Abstract
Chronic gastrointestinal (GI) complaints are often treated with proton pump inhibitors (PPIs), antibiotics, and other medications that offer temporary relief of acute symptoms. Although these drugs are prescribed to provide promising results, new research indicates the drug treatments often mask unresolved physiological problems and cause further complications. Complex GI cases require a comprehensive assessment and a multifaceted approach. This case reports on the development of PPI-induced hypochlorhydria secondary to a PPI prescription for unresolved gastritis in a patient with irritable bowel syndrome. The patient’s gastritis and hypochlorhydria symptoms resolved with the implementation of a comprehensive functional nutrition approach that incorporated dietary guidelines, lifestyle recommendations, and customized nutritional supplementation and herbs.
Proton pump inhibitors (PPIs)—inhibitor of gastric acid production1—are amongst the top 10 most commonly prescribed drugs in the world2 for the treatment and management of gastroesophageal reflux and peptic ulcers. They are widely prescribed and available over the counter for a range of gastrointestinal (GI) conditions from heartburn to gastritis, often with little or no testing.3 A total of 25% of the general population is said to experience heartburn at least once per month.2
PPIs were first thought to have few side effects.4 New evidence, particularly with extended use, has revealed a decrease in absorption of some key vitamins and minerals, gut dysbiosis, rebound stomach acid hypersecretion, increased reflux-like symptoms, and hypergastrinemia. Hypergastrinemia may be associated with an increased risk of stomach cancer.5
The current use of PPIs often focuses primarily on symptom management rather than addressing treatable factors that have contributed to the gastroesophageal reflux, gastritis, and even peptic ulcers.6
Patient Case
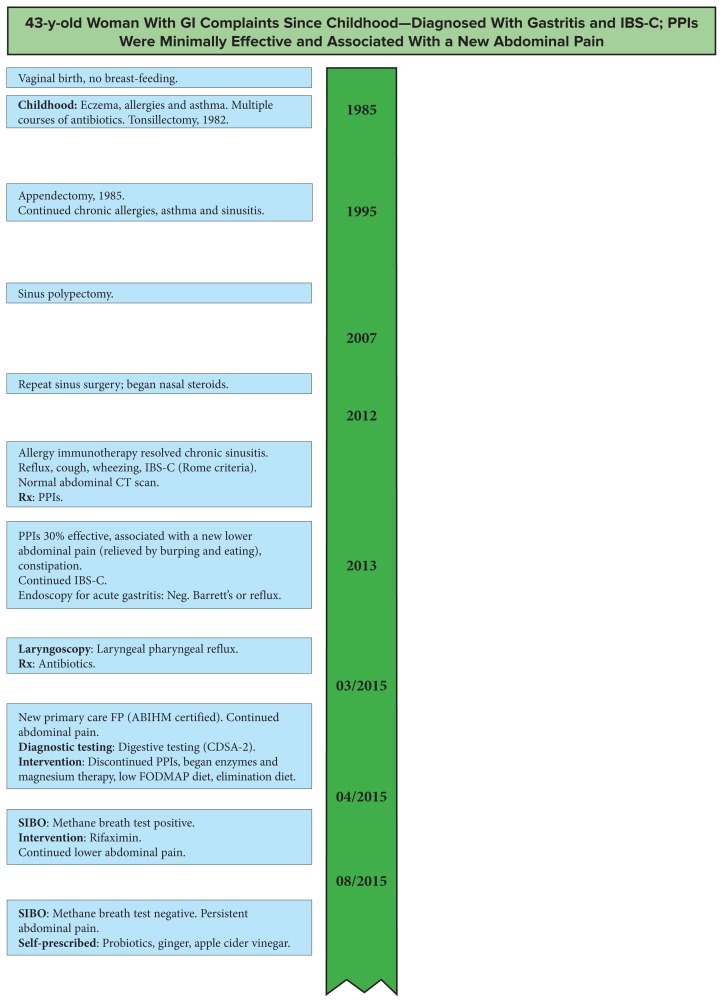
The patient was a 43-year-old female professional athlete, of healthy weight. She complained about anxiety, GI symptoms (dyspepsia, constant burping, abdominal pain, and constipation) and a recent diagnosis of small intestinal bacterial overgrowth (SIBO).
She had been born vaginally but was not breast-fed. She had history of allergies, childhood eczema, chronic sinusitis, asthma, repeated antibiotics much of her life, a tonsillectomy, an adenoidectomy, 2 sinus surgeries for polyps, and allergy shots during the past 2 years. She had previously tested negative for celiac disease but avoided gluten due to bloating. After she became a mother (age 39 y), with a daughter born prematurely, she developed postpartum anxiety, nervousness, and mood swings. Two years later, her father died and she developed abdominal pain, anxiety associated with a cough, and wheezing in her throat. During an emergency room visit for her symptoms, she had a normal computed tomography scan, was diagnosed with anxiety-related gastroesophageal reflux, and was given a prescription for PPIs. She did not receive any material or education related to nutritional support.
For the next 2 years, she tried 3 different PPIs, and all were minimally effective. She did note some improvement from self-prescribed dietary changes. Her anxiety continued and she developed new symptoms: a lower abdominal pain relieved only by forced burping and eating, tingling in her hands and feet, and constipation. She requested an endoscopy, which was positive for acute gastritis but negative for Helicobacter pylori, reflux, eosinophilic esophagitis, or Barrett’s esophagus. A pH monitor revealed no reflux and she was diagnosed with irritable bowel syndrome with constipation (IBS-C; Rome criteria). Laryngoscopy revealed laryngeal pharyngeal reflux and she was treated with antibiotics. She did not receive any material or education related to nutritional support.
In an attempt to get help with her ongoing symptoms, she consulted with a new primary care family practitioner who was board-certified in integrative medicine (American Board of Integrative Holistic Medicine). Laboratory testing revealed suboptimal B12 and magnesium, and a breath test was positive for methane, suggesting SIBO. She was started on magnesium and digestive enzymes (which improved her constipation) and was prescribed rifaximin 550 mg TID for 10 days. The medication; a low fermentable oligo-saccharides, di-saccharides, mono-saccharides, and polyols (FOMDAP) diet for SIBO; and an elimination diet decreased her complaints of gas but failed to resolve her upper and lower abdominal pains. These were relieved with only forced burping or food. Four months later, with ongoing symptoms, repeat breath test at Johns Hopkins University (Baltimore, MD, USA), she was negative for SIBO and methane. At that point, she self-prescribed probiotics, chewed ginger, and drank apple cider vinegar, all with mixed results.
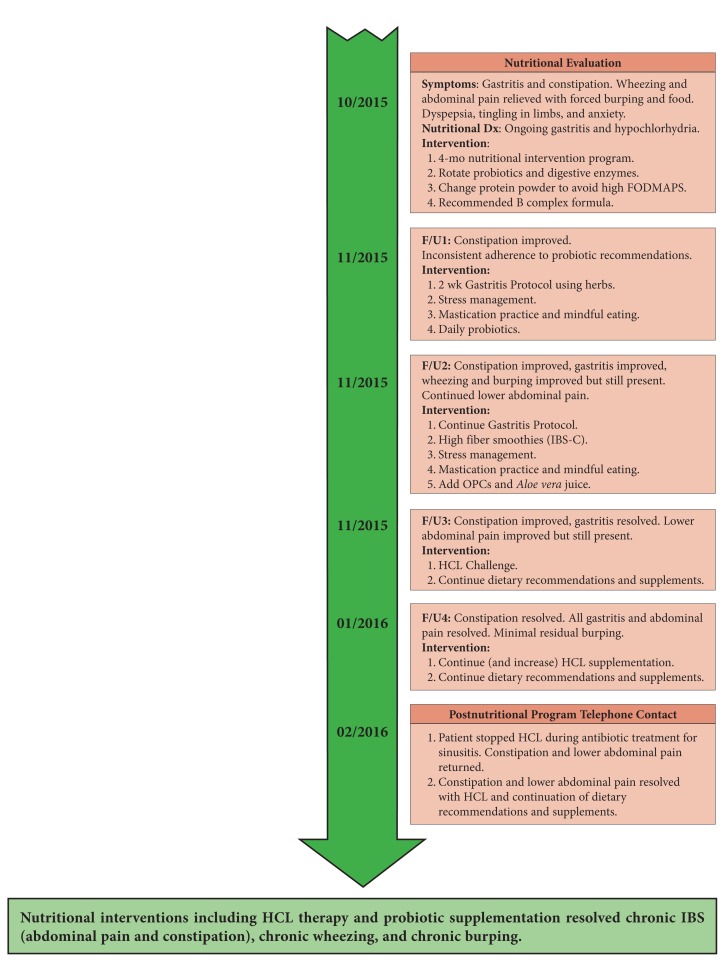
Her first nutrition consultation was 2 months later in October 2015. She was anxious and, based on her GI symptoms alone, she had dyspepsia, gastritis, possible PPI-induced hypochlorhydria, and a diagnosis of IBS-C. Her diet at the time was free of gluten, dairy, and legumes. She enjoyed vegetables, could not tolerate baked goods, and craved sugar. She was a fast eater and gulped her meals.
Initially, we discontinued the patient’s daily protein shake to avoid high FODMAPs. She was inconsistent with taking probiotics but believed that the enzymes she was taking were helping with her constipation. At a follow-up 2 weeks later, the patient was provided nutritional education and a 2-week dietary plan for gastritis, including 75 mg zinc carnosine BID and 1 smoothie per day with added l-glutamine, slippery elm, GI repair powder, and low-allergenic pea protein powder. Stress management tools were provided, including breathing techniques, phone apps, and materials on binaural rhythms, all designed to reduce anxiety. The patient was educated on potential effectiveness of probiotics to encourage compliance. In addition, she was asked to chew slowly and thoroughly and to practice mindful eating.
During the third consultation 2 weeks later, her gastritis pain, wheezing, and burping had decreased, but the lower abdominal pain continued. Her fiber and food intake were both inadequate, so additional strategies for an IBS-C appropriate fiber to reduce constipation were discussed. The patient also received education on the proper preparation of legumes to support digestion and optimize absorption. She was advised to continue the gastritis protocol and incorporate mindful eating and thorough chewing at meals. Supplementation with Aloe vera juice for additional GI support and oligomeric proanthocyanidins (OPCs) for allergies and sinuses was also recommended.
Two weeks later, her stomach pain had decreased and she reported improved mastication and slower paced chewing at meals. The sharp gastritis pain had resolved completely, but the refractory lower abdominal pain remained and still required forced burping or eating for relief.
Added legumes were well tolerated and enjoyed. Her constipation improved with increased fiber in her diet. She noticed that meat was hardest to digest. The new antihistamine OPC supplement and Aloe vera juice were well tolerated. With gastritis resolved an “HCL Challenge” was done to evaluate suspected hypochlorhydria prior to beginning supplementation with hydrochloric acid (HCL)/betaine/pepsin. In January 2016, she noted complete resolution of the refractory lower abdominal pain with daily HCL supplementation. She was encouraged to continue with current diet and supplements and her dose of HCL was increased in an attempt to treat her residual burping.
Epilogue
After another month, the patient contacted the functional nutritionist to advise that she had stopped HCL during another phase of antibiotics prescribed to treat her sinusitis. The lower abdominal pain returned, although this time without wheezing or burping. Approximately 30 days later, she sent another e-mail update stating that she was back on HCL (as well as a postantibiotic probiotic) and that the abdominal pain resolved again.
Patient Perspective
After being told I had IBS when several tests came back normal, I was determined to find the cause of my gut issues. The most valuable information and guidance came from working with my functional nutritionist. I can now live my life normally with no worries that I will have pain after I eat. After 2 years of mystery pains, I am pain free. I am forever grateful.
Discussion
Biomedical research has historically supported the hypothesis that heartburn is caused commonly by excessive stomach acid (often combined with a lax gastroesophageal sphincter tone). Suppression of acid production with PPIs to alleviate symptoms is the current standard of care. The use of PPIs (prescription and over the counter) to block stomach acid production is widespread and has been popularized by television advertisements. Stomach acid contains HCL, which is a digestive fluid secreted by stomach parietal cells that breaks down dietary proteins. Gastroesophageal reflux caused by regurgitation of HCL acid into the esophagus increases the risk of esophagitis, Barrett’s esophagus, and even esophageal cancer, and that is the main reason why PPIs remain the standard of care for any reflux.
Reflux can be caused by various factors and, depending on the cause, improved or eliminated without medications. For example, it can be caused by an erratic or weak lower esophageal sphincter, pressure from abdominal fat, hiatal hernia, or bile reflux. Moreover, overconsumption of fat in a meal can cause overexcretion of HCL, which can be mistaken for primary overproduction of HCL (hyperchlorhydria). Overuse of antacids and diet-induced hyperchlorhydria can lead to medication-induced hypochlorhydria. It is common to confuse reflux symptoms for hyperchlorhydria, when the symptom may actually be associated with hypochlorhydria.
Hypochlorhydria has detrimental health effects with long-term use, including autoimmune disorders.7 HCL plays a key role in many physiological processes; it triggers intestinal hormones; prepares folate and B12 for absorption; and is essential for the absorption of minerals, including calcium, magnesium, potassium, zinc, and iron. Lack or suppression of HCL has been associated with increase in osteoporosis; bone fractures; and impaired absorption of B12, iron and magnesium.8 HCL also prevents the overgrowth of pathogens in the upper GI tract, so hypochlorhydria can be a risk factor for SIBO.9 HCL is also responsible for breaking down dietary proteins aiding in the prevention of food allergies associated with incomplete digestion of protein. Acid-blocking medications are widely available and, despite the fact that they were initially approved for short-term use, they are often taken for extended periods, even years.
Functional medicine is an approach used to address underlying causes of disease, using a systems-oriented approach engaging patient and practitioner in a therapeutic partnership shifting the traditional disease-centered focus of medical practice to a more patient-centered approach.10 Functional nutrition is aligned with the functional medicine model and shares an emphasis that food is medicine, that a healthy GI tract is a critical foundation for health, and that many chronic complaints are linked to a compromised GI tract. Functional nutritionists use an evidence-based approach and are trained to assess patients empirically, symptomatically, and in response to specific dietary interventions, lifestyle changes, and dietary supplements.
It is not uncommon to see PPI-induced hypochlorhydria in patients on long-term PPIs. The “HCL Challenge” followed by supplementation (HCL/betaine/pepsin) is usually well tolerated and when used appropriately can stabilize physiological processes, support nutrient assimilation, and aid in the digestion of proteins (providing amino acids and preventing food allergies). It also helps prevent pathogenic infections through a restoration of a more normal GI microbiome. The resolution of this patient’s symptoms (including the forced burping) suggested that her gastritis and hypochlorhydria had resolved. Probiotic supplementation was also used to rebuild the microbiome severely affected by years of antibiotics and stress.
The simple noninvasive “HCL Challenge” can be used when hypochlorhydria is suspected. The patient starts with 1 capsule of betaine/HCL/pepsin (650–750 mg) with a meal containing protein and increases the dose to tolerance (a healthy stomach may produce 1.5 L of gastric acid daily).11 Heartburn, heat, or abdominal discomfort at a given dose will indicate an excessive dose, although heartburn with 1 capsule indicates normally secreted HCL, and the highest asymptomatic dose becomes the baseline dose for HCL supplementation, depending on the protein content of meals. The “HCL Challenge” is contraindicated in patients with active gastritis, esophagitis, or duodenitis.
Limitations
It is difficult to determine the mechanisms leading to the resolution of individual symptoms in a system-oriented approach to treatment with multiple interventions. It appears that the combination of therapies were associated with this patient’s successful outcome.
Conclusion
This case report demonstrates the complex nature of chronic conditions in patients, recognizing that some of the medications used to manage symptoms may actually create new medical imbalances. This is not atypical of the clinical practice of a functional nutritionist. In this case, the patient’s GI complaints resolved and, in the process, the patient became empowered to maintain her health.
Figure 1.
Patient Timeline
Abbreviations: IBS-C, irritable bowel syndrome with constipation; CT, computed tomography; PPIs, proton pump inhibitors; FP, family physician; ABIHM, American Board of Integrative Holistic Medicine; CDAS-2, Comprehensive Digestive Stool Analysis 2.0; FODMAP, fermentable oligo-saccharides, di-saccharides, mono-saccharides, and polyols; SIBO, small intestinal bacterial overgrowth; OPC, oligomeric proanthocyanidins; HCL, hydrochloric.
Acknowledgements
This case report followed the CARE guidelines for case reports. Kasia Kines and Tina Krupczak are doctoral students in clinical nutrition at the Maryland University in Integrative Health (Laurel, MD, USA). The authors wish to acknowledge David Riley, md, for his support with this case report.
References
- 1.Weekes LM. Proton pump inhibitors: Too much of a good thing? Med J Aust. 2015;202(9):464. doi: 10.5694/mja15.00477. [DOI] [PubMed] [Google Scholar]
- 2.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimer C. Safety of long-term PPI therapy. Best Pract Res Clin Gastroenterol. 2013;27(3):443–454. doi: 10.1016/j.bpg.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbol DE. Strategies for discontinuation of proton pump inhibitors: A systematic review. Fam Pract. 2014;31(6):625–630. doi: 10.1093/fampra/cmu050. [DOI] [PubMed] [Google Scholar]
- 6.Ho CE, Goh YL, Zhao XX, Yu CY, Zhang C. GERD: An Alternative Perspective. Psychosomatics. 2016;57(2):142–151. doi: 10.1016/j.psym.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Sandberg-Lewis S. Functional Gastroenterology: Assessing and Addressing the Causes of Functional Gastrointestinal Disorders. Portland, OR: NCNMC Press; 2009. pp. 152–153. [Google Scholar]
- 8.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12(6):448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol Hepatol. 2007;3(2):112–122. [PMC free article] [PubMed] [Google Scholar]
- 10.The Institute for Functional Medicine. IFM Web site. [Accessed April 3, 2016]. http://www.functionalmedicine.org.
- 11.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134(7):1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]