‡ | 924 ± 260 |
| Annular circumference, mm | 103 ±11 | 114 ± 13‡ | 115 ± 14§ |
| Mitral valve tethering volume, mm3 | 1771 ± 689 | 2812 ± 1499‡ | 3744 ± 1541§ |
| Mitral valve tethering index | 2.25 ± 0.70 | 2.90 ± 1.17‡ | 3.91 ± 1.01†, § |
| Segmental tethering angle, ° | |||
| A1 | 18.4 ± 9.2 | 19.4 ± 8.6 | 24.7 ± 6.8§ |
| A2 | 15.0 ± 8.2 | 26.9 ± 11.6‡ | 33.3 ± 10.6§ |
| A3 | 9.5 ± 6.4 | 14.4 ±11.4 | 23.5 ± 8.9†, § |
| P1 | 16.5 ± 8.5 | 24.0 ± 12.3 ‡ | 30.6 ± 6.3§ |
| P2 | 17.9 ± 12.0 | 28.2 ± 17.0‡ | 44.4 ± 8.8†, § |
| P3 | 14.0 ± 7.6 | 18.6 ± 12.7 | 35.2 ± 6.0†, § |
IMR, Ischemic mitral regurgitation; 2DE, 2-dimensional echocardiography; PTA, posterior tethering angle; 3DE, 3-dimensional echocardiography.
Data are presented as mean ± standard deviation.
P < .05 recurrent versus nonrecurrent.
P < .05 nonrecurrent versus normal.
P < .05 recurrent versus normal.
Leaflet Tethering
Leaflet tethering parameters are summarized in Table 2. Patients with recurrent IMR after annuloplasty had more severe preoperative global and regional leaflet tethering than patients without recurrent IMR. On 2DE (2-chamber view), tethering height, tethering area, and anterior and PTAs were significantly higher in patients with recurrent IMR. On 3DE, the preoperative mitral valve tethering index and preoperative tethering angles of A3, P2, and P3 were significantly higher in patients with recurrent IMR.
Two-Dimensional Geometric Predictors of Ischemic Mitral Regurgitation Recurrence
Univariate and multivariate logistic regression analyses of IMR recurrence are shown in Table 3. Multivariate analysis revealed preoperative PTA (measured in the 2-chamber view) as an independent predictor of IMR recurrence after undersized annuloplasty (OR, 1.17; 95% CI, 1.04–1.32; Wald chi-square 6.72; P = .010). The Hosmer-Lemeshow goodness-of-fit test was nonsignificant, indicating that this multivariate model is a good fit (chi-square = 4.74, degrees of freedom [df] = 8, P = .785). An ROC curve was calculated for preoperative PTA to single out the optimal cutoff value of predicting IMR recurrence (Figure 3). The optimal cutoff value was 32.0° with an AUC of 0.81 (95% CI, 0.68–0.95; P = .002), a sensitivity of 90.9%, and a specificity of 61.1%.
TABLE 3.
Two- and 3-dimensional echocardiography geometric predictors of ischemic mitral regurgitation recurrence by univariate and multivariate logistic regression analyses
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| 2DE geometric parameters | OR | 95% CI | P value | OR | 95% CI | P value |
| Anterior tethering angle, ° (4CH view) | 1.10 | 1.00–1.22 | .061 | - | - | - |
| Tethering height, mm (2CH view) | 5.04 | 0.94–26.99 | .059 | - | - | - |
| Tethering area, cm2 (2CH view) | 3.05 | 1.25–7.46 | .015 | - | - | - |
| Anterior tethering angle, ° (2CH view) | 1.09 | 1.00–1.18 | .053 | - | - | - |
| PTA, ° (2CH view) | 1.18 | 1.05–1.34 | .008 | 1.17 | 1.04–1.32 | .010 |
| 3DE geometric parameters | ||||||
| Septolateral diameter, mm | 1.00 | 0.86–1.17 | .975 | - | - | - |
| Commissural width, mm | 0.98 | 0.88–1.10 | .772 | - | - | - |
| Mitral transverse diameter, mm | 0.97 | 0.84–1.12 | .688 | - | - | - |
| Mitral annular area, mm2 | 1.00 | 1.00–1.00 | .781 | - | - | - |
| Annular circumference, mm | 1.01 | 0.96–1.06 | .846 | - | - | - |
| Mitral valve tethering volume, mm3 | 1.00 | 1.00–1.00 | .069 | - | - | - |
| Mitral valve tethering index | 2.48 | 1.19–5.17 | .015 | - | - | - |
| Segmental tethering angle, ° | ||||||
| A1 | 1.09 | 1.00–1.19 | .058 | - | - | - |
| A2 | 1.05 | 0.99–1.12 | .094 | - | - | - |
| A3 | 1.10 | 1.02–1.19 | .019 | - | - | - |
| P1 | 1.07 | 0.99–1.16 | .076 | - | - | - |
| P2 | 1.13 | 1.04–1.22 | .005 | - | - | - |
| P3* | 1.28 | 1.11–1.49 | .001 | 1.28 | 1.11–1.49 | .001* |
2DE, 2-Dimensional echocardiography; OR, odds ratio; CI, confidence interval; 4CH, 4-chamber; 2CH, 2-chamber; PTA, posterior tethering angle; 3DE, 3-dimensional echocardiography.
When the univariate functional parameter “basal aneurysm/dyskinesis” (OR, 42.00; 95% CI, 4.35–405.13; P= .001) was added to the geometric 3DE analysis, multivariate analysis revealed preoperative P3TA (OR, 1.24; 95% CI, 1.07–1.45; P= .005) and basal aneurysm/dyskinesis (OR, 16.47; 95% CI, 1.34–202.74; P= .029) as independent predictors of IMR recurrence.
Figure 3.
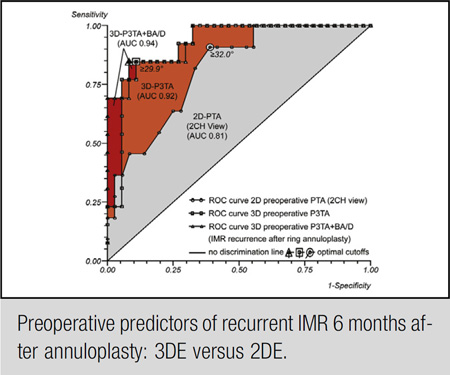
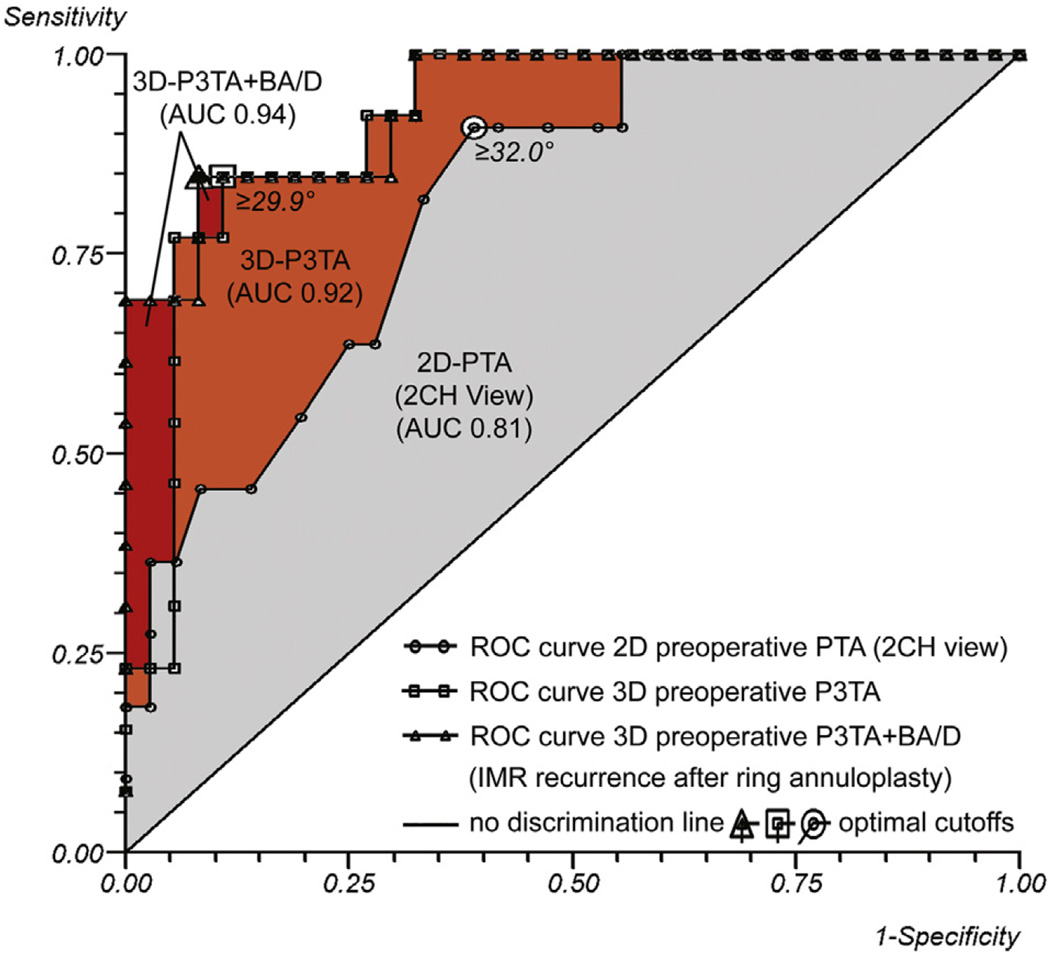
ROC curves. ROC curve for preoperative PTA (optimal cutoff value 32.0°, AUC 0.81), preoperative P3TA (optimal cutoff value 29.9°, AUC 0.92), and preoperative P3TA and basal aneurysm/dyskinesis (AUC 0.94) as predictors of IMR recurrence after undersized mitral ring annuloplasty. The incremental value of preoperative 3DE over 2DE (orange area) in predicting IMR recurrence after mitral annuloplasty becomes apparent immediately. 3D, 3-Dimensional; P3TA, P3 tethering angle; BA/D, basal aneurysm/dyskinesis; AUC, area under the curve; 2D, 2-dimensional; 2CH, 2-chamber; PTA, posterior tethering angle; ROC, receiver operating characteristic; IMR, ischemic mitral regurgitation.
Three-Dimensional Geometric Predictors of Ischemic Mitral Regurgitation Recurrence
Univariate and multivariate logistic regression analyses of IMR recurrence are shown in Table 3. Multivariate analysis revealed preoperative regional tethering of segment P3 (preoperative P3 tethering angle [P3TA]) as an independent predictor of IMR recurrence after undersized annuloplasty (OR, 1.28; 95% CI, 1.11–1.49; Wald chi-square 11.14; P = .001). The Hosmer–Lemeshow goodness-of-fit test was nonsignificant, indicating that this multivariate model is a good fit (chi-square = 2.13, df = 8, P = .977). An ROC curve was calculated for preoperative P3TA to single out the optimal cutoff value of predicting IMR recurrence (Figure 3). The optimal cutoff value was 29.9° with an AUC of 0.92 (95% CI, 0.84–1.00; P < .001), a sensitivity of 84.6%, and a specificity of 89.2%. In Figure 3, the incremental value of preoperative 3DE over 2DE in predicting IMR recurrence after mitral annuloplasty becomes apparent immediately (orange area).
Three-Dimensional Geometric and Functional Predictors of Ischemic Mitral Regurgitation Recurrence
When the functional parameter “basal aneurysm/dyskinesis” was added to the geometric 3DE analysis, multivariate analysis revealed preoperative P3TA (OR, 1.24; 95% CI, 1.07–1.45; Wald chi-square 8.01; P = .005) and basal aneurysm/dyskinesis (OR, 16.47; 95% CI, 1.34–202.74; Wald chi-square 4.79; P = .029) as independent predictors of IMR recurrence after undersized annuloplasty (Table 3). The Hosmer-Lemeshow goodness-of-fit test was nonsignificant, indicating that this multivariate model is a good fit (chi-square = 5.38, df = 8, P = .716). An ROC curve for both parameters revealed an AUC of 0.94 (95% CI, 0.87–1.00; P < .001), a sensitivity of 84.6%, and a specificity of 91.9% (Figure 3). In Figure 3, the incremental value of the combined model over preoperative 3DE geometrics in predicting IMR recurrence after mitral annuloplasty becomes apparent immediately (red area).
Systematic Review of the Literature
Table 4 provides a systematic review of the literature on 2DE and 3DE predictors of IMR recurrence after mitral annuloplasty.
TABLE 4.
Systematic review of the literature on preoperative 2- and 3-dimensional echocardiography predictors of ischemic mitral regurgitation recurrence after mitral ring annuloplasty
| Reference, Year N =; 2/3DT(T)(E)E; IMR grading; % IMR recurrence* (grade) (follow-up) |
Predictors | View | Independent predictors | View | Cutoff value | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| Matsunaga and colleagues, 13 2004 |
Posterior PM depth (indexed), mm/m2 |
AP2CH | — | |||||
| 48; 2D TTE; JA/LAA | Posterior PM angle, ° | AP2CH | ||||||
| 31% (≥3±) (NR) | ||||||||
| McGee and colleagues, 6 2004 |
Degree of IMR | - | — | |||||
| 422; 2D TTE; JA/LAA | Degree of LV dysfunction | - | ||||||
| ±28% (≥3±) (6 mo) | Jet direction (central or complex) |
- | ||||||
| Zhu and colleagues, 14 2005 | LVEDV index and LVESV index, mL/m2 |
Tethering height (indexed), mm/m2 |
PLAX | NR | NR | NR | NR | |
| 31; 2D TTE; VC | Systolic sphericity index | AP4CH | Coaptation length (indexed), mm/m2 |
PLAX | NR | NR | NR | NR |
| 19% (≥2±) (2–8 wks) | Annular area index, cm2/m2 | AP2/4CH | ||||||
| Tethering distance (indexed), mm/m2 |
PLAX | |||||||
| Basal anterior tethering angle, ° |
PLAX | |||||||
| PTA, ° | PLAX | |||||||
| Anterior bending angle, ° | PLAX | |||||||
| Anterior and post leaflet excursion angle, ° |
PLAX | |||||||
| Coaptation length (indexed), mm/m2 |
PLAX | |||||||
| Ereminiene and colleagues, 15 2005 |
LVEDD index and LVESD index, mm/m2 |
PLAX | Restrictive LV diastolic filling |
AP4CH | - | - | - | - |
| 53; 2D TTE; JA/LAA, ERO |
Deceleration time, ms | — | ||||||
| ±57%(≥2±)(1y) | ||||||||
| Kongsaerepong and colleagues, 16 2006 |
LVEDV, cm3 | - | Degree of IMR | - | ≥3.5 | NR | 42.0 | 81.0 |
| 365; 2D TEE; JA/LAA, PISA |
LVESV, cm3 | - | Mitral annular diameter, cm | 4CH | ≥3.7 cm | NR | 84.0 | 76.0 |
| 19% (≥2+) (mean 269 d) | Tethering height, cm | 2/4CH, LAX | Tethering area, cm | LAX | ≥1.6 cm | NR | 80.0 | 54.0 |
| Serri and colleagues, 17 2006 |
Anterior annulus-PPM distance, mm |
AP2CH | — | |||||
| 73; 2D TEE; JA/LAA, PISA | ||||||||
| 57% (≥2±) (28 ± 23 mo) | ||||||||
| Roshanali and colleagues, 18 2007 |
Tethering height, mm | NR | IPMD, mm | PSAX | ≥20 mm | 0.99 | 95.7 | 97.2 |
| 95; 2D TTE; VC | Concavity area anterior leaflet, mm |
NR | ||||||
| 24% (≥2±) (±1.5–2 y) | ||||||||
| Magne and colleagues, 19 2007 |
Tethering area, cm | AP4CH/PLAX | Tethering height, mm | AP4CH/PLAX | ≥1 cm | 0.87 | 64.0 | 90.0 |
| 51; 2D TTE; JA/LAA, VC |
Anterior leaflet angle, ° | AP4CH/PLAX | Posterior leaflet angle, ° | AP4CH/PLAX | ≥45° | 0.98 | 100.0 | 95.0 |
| 22% (≥2±) (mean 9 d) | ||||||||
| Ueno and colleagues, 20 2008 |
LVEDD and LVESD, mm | PLAX | - | |||||
| 20; 2D TTE; NR | LVEDV index and LVESV index, mL/m2 |
- | ||||||
| 40%† (±1–2 wk) | ||||||||
| Gelsomino and colleagues, 21 2008 |
Tethering area, cm2 | PLAX | LVESV, mL | ≥145mL | 0.87 | 90.0 | 90.0 | |
| 220; 2D TTE; ERO, RV | Tethering height, mm | PLAX | Systolic sphericity index | AP4CH | ≥0.7 | 1.00 | 100.0 | 100.0 |
| 33%(≥2+)(3y) | LVEDD and LVESD, mm | PLAX | Myocardial performance index |
- | ≥0.9 | 0.94 | 85.0 | 84.0 |
| 72% (≥2+) (5 y) (median follow-up 33 mo) |
LVEF, % Diastolic sphericity index |
AP4CH | WMSI | - | ≥1.5 | 0.81 | 80.0 | 82.0 |
| APM lateral distance, cm | PSAX | |||||||
| APM WMSI | - | |||||||
| Gelsomino and colleagues, 22 2008 |
Jet direction (central or anterior) |
PLAX | Tethering height, mm | PLAX | ≥11 mm | 0.89 | 81.0 | 84.0 |
| 230; 2D TTE; ERO, RV | APM posterior and lateral distance, cm |
PSAX | Basal anterior tethering angle, ° |
PLAX | ≥39.5° | 0.99 | 98.0 | 97.0 |
| 23% (≥2+) (median 33 mo) |
IPMD, cm | PSAX | Basal ATA/PTA ratio | PLAX | ≥0.76 | 0.92 | 87.0 | 86.0 |
| APM and PPM WMSI | - | Anterior leaflet excursion angle, ° |
PLAX | <35° | 0.87 | 85.0 | 83.0 | |
| Tethering area, cm2 | PLAX | |||||||
| Coaptation length, mm | PLAX | |||||||
| Anterior leaflet bending angle, ° |
PLAX | |||||||
| PTA, ° | PLAX | |||||||
| Anterior leaflet excursion, mm |
PLAX | |||||||
| Gelsomino and colleagues, 23 2009 |
- | - | Deceleration time, ms‡ | - | <142 ms | 0.94 | 87.0 | 80.0 |
| 234; 2D TTE; ERO, RV | ||||||||
| 23% (≥2+) (median 38 mo) |
||||||||
| Onorati and colleagues, 24 2009 |
Degree of IMR | - | LVEDD, mm | NR | ≥70 mm | NR | NR | NR |
| 82; 2D TTE; JA/LAA | Systolic PAP, mm Hg | - | ||||||
| 26% (≥2+) (18 ± 15 mo) | LVESD, mm | NR | ||||||
| Ciarka and colleagues, 25 2010 |
Basal anterior tethering angle, ° |
AP4CH | Distal anterior tethering angle, ° |
AP4CH | NR | NR | NR | NR |
| 109§; 2D TTE; VC, ERO, RV |
Tethering area, cm2 | PLAX | PTA, ° | AP4CH | NR | NR | NR | NR |
| 19% (≥2+) (2.6 ± 1.6 y) | ||||||||
| Tethering height, mm Coaptation length, mm |
PLAX PLAX |
|||||||
| Systolic and diastolic sphericity index |
- | |||||||
| Gelsomino and colleagues, 26 2011 |
- | - | Basal anterior tethering angle, ° |
PLAX | ≥39.5° | NR | NR | NR |
| 362; 2D TTE; ERO, RV | ||||||||
| 19% (≥2+) (median 14 mo) |
||||||||
| Troubil and colleagues, 27 2012 |
LVEDD index, mm/m | PLAX | Anterior tethering angle, ° | AP4CH | ≥27° | 0.72 | 67.0 | 76.0 |
| 87; 2D TTE; JA/LAA, VC, ERO, RV |
LVEF, % | - | ||||||
| 32% (≥2+) (24 ± 2 mo) | Jet direction (not central) PTA, ° |
NR AP4CH |
||||||
| Lee and colleagues, 28 2012 250; 2D TTE; NR |
- | - | LVEDD index, cm/m2 | PLAX | ≥3.5 cm/m | NR | NR | NR |
| 13% (≥3+) (mean ± 2 y) | ||||||||
| van Garsse and colleagues, 29 2012 |
Basal anterior tethering angle, ° |
PLAX | ≥36.9 | NR | NR | NR | ||
| 435; 2D TTE; ERO, RV | Basal ATA/PTA ratio | PLAX | NR | NR | NR | NR | ||
| 23% (≥2+) (median45 mo) | Anterior leaflet excursion angle, ° |
PLAX | <35° | NR | NR | NR | ||
| van Garsse and colleagues, 30 2012 |
Papillary muscle dyssynchrony, ms |
AP4CH/PLAX | ≥58 ms | 0.92 | 100.0 | 83.0 | ||
| 144; 2D TTE; ERO, RV | Basal anterior tethering angle, ° |
PLAX | ≥39.5° | 0.86 | 95.0 | 80.0 | ||
| 68% (≥2+) (median 39 mo)║ |
Basal ATA/PTA ratio | PLAX | ≥0.75 | 0.82 | 88.0 | 79.0 | ||
| van Garsse and colleagues, 31 2013 |
LVEDV index and LVESV index, mL/m2 |
- | Left atrial peak global strain, % |
AP2/4CH | ≤25% | 0.90 | 92.0 | 87.0 |
| 95; 2D TTE; ERO, RV | WMSI | - | Peak systolic strain rate, s−1 | AP2/4CH | ≤1.50s−1 | 0.85 | 90.0 | 82.0 |
| 32% (≥2+) (median 42 mo) | Systolic and diastolic sphericity index |
- | Peak early diastolic strain rate, s−1 |
AP2/4CH | ≤1.11 s −1 | 0.79 | 86.0 | 80.0 |
| Tethering area, cm2 | PLAX | |||||||
| Coaptation length, mm | PLAX | |||||||
| Tethering height, mm | PLAX | |||||||
| Left atrial diameter, mm | PLAX | |||||||
| Different left atrial volumes, mL/m2 |
AP2/4CH | |||||||
| Peak late diastolic strain rate, s−1 |
AP2/4CH | |||||||
| van Garsse and colleagues, 32 2013 |
LVESVand LVEDV, mL | - | Papillary muscle dyssynchrony, ms |
AP4CH/PLAX | ≥58 ms | 0.97 | 98.0 | 90.0 |
| 524; 2D TTE; ERO, RV | Diastolic sphericity index | - | Systolic sphericity index | - | ≥0.72 | 0.85 | 88.0 | 71.0 |
| 21% (≥2+) (median 45 mo) |
WMSI | - | Basal anterior tethering angle, ° |
PLAX | ≥39° | 0.93 | 90.0 | 86.0 |
| Tethering area, cm2 | PLAX | Basal ATA/PTA ratio | PLAX | ≥0.76 | 0.89 | 93.0 | 83.0 | |
| Coaptation length, mm | PLAX | |||||||
| Tethering height, mm | PLAX | |||||||
| Kron and colleagues, 33 2015 |
- | - | Basal aneurysm/dyskinesis | - | - | - | - | - |
| 110; 2D TTE; JA/LAA, VC, ERO |
||||||||
| 60% (≥3+) (2 y) | ||||||||
| Hajsadeghi and colleagues, 34 2015 |
LVEF, % | - | LVESV, cm3 | - | ≥3.85 cm3 | 0.69 | 83.0 | 57.0 |
| 126; 2D TTE; VC, ERO | Basal-IPMD diastolic- systolic ratio |
PSAX | ≥1.25 | 0.95 | 100.0 | 95.0 | ||
| 46% (≥2+) (30 d) | ||||||||
| Wijdh-den Hamer, 2016 | Tethering height, mm | 2CH | PTA, ° | 2CH | ≥32.0 | 0.81 | 90.9 | 61.1 |
| 50; 2D TEE; JA/LAA | Tethering area, cm2 | 2CH | ||||||
| 26% (≥2+) (6 mo) | ||||||||
| 50; 3D TEE | Annular circumference, mm | 3D TEE | P3TA, ° | 3D TEE | ≥29.9° | 0.92 | 84.6 | 89.2 |
| Mitral valve tethering volume, mm |
3D TEE | Basal aneurysm/dyskinesis | 3D TEE | - | - | - | - | |
| Mitral valve tethering index | 3D TEE | |||||||
| A1, A2, A3, P1, P2 tethering angles, ° |
3D TEE |
2D, 2-dimensional; 3D, 3-dimensional; IMR, ischemic mitral regurgitation; AUC, area under the curve; (A)PM, (anterior) papillary muscle; AP2CH, apical 2-chamber view; TTE, transthoracic echocardiography; JA/LAA,jet area/left atrial area; NR, not reported; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; PLAX, parasternal long-axis view; VC, vena contracta; AP4CH, apical 4-chamber view; PTA, posterior tethering angle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; ERO, effective regurgitant orifice; PISA, proximal isovelocity surface area; PPM, posterior papillary muscle; IPMD, interpapillary muscle distance; PSAX, parasternal short-axis view; RV, regurgitant volume; LVEF, left ventricular ejection fraction; WMSI, wall motion score index; ATA, anterior tethering angle; PAP, pulmonary artery pressure; TEE, transesophageal echocardiography.
Defined as postoperative persistence or recurrence (within 1–5 years) IMR grade ≥2+.
Any increase in MR compared with the immediate postoperative phase.
Additional independent predictors from the same cohort were mentioned by Ueno and colleagues.20
A total of 53 patients with IMR and 56 patients with functional MR.
All patients had LV ejection fraction <35%.
DISCUSSION
Ring annuloplasty to reduce mitral annular size has been the most commonly used surgical treatment for IMR. A growing body of literature has documented that the risk of IMR persistence or recurrence after reduction annuloplasty is unacceptably high6–9; however, in patients who do not experience recurrent IMR, repair may offer benefits over valve replacement especially with regard to LV remodeling and function. This strongly suggests that an imaging strategy capable of reliably determining the risk of annuloplasty failure preoperatively would improve surgical results. Such a strategy would allow patients at high risk for valve repair failure to be treated with valve replacement.
The global and regional 3D pathologic anatomy of IMR is highly complex and varies greatly between patients. Annular dilatation and leaflet tethering occur to some degree in all patients with IMR, but the relative contribution of these parameters to valve incompetence differs significantly between patients. Reduction annuloplasty effectively treats annular dilatation, but it can exacerbate leaflet tethering by causing the anterior displacement of the posterior leaflet.11, 12, 25
The complex and varied 3D valvular pathology that causes IMR has likely contributed to the difficulty in establishing the usefulness of 2DE as a tool for preoperative repair failure risk stratification. These results are likely because 2DE measurements are highly dependent on viewing plane selection. Studies reporting on preoperative 2DE predictors of IMR recurrence after annuloplasty show inconsistent, frequently nonreproducible, and sometimes conflicting results (Table 4).6, 13–34 Some studies identify certain valvular, subvalvular, ventricular, or functional 2DE parameters as independent predictors, whereas the same predictors are not found to be predictive in other studies.6, 13–34 The current study clearly demonstrates the influence of viewing plane selection. Two-dimensional tethering parameters measured in the 2-chamber view are predictive of IMR recurrence, whereas the same 2-dimensional tethering parameters measured in the 4-chamber view are not predictive. The limitation of preoperative 2DE parameters to predict recurrent IMR was most recently demonstrated by a subgroup analysis of the Cardiothoracic Surgical Trials Network (CTSN) trial.33 In that study of 110 patients, leaflet tethering was determined to be the cause of recurrent IMR; however, none of the baseline 2DE measures of leaflet tethering were predictive of recurrent IMR.33 The lack of reliability of 2DE predictors is reflected in the fact that they have not been incorporated into current guidelines on surgical treatment of ischemic mitral valve disease.5 To achieve effective predictive models for a patient-specific approach, reliable preoperative (echocardiographic) predictors of IMR recurrence are needed.
We have previously shown that real-time 3DE combined with custom valve-modeling algorithms provides a useful tool for quantifying the complex 3D geometry of the entire mitral valve and, more important, is able to effectively predict the risk of IMR recurrence after undersized ring annuloplasty.10 Unlike 2DE, 3DE is not influenced by viewing plane selection, regional asymmetry, or localized annular distortions. In the current study, 2DE PTA (measured in the 2-chamber view) independently predicts IMR recurrence with an AUC of 0.81. A fitted combined clinical and 2DE model in the subanalysis of the CTSN trial yielded a predictive model with a similar AUC of 0.83.33 Our 3D P3TA has proven to be a stronger independent predictor of IMR recurrence with an AUC of 0.92.
Results from this study and the CTSN trial suggest that the functional parameter “basal (inferior) aneurysm/dyskinesis” is an important determinant of IMR recurrence.33 The strong and reproducible predictive value of this parameter may be due to the fact that it reflects both mitral valve tethering and LV ischemic remodeling. Adding this functional parameter to the 3DE geometric multivariate model yields a new predictive model with an augmented predictive value (AUC increases from 0.92 to 0.94, sensitivity remains 84.6%, and specificity increases from 89.2% to 91.9%).
On the basis of these results presented, the prospective application of the 3DE imaging and mitral valve modeling algorithm described in this study should greatly reduce the incidence of recurrent IMR. Patients with severe leaflet tethering could be treated with chordal-sparing valve replacement. Alternatively, in centers where the expertise exists, more complex repair techniques that use leaflet and subvalvular maneuvers also could be used in appropriately selected patients.
Study Limitations
This study had several limitations. (1) Although it has many advantages, the imaging and modeling approach used in this study requires time-consuming off-line analysis. Therefore, work is in progress to develop automated segmentation techniques that will allow image processing and mitral leaflet segmentation in minutes rather than hours. (2) The end point was an echocardiographic measure of IMR recurrence, not a clinical outcome such as survival. However, there is strong evidence correlating IMR with reduced survival.1, 2 (3) IMR recurrence after repair was evaluated with transthoracic echocardiography and measured semiquantitatively with jet area/left atrium area. Alternative validated methods for quantitative IMR severity assessment, including regurgitant volume and effective regurgitant orifice, were not available in this study. (4) The number of patients was relatively small (n = 50), and follow-up was relatively short (6 months). (5) The predictive models described in this study require validation in future studies.
CONCLUSIONS
Both preoperative 2DE and 3DE combined with valve modeling are predictive of recurrent IMR, but 2DE results are highly influenced by viewing plane selection. Preoperative 3DE P3TA is a stronger independent predictor of IMR recurrence after undersized ring annuloplasty than preoperative 2DE PTA. In patients with a preoperative P3TA of 29.9° or larger (especially when combined with the presence of a basal aneurysm or dyskinesis), chordal-sparing valve replacement or additional (subvalvular) repair techniques should be strongly considered.
Supplementary Material
VIDEO 1.
The value of preoperative 3-dimensional over 2-dimensional valve analysis in predicting recurrent IMR after mitral annuloplasty.
Central Message.
Preoperative 3DE P3TA is a stronger independent predictor of IMR recurrence 6 months after annuloplasty than preoperative 2DE PTA.
Perspective.
Preoperative 3DE P3TA is a stronger predictor of IMR recurrence 6 months after annuloplasty than preoperative 2DE PTA, which is highly influenced by viewing plane selection. In patients with a preoperative P3TA of 29.9° or larger (especially when combined with the presence of a basal aneurysm/dyskinesis), chordal-sparing valve replacement should be strongly considered.
Acknowledgments
Funding was provided by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, Maryland (HL 103723, HL73021, and HL063954).
Abbreviations and Acronyms
- AUC
area under the curve
- CI
confidence interval
- CTSN
Cardiothoracic Surgical Trials Network
- df
degrees of freedom
- IMR
ischemic mitral regurgitation
- LV
left ventricular
- MR
mitral regurgitation
- OR
odds ratio
- P3TA
P3 tethering angle
- PTA
posterior tethering angle
- ROC
receiver operating characteristic
- TEE
transesophageal echocardiography
- 3D
3-dimensional
- 2DE
2-dimensional echocardiography
- 3DE
3-dimensional echocardiography
Footnotes
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
References
- 1.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Conner CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 3.Gillinov AM, Wierup PN, Blackstone EH, Bishay ES, Cosgrove DM, White J, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–1141. doi: 10.1067/mtc.2001.116557. [DOI] [PubMed] [Google Scholar]
- 4.Grossi EA, Goldberg JD, LaPietra A, Ye X, Zakow P, Sussman M, et al. Ischemic mitral valve reconstruction and replacement: comparison of long-term survival and complications. J Thorac Cardiovasc Surg. 2001;122:1107–1124. doi: 10.1067/mtc.2001.116945. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 6.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 8.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–353. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouma W, Lai EK, Levack MM, Shang EK, Pouch AM, Eperjesi TJ, et al. Preoperative three-dimensional valve analysis predicts recurrent ischemic mitral regurgitation after mitral annuloplasty. Ann Thorac Surg. 2016;101:567–575. doi: 10.1016/j.athoracsur.2015.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouma W, van der Horst ICC, Wijdh-den Hamer IJ, Erasmus ME, Zijlstra F, Mariani MA, et al. Chronic ischaemic mitral regurgitation. Current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg. 2010;37:170–185. doi: 10.1016/j.ejcts.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Kuwahara E, Otsuji Y, Iguro Y, Ueno T, Zhu F, Mizukami N, et al. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation. 2006;114:I529–I534. doi: 10.1161/CIRCULATIONAHA.105.000729. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga A, Tahta SA, Duran CM. Failure of reduction annuloplasty for functional ischemic mitral regurgitation. J Heart Valve Dis. 2004;13:390–397. [PubMed] [Google Scholar]
- 14.Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, Yu B, et al. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation. 2005;112:I396–I401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]
- 15.Ereminiene E, Vaskelyte J, Benetis R, Stoskute N. Ischemic mitral valve repair: predictive significance of restrictive left ventricular diastolic filling. Echocardiography. 2005;22:217–224. doi: 10.1111/j.0742-2822.2005.03108.x. [DOI] [PubMed] [Google Scholar]
- 16.Kongsaerepong V, Shiota M, Gillinov AM, Song JM, Fukuda S, McCarthy PM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol. 2006;98:504–508. doi: 10.1016/j.amjcard.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 17.Serri K, Bouchard D, Demers P, Coutu M, Pellerin M, Carrier M, et al. Is a good perioperative echocardiographic result predictive of durability in ischemic mitral valve repair? J Thorac Cardiovasc Surg. 2006;131:565–573. doi: 10.1016/j.jtcvs.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Roshanali F, Mandegar MH, Yousefnia MA, Rayatzadeh H, Alaeddini F. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg. 2007;84:745–749. doi: 10.1016/j.athoracsur.2007.04.106. [DOI] [PubMed] [Google Scholar]
- 19.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Senechal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–791. doi: 10.1161/CIRCULATIONAHA.106.649236. [DOI] [PubMed] [Google Scholar]
- 20.Ueno T, Sakata R, Iguro Y, Yamamoto H, Ueno M, Ueno T, et al. Preoperative advanced left ventricular remodeling predisposes to recurrence of ischemic mitral regurgitation with less reverse remodeling. J Heart Valve Dis. 2008;17:36–41. [PubMed] [Google Scholar]
- 21.Gelsomino S, Lorusso R, De Cicco G, Capecchi I, Rostagno C, Caciolli S, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J. 2008;29:231–240. doi: 10.1093/eurheartj/ehm468. [DOI] [PubMed] [Google Scholar]
- 22.Gelsomino S, Lorusso R, Caciolli S, Capecchi I, Rostagno C, Chioccioli M, et al. Insights on left ventricular and valvular mechanisms of recurrent ischemic mitral regurgitation after restrictive annuloplasty and coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2008;136:507–518. doi: 10.1016/j.jtcvs.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Gelsomino S, Lorusso R, Bille G, Rostagno C, De Cicco G, Romagnoli S, et al. Left ventricular diastolic function after restrictive mitral ring annuloplasty in chronic ischemic mitral regurgitation and its predictive value on outcome and recurrence of regurgitation. Int J Cardiol. 2009;132:419–428. doi: 10.1016/j.ijcard.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 24.Onorati F, Rubino AS, Marturano D, Pasceri E, Santarpino G, Zinzi S, et al. Midterm clinical and echocardiographic results and predictors of mitral regurgitation recurrence following restrictive annuloplasty for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2009;138:654–662. doi: 10.1016/j.jtcvs.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Ciarka A, Braun J, Delgado V, Versteegh M, Boersma E, Klautz R, et al. Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol. 2010;106:395–401. doi: 10.1016/j.amjcard.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Gelsomino S, van Garsse L, Luca F, Lorusso R, Cheriex E, Rao CM, et al. Impact of preoperative anterior leaflet tethering on the recurrence of ischemic mitral regurgitation and the lack of left ventricular reverse remodeling after restrictive annuloplasty. J Am Soc Echocardiogr. 2011;24:1365–1375. doi: 10.1016/j.echo.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Troubil M, Marcian P, Gwozdziewicz M, Santavy P, Langova K, Nemec P, et al. Predictors of failure following restrictive annuloplasty for chronic ischemic mitral regurgitation. J Card Surg. 2012;27:6–12. doi: 10.1111/j.1540-8191.2011.01342.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee LS, Kwon MH, Cevasco M, Schmitto JD, Mokashi SA, McGurk S, et al. Postoperative recurrence of mitral regurgitation after annuloplasty for functional mitral regurgitation. Ann Thorac Surg. 2012;94:1211–1216. doi: 10.1016/j.athoracsur.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 29.van Garsse L, Gelsomino S, Luca F, Lorusso R, Rao CM, Stefano P, et al. Importance of anterior leaflet tethering in predicting recurrence of ischemic mitral regurgitation after restrictive annuloplasty. J Thorac Cardiovasc Surg. 2012;143:S54–S59. doi: 10.1016/j.jtcvs.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 30.van Garsse L, Gelsomino S, Parise O, Luca F, Cheriex E, Lorusso R, et al. Systolic papillary muscle dyssynchrony predicts recurrence of mitral regurgitation in patients with ischemic cardiomyopathy (ICM) undergoing mitral valve repair. Echocardiography. 2012;29:1191–1200. doi: 10.1111/j.1540-8175.2012.01789.x. [DOI] [PubMed] [Google Scholar]
- 31.van Garsse L, Gelsomino S, Luca F, Parise O, Cheriex E, Rao CM, et al. Left atrial strain and strain rate before and following restrictive annuloplasty for ischaemic mitral regurgitation evaluated by two-dimensional speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:534–543. doi: 10.1093/ehjci/jes206. [DOI] [PubMed] [Google Scholar]
- 32.van Garsse L, Gelsomino S, Luca F, Parise O, Lorusso R, Cheriex E, et al. Left ventricular dyssynchrony is associated with recurrence of ischemic mitral regurgitation after restrictive annuloplasty. Int J Cardiol. 2013;168:176–184. doi: 10.1016/j.ijcard.2012.09.098. [DOI] [PubMed] [Google Scholar]
- 33.Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:752–761. doi: 10.1016/j.jtcvs.2014.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajsadeghi S, Samiee N, Hosseini SS, Hassanzadeh M, Kerman SR. Novel Echocardiographic indices as predictors of immediate recurrence after undersized ring annuloplasty for ischemic mitral regurgitation. Echocardiography. 2015;32:1339–1346. doi: 10.1111/echo.12879. [DOI] [PubMed] [Google Scholar]
- 35.Miyatake K, Izumi S, Okamoto M, Kinoshita N, Asonuma H, Nakagawa H, et al. Semiquantitative grading of severity of mitral regurgitation by real-time two-dimensional Doppler flow imaging technique. J Am Coll Cardiol. 1986;7:82–88. doi: 10.1016/s0735-1097(86)80263-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.