Abstract
STUDY QUESTION
Is protein expression of the muscle segment homeobox gene family member MSX1 altered in the human secretory endometrium by cell type, developmental stage or fertility?
SUMMARY ANSWER
MSX1 protein levels, normally elevated in the secretory phase endometrium, were significantly reduced in endometrial biopsies obtained from women of infertile couples.
WHAT IS KNOWN ALREADY
Molecular changes in the endometrium are important for fertility in both animals and humans. Msx1 is expressed in the preimplantation mouse uterus and regulates uterine receptivity for implantation. The MSX protein persists a short time, after its message has been down-regulated. Microarray analysis of the human endometrium reveals a similar pattern of MSX1 mRNA expression that peaks before the receptive period, with depressed expression at implantation. Targeted deletion of uterine Msx1 and Msx2 in mice prevents the loss of epithelial cell polarity during implantation and causes infertility.
STUDY DESIGN, SIZE DURATION
MSX1 mRNA and cell type-specific levels of MSX1 protein were quantified from two retrospective cohorts during the human endometrial cycle. MSX1 protein expression patterns were compared between fertile and infertile couples. Selected samples were dual-labeled by immunofluorescence microscopy to localize E-cadherin and β-catenin in epithelial cells.
PARTICIPANTS/MATERIALS, SETTING METHODS
MSX1 mRNA was quantified by PCR in endometrium from hysterectomies (n = 14) determined by endometrial dating to be in the late-proliferative (cycle days 10–13), early-secretory (cycle days 14–19) or mid-secretory (cycle days 20–24) phase. MSX1 protein was localized using high-throughput, semi-quantitative immunohistochemistry with sectioned endometrial biopsy tissues from fertile (n = 89) and infertile (n = 89) couples. Image analysis measured stain intensity specifically within the luminal epithelium, glands and stroma during the early-, mid- and late- (cycle days 25–28) secretory phases.
MAIN RESULTS AND THE ROLE OF CHANCE
MSX1 transcript increased 5-fold (P < 0.05) between the late-proliferative and early secretory phase and was then down-regulated (P < 0.05) prior to receptivity for implantation. In fertile patients, MSX1 protein displayed strong nuclear localization in the luminal epithelium and glands, while it was weakly expressed in nuclei of the stroma. MSX1 protein levels accumulated throughout the secretory phase in all endometrial cellular compartments. MSX1 protein decreased (P < 0.05) in the glands between mid- and late-secretory phases. However, infertile patients demonstrated a broad reduction (P < 0.001) of MSX1 accumulation in all cell types throughout the secretory phase that was most pronounced (∼3-fold) in stroma and glands. Infertility was associated with persistent co-localization of E-cadherin and β-catenin in epithelial cell junctions in the mid- and late-secretory phases.
LIMITATIONS, REASONS FOR CAUTION
Details of the infertility diagnoses and other patient demographic data were not available. Therefore, patients with uterine abnormalities (Mullerian) could not be distinguished from other sources of infertility. Antibody against human MSX2 is not available, limiting the study to MSX1. However, both RNAs in the human endometrium are similarly regulated. In mice, Msx1 and Msx2 are imperative for murine embryo implantation, with Msx2 compensating for genetic ablation of Msx1 through its up-regulation in a knockout model.
WIDER IMPLICATIONS OF THE FINDINGS
This investigation establishes that the MSX1 homeobox protein accumulation is associated with the secretory phase in endometrium of fertile couples, and is widely disrupted in infertile patients. It is the first study to examine MSX1 protein localization in the human endometrium, and supported by genetic findings in mice, suggests that genes regulated by MSX1 are linked to the loss of epithelial cell polarity required for uterine receptivity during implantation.
STUDY FUNDING/COMPETING INTERESTS
This research was supported by the NICHD National Cooperative Reproductive Medicine Network grant HD039005 (M.P.D.), NIH grants HD068524 (S.K.D.), HD071408 (D.R.A., M.P.D.), and HL128628 (S.D.), the Intramural Research Program of the NICHD, March of Dimes (S.K.D., S.D.) and JSPS KAKENHI grant 26112506 (Y.H.). There were no conflicts or competing interests.
Keywords: endometrium, implantation, menstrual cycle, secretory phase, female infertility, transcription factor, muscle segment homeobox 1, messenger RNA, immunohistochemistry, E-cadherin
Introduction
Abnormalities in the process of implantation are believed to be the basis of much unexplained infertility (Simon and Laufer, 2012). It has been established that histological dating of endometrial biopsies is a poor predictor of infertility (Coutifaris et al., 2004). The current literature indicates that the endometrium can be accurately evaluated at the molecular level to identify the receptive phase and changes associated with abnormal implantation. Transcriptomic analysis of endometrial biopsies by gene array has documented significant changes across the normal endometrial cycle (Punyadeera et al., 2005; Yanaihara et al., 2005; Henriquez et al., 2006; Talbi et al., 2006; Lai et al., 2007), during uterine receptivity for implantation (Kao et al., 2002; Dominguez et al., 2003; Riesewijk et al., 2003; Mirkin et al., 2005; Simon et al., 2005; Henriquez et al., 2006), with gonadotrophin therapy (Mirkin et al., 2004; Horcajadas et al., 2005; Simon et al., 2005), in endometriosis (Eyster et al., 2002; Arimoto et al., 2003; Matsuzaki et al., 2004, 2005; Wu et al., 2006), and in endometrial cancer (Hever et al., 2006). Several genes contributing to endometrial development have been identified that appear to become dysregulated in disease. Proteins that accumulate during the secretory phase have been strongly implicated in various etiologies of infertility, including endometriosis (Kao et al., 2003), idiopathic infertility (Laird et al., 1997; Mikolajczyk et al., 2007), and failed uterine receptivity for implantation (Das et al., 1994; Leach et al., 1999, 2012; Lessey, 2002; Wang and Armant, 2002; Kumar et al., 2003; Franco et al., 2008).
Successful implantation requires establishment of efficient, bidirectional communication between an implantation-competent blastocyst and the receptive uterus (Cha et al., 2012; Zhang et al., 2013). The uterine lining, which consists of luminal epithelium (LE), glandular epithelium (GE) and stroma (STR; mesenchyme, leukocytes, endothelium and vascular smooth muscle), undergoes a series of biological transformations during the secretory component of the menstrual cycle that is crucial for the success of embryo implantation (Gellersen and Brosens, 2014; Leach et al., 2012). The human endometrium enters into a ‘receptive’ interval comprised of only 4 days during the mid-secretory phase (MSP) (Cha et al., 2012). Pregnancy success is negatively impacted when the receptive interval is disrupted. For example, if embryo implantation occurs 1 day after the receptive interval, the rate of early pregnancy loss increases from 25 to 40%, and is further elevated to 80% after a delay of 2 days (Wilcox et al., 1999). Few studies have been published involving the receptive endometrium and its disruption, which potentially leads to infertility and recurrent pregnancy loss (Martel and Psychoyos, 1981; Nikas et al., 1995; Lessey, 2010; Aghajanova et al., 2011).
Muscle segment homeobox gene family members msh homeobox 1 (Msx1) and msh homeobox 2 (Msx2) encode transcription factors that control organogenesis and tissue interactions during embryonic development (Alappat et al., 2003). Msx1 and Msx2 are expressed in the preimplantation mouse uterus, and are critical for fertility in mice (Daikoku et al., 2011; Nallasamy et al., 2012). It has been suggested that these transcription factors have cell-specific functions in the pregnant uterus, and its subsequent morphological and functional changes (Cha et al., 2013). The Msx genes are expressed at very low levels in uteri of nonpregnant mice, increase dramatically prior to implantation (Days 3–4 of pregnancy), and are rapidly down-regulated approaching implantation and thereafter (Daikoku et al., 2011; Nallasamy et al., 2012). Conditional uterine ablation of both Msx1 and Msx2 induces infertility in mice due to a failure of implantation, and is accompanied by persistent polarized organization of epithelial cell junctions with co-localized E-cadherin and β-catenin (Daikoku et al., 2011).
In the human endometrial cycle, MSX1 mRNA expression appears to be similarly down-regulated before the window of implantation, at MSP (Kao et al., 2002; Riesewijk et al., 2003; Mirkin et al., 2005; Talbi et al., 2006). We hypothesized that MSX1 transcript and protein expression in the human endometrium correlates with uterine receptivity for implantation, and is disrupted in a subset of infertile couples. To address this idea, the expression pattern of human MSX1 was first determined during the endometrial cycle. We then utilized 178 secretory phase endometrial biopsies obtained by the National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network's Endometrial Biopsy Project, equally distributed between women from fertile and infertile couples, to delineate the developmental dynamics of MSX1 protein with regard to cyclic, cell type and pathology-associated expression levels.
Materials and Methods
Sample collection
Endometrial tissues were obtained from two retrospective cohorts. The first cohort of endometrial samples was collected for MSX1 mRNA analysis with institutional review board approval at the University of Tokyo. Fertile women (aged 38.8 ± 8.8 years; mean ± SD; N = 14) underwent hysterectomy due to uterine fibroids. All subjects had regular menstrual cycles, and received no hormonal treatment for at least 6 months before surgery. Endometrial samples were dated and grouped, according to the women's menstrual history and standard histological criteria (Noyes et al., 1950). Tissues were collected during the late-proliferative phase (LPP; cycle days 10–13, N = 4), ESP (N = 6), and MSP (N = 4), and snap-frozen for RNA isolation. RNA was isolated from the tissues using ISOGEN (Nippongene, Toyama, Japan).
A second cohort of endometrial biopsies was obtained for MSX1 protein analysis with approval of the Wayne State University Human Investigation Research Board, and those of all universities that contributed human biopsy tissues. To determine cell-, cycle- and fertility-specific expression of MSX1 protein, timed endometrial biopsies, originally collected by the Reproductive Medicine Network as part of a study examining the incidence of out of phase endometrial biopsies, were used from fertile couples who had conceived and delivered in the preceding 2 years (controls, n = 89) and infertile couples (n = 89) (Coutifaris et al., 2004; Leach et al., 2012). The basis for sample inclusion was (i) consent from the research participants to utilize their tissues for additional research beyond the primary purpose of the original study of endometrial dating, and (ii) sufficient tissue in the paraffin blocks for preparation of supplemental slides. The mean ages of the women from fertile and infertile couples were 31.9 and 30.2 years, respectively; however, additional demographic or etiological patient information was not available. Timed endometrial biopsies were obtained, fixed and embedded in paraffin as previously described (Coutifaris et al., 2004; Leach et al., 2012). The biopsies were assigned cycle days, using histological dating criteria (Noyes et al., 1950), and were grouped into early secretory phase (ESP; cycle days 14–19), MSP (cycle days 20–24), and late-secretory phase (LSP; cycle days 25–28). While the cycle phase of the endometrial biopsies based on timing to the LH surge had been estimated as MSP and LSP, the histological evaluation revealed that 57 women were in the ESP, 80 in the MSP and 41 in the LSP of the cycle (Leach et al., 2012).
Quantitative PCR (qPCR)
Quantitative PCR was performed as previously described (Hirota et al., 2008). A house-keeping gene, GAPDH, was used as an internal standard. The following primers were used: MSX1, sense, 5′-TCCTCAAGCTGCCAGAAGAT-3′; antisense, 5′-TACTGCTTCTGGCGGAACTT-3′; GAPDH, sense, 5′-ACCACAGTCCATGCCATCAC-3′, antisense, 5′-TCCACCACCCTGTTGCTGTA-3′.
Immunohistochemistry (IHC)
Tissue sections (5 µm) from endometrial biopsies were deparaffinized and rehydrated through a series of xylene and ethanol, as previously described (Leach et al., 2012). Antigen retrieval was carried out by heating slides at 15 psi and 121°C for 15 min in modified citrate buffer, pH 6.1 (DAKO, Carpinteria, CA, USA). Slides were cooled for 20 min and washed three times with Tris-buffered saline (TBS). IHC was performed on sections prepared from each specimen using an antibody against MSX1 (Santa Cruz Biotechnology, Dallas, TX, USA). Negative controls were performed using 10 µg/ml nonimmune rabbit immunoglobulin IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The antibody was titered and an optimal concentration chosen based on the linear region of the IHC labeling curve, prepared using paraformaldehyde-fixed HTR-8/SVneo human trophoblast cells.
Antibody staining, to avoid operation bias, was performed using a DAKO Autostainer Universal Staining System, as previously described (Leach et al., 2012). Briefly, slides were incubated for 30 min in 3% H2O2 (v/v) followed by application of primary antibody for 1 h at room temperature. All samples were then incubated for 30 min with an EnVision Systems peroxidase-conjugated polymer coupled to anti-rabbit IgG (DAKO), and visualized with 3,3-diaminobenzidine (DAB, DAKO). All slides were then rinsed in Tris-buffered saline (TBS), mounted and cover slipped.
Immunofluorescence
Biopsies from selected fertile and infertile couples obtained during the MSP and LSP were examined for the expression of E-cadherin and β-catenin. Slides with deparaffinized/rehydrated tissue sections were dual labeled by immunofluorescence microscopy, using a mouse antibody against E-cadherin and a rabbit antibody against β-catenin (Abcam, Cambridge, MA, USA). Negative controls were labeled with 10 µg/ml each of nonimmune mouse and rabbit IgG (Jackson ImmunoResearch). Primary antibodies were visualized using fluorescein isothiocyanate (FITC)- and Texas red-conjugated secondary antibodies, with a 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain. Images of each fluorophore were captured by epifluorescence microscopy on a Leica (Wetzlar, Germany) DM IRB epifluorescence microscope with a Hamamatsu Orca cooled-chip digital camera (Hamamatsu City, Japan).
Semi-quantitative image analysis of MSX1 protein
The stain intensity obtained by IHC was quantified by image analysis, as previously detailed (Leach et al., 2012). This method of estimating tissue-specific relative antigen levels compares linearly with enzyme-linked immunosorbent assay (ELISA) results, although there is some overestimation at very low levels. Sections of each specimen stained with hematoxylin and eosin were used to identify and photo-document four to six random regions that contained all three of the tissue features (glands (GE), stroma (STR) and luminal epithelium (LE)) to be studied by IHC. Digital images of each selected region were obtained at ×200 using a Leica DM IRB microscope and mapped using a 1.5 × 1.0 inch glass coverslip containing a 6 × 4 grid. The left edge of the grid was aligned flush with the left edge of the tissue section to locate the regions of interest identified by hematoxylin and eosin staining on adjacent tissue sections. Monochromatic bright field images of the antibody/DAB stained tissues were obtained at ×400 using a Hamamatsu Orca digital camera. Brightness was adjusted in a region of each slide devoid of tissue by setting the gray level to 255 and kept constant for all samples. Using Simple PCI imaging software (Hamamatsu Corp., Sewickley, PA, USA), areas determined to be LE, GE and STR were each digitally traced to determine the mean gray level of the circumscribed areas on the DAB-labeled images.
Statistics
A significant P value from Levene's test demonstrated the inequality of variances among groups assessed for MSX1 mRNA. Therefore, the nonparametric Kruskal–Wallis test and post hoc multiple pairwise Wilcoxon test were performed to compare the MSX1 transcript levels. The IHC data were non-normally distributed with unequal variances according to the Shapiro–Wilk and Levene tests, respectively. The generalized estimating equation, which provides robust modeling and valid inferences when distribution assumptions are violated, was used to examine the main effects and interactions among fertility groups, tissue types and endometrial phases. The significant interaction between fertility groups and tissue types allowed separate comparisons among tissue types for fertile and infertile groups with Tukey's post hoc test. To examine the trend of MSX1 expression (gray level) throughout the endometrial cycle, linear regression was employed. Receiver operating characteristic (ROC) curves were used to assess the accuracy of MSX1 expression in predicting infertile patients. Best criterion for threshold values (cutoff point) was determined by the Youden index (Youden, 1950; Hughes, 2015). Statistical computations were performed using either the open-source R software (http://www.r-project.org/) or MedCalc ver 13.0.6 (MedCalc Software, Mariakerke, Belgium). Post hoc power of the study was estimated using G*Power software (version 3.1). A P value of < 0.05 was considered significant.
Results
MSX1 mRNA expression in fertile women
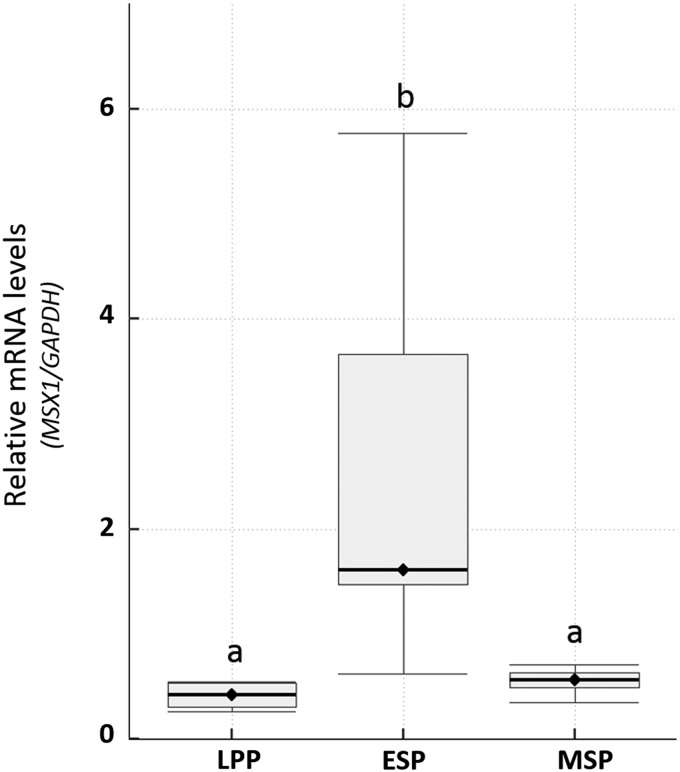
Uterine tissue obtained from hysterectomies performed in fertile women was used for qPCR analysis of RNA in the endometrial tissue. MSX1 mRNA was quantified during the LPP, ESP and MSP as receptivity for implantation emerges. Transcript increased ∼5-fold (P < 0.05) between the LPP and ESP, and then returned to low levels by the MSP (Fig. 1).
Figure 1.
Expression of homeobox protein MSX1 mRNA in endometrial tissues during the menstrual cycle. MSX1 mRNA was quantified by PCR in extracts prepared from endometrial tissue of women with normal fertility, which were obtained during the late-proliferative phase (LPP), early secretory phase (ESP) or mid-secretory phase (MSP). Boxes marked by different letters were significantly different (P < 0.05), as determined by the Kruskal–Wallis test and a post hoc multiple pairwise Wilcoxon test. Box = 25–75th percentiles; horizontal line within the box = median; whisker = 1.5 × Interquartile range (third quartile to first quartile).
Cellular expression of MSX1 in fertile couples
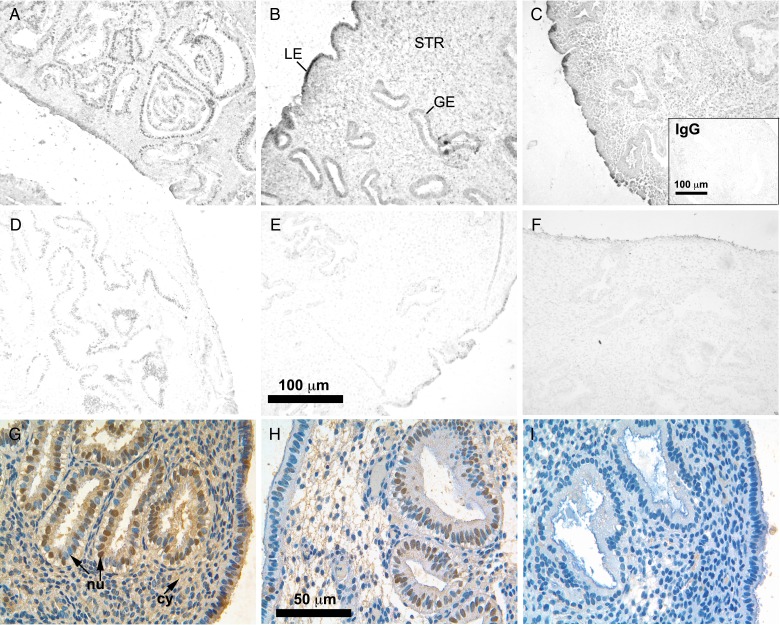
The expression of MSX1 protein was examined during the ESP, MSP and LSP in each cellular compartment (LE, GE and STR) of the endometrium in women from fertile couples. Although MSX1 mRNA declined in the MSP, the protein remained present and persisted to the LSP in all compartments, based on images of IHC labeling without a counterstain (Fig. 2A–C). Substitution of nonimmune IgG for primary antibody resulted in minimal IHC staining (Fig. 2C, inset). At high magnification with a hematoxylin counterstain, nuclear localization of MSX1 was apparent in the LE and GE, while cytoplasmic labeling was more predominant than nuclear labeling in the STR (Fig. 2G). Epithelial cells also contained unlabeled nuclei and exhibited labeling of the cytoplasm.
Figure 2.
Localization of homeobox protein MSX1 labeled by immunohistochemistry in endometrial biopsies of women from fertile and infertile couples. Representative images of MSX1 in fertile controls (A–C) and in women from infertile couples (D–F) during the early- (A, D), mid- (B, E), and late- (C, F) secretory phases. Images in (A–F) were not counterstained. Glands (GE), stroma (STR) and luminal epithelium (LE) are indicated in (B). Inset in (C) shows endometrial tissue from a fertile control labeled with nonimmune IgG in place of anti-MSX1 antibody, as a negative control. High magnification, color images of MSX1 labeling from fertile (G) and infertile (H, I) couples with a hematoxylin counterstain are shown in the bottom row. Nuclei (nu) in GE, indicated by arrows, were both labeled (brown) and unlabeled (blue) throughout all epithelia of fertile couples (G), and poorly labeled in STR. The cytoplasm (cy) was labeled in the STR, as well as epithelia. Reduced MSX1 labeling was found in the infertile group, ranging from low (H) to nearly absent (I).
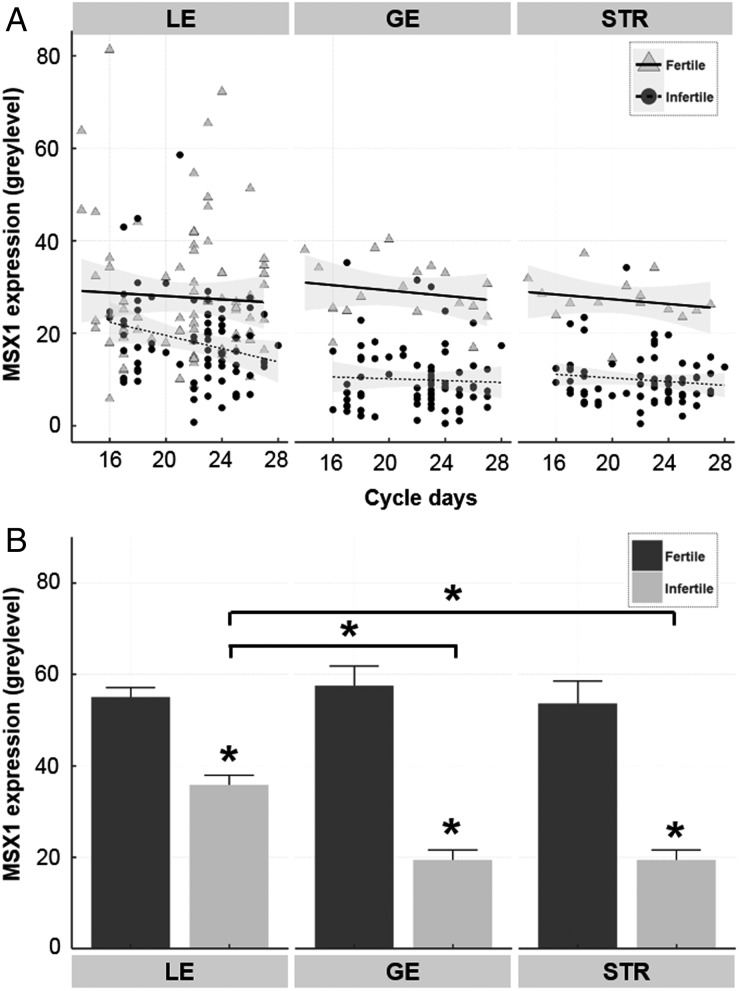
MSX1 protein expression was documented by semi-quantitative measurement of the IHC stain intensity. MSX1 was expressed in LE, GE and STR of fertile couples across the secretory phase, with no significant trend (Fig. 3A), according to regression analysis. Expression in all tissue compartments was highest during the MSP, with a significant decrease in stain intensity from an average gray level of 32.6 ± 3.8 to 24.7 ± 4.2 in the GE between the MSP and LSP for fertile patients (P < 0.05).
Figure 3.
Semi-quantitative analysis of homeobox protein MSX1 in endometrial biopsies of women from fertile and infertile couples during the secretory phase. MSX1 protein expression was calculated from gray levels measured by immunohistochemistry and image analysis, as described in the Materials and Methods. (A) MSX1 levels are shown for individual women by cycle day in the luminal epithelium (LE), glandular epithelium (GE) and stroma (STR). Cycle day was determined by histological dating. Regression lines are drawn with 95% CIs, indicated by shadowing for fertile and infertile couples. (B) Data were grouped as indicated, according to tissue region and fertility, showing the mean ± SEM values for MSX1. *P < 0.001 compared with fertile couples, or compared between tissue regions in the infertile couples.
Cellular expression of MSX1 in infertile couples
IHC analysis revealed dysregulation of MSX1 expression in women of infertile couples. MSX1 protein levels appeared to be noticeably reduced throughout endometrial tissues of women from infertile couples (Fig. 2D–F). MSX1 was reduced in epithelial cell nuclei of some specimens (Fig. 2H), while in others MSX1 was nearly absent (Fig. 2I). Very little cytoplasmic MSX1 was observed in the STR, with no nuclear labeling.
Semi-quantitative analysis of MSX1 IHC labeling confirmed its dysregulation with infertility. Infertile patients demonstrated a significant (P < 0.001) reduction in MSX1 expression throughout the endometrium during the secretory phase, compared with fertile couples (Fig. 3B), although there were several samples from infertile couples within the normal range (Fig. 3A). The greatest reductions (∼3-fold) were found in GE and STR, which demonstrated lower (P < 0.001) labeling by MSX1 antibody than the LE (Fig. 3B). Post hoc power analysis was performed to confirm statistically adequate sample size for each cell type, using mean expression and standard deviation values. Using a two-tail analysis at α = 0.01, and effect sizes of 0.788, 3.269 and 2.803, power values of 98.6, 100 and 100% were obtained for LE, GE and STR, respectively.
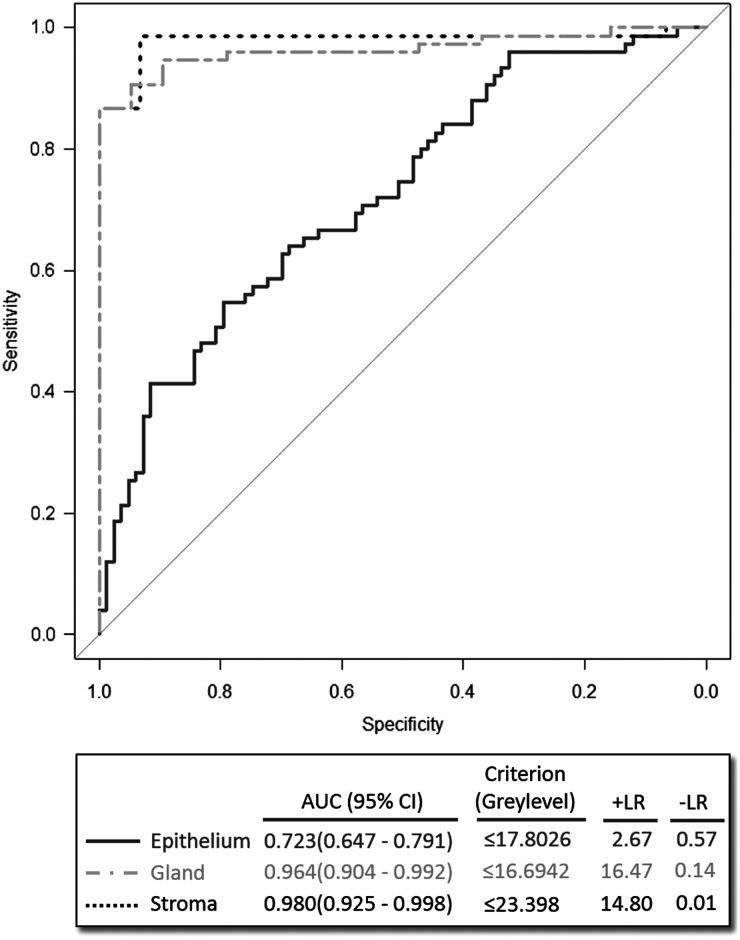
Diagnostic potential of MSX1 for the detection of infertility
The ROC curve analysis was performed for each cellular compartment (LE, GE and STR). The area under the curve (AUC) with 95% confidence intervals (CIs) was calculated for each region (Fig. 4). The results reveal a higher AUC for STR and GE regions (0.980 and 0.964, respectively) compared with LE (0.723). The accuracy of classifier prediction was cross-validated, using a bootstrap-based, Monte-Carlo sampling simulation with 1000 iterations. In each iteration, the data were split into a testing set that included 25% of randomly drawn cases, and a training set that comprised the remaining 75% of samples. The training set was used to train the classifier, which was subsequently evaluated by prediction of the group of samples (fertile/infertile) in the testing set. Using this method, the mean successful classification rates ± standard error of mean (SEM) for STR and GE were estimated as 93.7 ± 0.19% and 88.1 ± 0.17%, respectively.
Figure 4.
Receptor operator characteristic curve (ROC) analysis to distinguish infertility. ROC analysis was performed with gray level values obtained from luminal epithelium, glandular epithelium or stroma, as indicated, and plotting specificity against sensitivity. Areas under the curves (AUC) with 95% CIs were calculated for each cellular region, and the best criteria for threshold values were determined in the box below. Positive likelihood ratios (+LR) and negative likelihood ratios (−LR) were determined to assess diagnostic performance.
Expression of E-cadherin and β-catenin in fertile and infertile couples
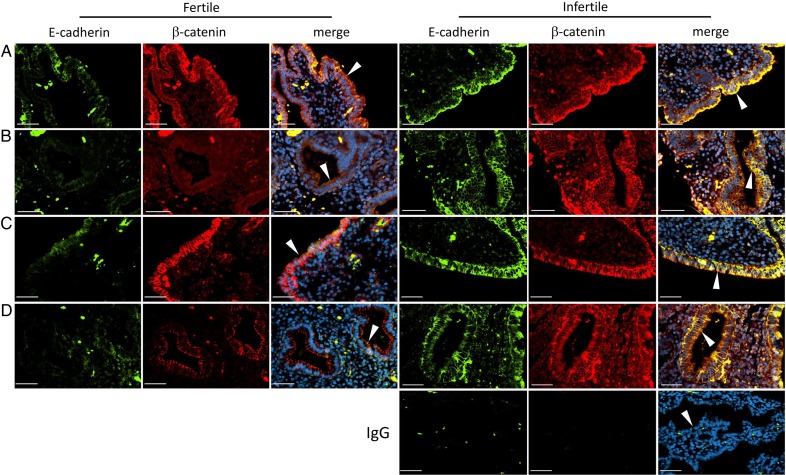
Deletion of Msx1 and Msx2 in mice disrupts the normal down-regulation of E-cadherin and β-catenin during implantation (Daikoku et al., 2011). To determine whether E-cadherin and β-catenin are dysregulated in endometrial epithelial cells from couples with infertility, selected fertile and infertile subjects were examined at MSP and LSP (n = 3, each). E-cadherin and β-catenin were not co-localized in epithelial cell junctions during the MSP and LSP in fertile couples, as shown in representative images (Fig. 5). E-cadherin expression was low in LE and GE (arrowheads in Fig. 5), while β-catenin was down-regulated in the GE, but remained present in LE junctions. In couples with infertility, both E-cadherin and β-catenin remained highly expressed and were co-localized in epithelial cell junctions during the MSP and LSP. Tissue sections of biopsies obtained at LSP from infertile patients that were labeled with nonimmune IgG of both species used for primary antibodies were negative (shown in Fig. 5).
Figure 5.
Expression and localization of E-cadherin and β-catenin in endometrial biopsies of women from fertile and infertile couples. Immunofluorescence images are shown of E-cadherin (green), β-catenin (red) and merged fields in fertile (left three columns) and infertile (right three columns) couples during the mid- (rows A and B) and late- (rows C and D) secretory phases, as indicated. Luminal epithelium (rows A and C) and glandular epithelium (rows B and D) are indicated by arrowheads in the merged images. Negative controls (IgG, bottom right three panels) were labeled with nonimmune IgG of both species used for the primary antibodies. 4′,6-Diamidino-2-phenylindole (DAPI) nuclear counterstain (blue) is included in the merged images. Bars 50 µM.
Discussion
This is the first report to describe the dynamic regulation of the MSX1 transcription factor in human endometrium, specifically focusing on the secretory phase in fertile and infertile couples. As predicted by studies in a mouse model (Daikoku et al., 2011; Nallasamy et al., 2012), MSX1 was elevated during the period of receptivity for implantation at the MSP, and appeared to be regulated through a brief period of heightened transcription at the ESP, prior to implantation. The protein appeared to be more stable than the mRNA, as in mice (Nallasamy et al., 2012), although it is not known whether it is down-regulated after an embryo implants. Consistently low levels of MSX1 protein were found throughout the secretory phase in the endometrium of women from infertile couples, in accordance with the infertility of mice lacking uterine Msx1/Msx2. Power analysis revealed that the sample size was adequate to distinguish infertile from fertile patients. Furthermore, ROC analysis demonstrated extremely high sensitivity and specificity to discriminate fertility status based on MSX1 accumulation in the STR and GE. The likelihood ratios (+LR; −LR) calculated in STR (14.80; 0.01) and GE (16.47; 0.14) regions classified MSX1 measurement as a ‘very useful’ and conclusive diagnostic test (Chien and Khan, 2001). The findings reveal that endometrial MSX1 deficiency could contribute clinically to infertility in women. Several proteins thought to function in embryo implantation were examined in this same set of 178 endometrial biopsies in a previous study (Leach et al., 2012). Although significant cyclic changes were documented, none were comparable with the relationship of MSX1 levels to fertility, suggesting that dysregulation of this transcription factor could represent a fundamental problem in human uterine receptivity.
Msx genes are crucial for transitioning the uterus between the pre-receptive and receptive states in mice (Daikoku et al., 2011), thereby regulating uterine receptivity and maintaining uterine readiness for implantation, independent of the ovarian hormone levels. Because the expression of MSX genes in the human endometrium is variable during the menstrual cycle, there are potentially implications for female fertility. Ablation of uterine Msx1/Msx2 in mice undergoing experimentally induced diapause alters the uterine proteome profile, inducing proteasomal stress and inflammation to confer a pseudoimplantation state that ultimately succumbs to resorption (Cha et al., 2015). Interestingly, in the mice lacking Msx1/Msx2, the phenotypic responses were attenuated by treatment with dexamethasone. The physiological responses in mice are consistent with the observations of inflammation and reduced fertility in endometrium of women with endometriosis (Macer and Taylor, 2012). A lack of uterine Msx1/Msx2 expression prevents the loss of epithelial polarity, which normally occurs on Day 4 of pregnancy in mice, with persistence of E-cadherin and β-catenin co-localization in cell junctions until Day 6 (Daikoku et al., 2011). We found a similar persistence of E-cadherin co-localization with β-catenin at epithelial cell junctions in couples with infertility. These studies provide evidence that the MSX gene family constitutes a conserved molecular mediator of female reproductive fitness, and support the view that MSX dysregulation contributes to infertility in humans, rather than being caused by infertility.
MSX1 protein expression was reduced in infertile patients, suggesting that its target genes might be critical to sustain blastocyst implantation and decidualization. Two other transcription factors essential for decidualization and successful implantation, homeobox A10 (HOXA10) and progesterone receptor B (PGRB) (Benson et al., 1996; Wetendorf and DeMayo, 2014), were previously examined in the same endometrial biopsy specimens, revealing both cyclic and fertility-dependent variations (Leach et al., 2012). A decline in PGRB at the ESP was delayed in the infertile group until MSP, suggestive of progesterone resistance. HOXA10, a target gene of PGRB (Benson et al., 1996; Wetendorf and DeMayo, 2014), rose significantly in the LE between MSP and LSP, but failed to do so in infertile couples. Additionally, leukemia inhibitory factor (LIF), a cytokine of the IL-6 family that is essential for uterine receptivity in mice (Stewart et al., 1992) was consistently lower in endometrial biopsies of infertile couples, although the decline did not reach statistical significance (Leach et al., 2012). Whether there is a correlation between the altered expression patterns of these regulatory proteins and the reduced expression of MSX1 in infertile couples is not known. Compromised uterine receptivity is a major cause of pregnancy failure in human in vitro fertilization (IVF) programs (Margalioth et al., 2006). Our findings suggest a potential avenue to improve implantation rates in IVF programs by manipulating uterine levels of MSX1 or its gene targets in embryo transfer cycles to improve uterine responsiveness for implantation.
The analysis of MSX1 in clinical specimens reported here has several limitations. Patient demographics were not available. The study included a population of individuals with infertility; however, the basis of the diagnosis of infertility was not available, and patients with uterine (Mullerian) abnormalities could not be distinguished from other sources of infertility. Infertility in about 20–30% of couples is due solely to a male factor without a uterine component (Agarwal et al., 2015). The ostensive inclusion of couples with nonuterine etiologies might account for MSX1 values of some infertile couples that fell within the range of fertile couples, evident in Fig. 3A. Our results were also limited to an analysis of only one MSX homeobox gene family member, as a specific antibody to human MSX2 is not available. Since microarray studies suggest that MSX1 and MSX2 expression levels are similarly altered in women during the window of implantation (Kao et al., 2002; Riesewijk et al., 2003; Mirkin et al., 2005; Talbi et al., 2006), we can speculate that MSX2 protein might also be down-regulated in infertile patients. An important future goal would be to investigate whether MSX1 protein expression is altered in the secretory endometrium of infertile patients because of implantation failure. With further validation, MSX1 could prove to be a useful biomarker of endometrial insufficiency.
This study provides an impetus for additional basic and translational research on the molecular function of MSX1 in human fertility. Protein expression and localization provided insights into the role of this gene in development of the endometrium that is not attainable from transcriptome analysis, potentially establishing a basis for MSX1 as a useful biomarker for evaluating women with reproductive abnormalities. Localization of MSX1 in endometrial biopsies by IHC indicated its presence in both nuclei and cytoplasm, similar to its staining patterns in mice (Nallasamy et al., 2012). Evidence from studies in mice indicating that Msx1/Msx2 signaling operates downstream through noncanonical Wnt5a and FGF pathways (Daikoku et al., 2011; Nallasamy et al., 2012) supports the possibility of a similar mechanism in human implantation. Indeed, there is ample evidence that the WNT pathway is widely expressed in the human endometrium, and regulates uterine cellular function (Tulac et al., 2003; Matsuzaki et al., 2010; Sonderegger et al., 2010; Li et al., 2013). An analysis of gene expression in pseudo-pregnant mouse uteri with ablated Msx1/Msx2 demonstrates changes in cell adhesion and the extracellular matrix (Sun et al., 2015) that could signify a general defect in the remodeling of the uterus required for successful trophoblast invasion during the peri-implantation period. These studies suggest that Msx1/Msx2 gene expression in humans is critical for uterine receptivity for implantation, closely paralleling its regulation and function in mice.
Authors’ roles
The study was designed, analyzed and written by A.D.B, J.M.B., B.A.K., H.-R.K.-G., J.D., M.P.D., Y.H., S.D., S.K.D. and D.R.A. It was executed by A.D.B, J.M.B., B.A.K., T.S., J.O., J.R. and Y.H. All authors read and approved the manuscript.
Funding
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Cooperative Reproductive Medicine Network grant HD039005 (M.P.D.), NIH grants HD068524 (S.K.D.), HD071408 (D.R.A., M.P.D.), and HL128628 (S.D.), the Intramural Research Program of the NICHD, March of Dimes (S.K.D., S.D.) and the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research Program grant 26112506 (Y.H.).
Conflict of interest
None declared.
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod 2011;84:801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res 2003;13:429–442. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y et al. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. Int J Oncol 2003;22:551–560. [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996;122:2687–2696. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Bartos A, Fenelon J, Lefevre P, Daikoku T, Shaw G, Maxson R, Murphy BD, Renfree MB et al. A new role for muscle segment homeobox genes in mammalian embryonic diapause. Open Biol 2013;3:130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Burnum-Johnson KE, Bartos A, Li Y, Baker ES, Tilton SC, Webb-Robertson BJ, Piehowski PD, Monroe ME, Jegga AG et al. Muscle segment homeobox genes direct embryonic diapause by limiting inflammation in the uterus. J Biol Chem 2015;290:15337–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien PF, Khan KS. Evaluation of a clinical test. II: Assessment of validity. BJOG 2001;108:568–572. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 2004;82:1264–1272. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell 2011;21:1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Mukhopadhyay PK, Chowdhury M. Carbohydrate-binding profile of a pregnancy-associated rat uterine glycoprotein. Mol Cell Biochem 1994;137:91–99. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Avila S, Cervero A, Martin J, Pellicer A, Castrillo JL, Simon C. A combined approach for gene discovery identifies insulin-like growth factor-binding protein-related protein 1 as a new gene implicated in human endometrial receptivity. J Clin Endocrinol Metab 2003;88:1849–1857. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril 2002;77:38–42. [DOI] [PubMed] [Google Scholar]
- Franco ES, Hyppolito SB, Franco RG, Oria MO, Almeida PC, Pagliuca LM, Rocha NF. Digital cervicography criteria: improving sensitivity in uterine cervical cancer diagnosis. Cad Saude Publica 2008;24:2653–2660. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Henriquez S, Tapia A, Quezada M, Vargas M, Cardenas H, Rios M, Salvatierra AM, Croxatto H, Orihuela P, Zegers-Hochschild F et al. Deficient expression of monoamine oxidase A in the endometrium is associated with implantation failure in women participating as recipients in oocyte donation. Mol Hum Reprod 2006;12:749–754. [DOI] [PubMed] [Google Scholar]
- Hever A, Roth RB, Hevezi PA, Lee J, Willhite D, White EC, Marin EM, Herrera R, Acosta HM, Acosta AJ et al. Molecular characterization of human adenomyosis. Mol Hum Reprod 2006;12:737–748. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol 2008;173:1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, Simon C. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod 2005;11:195–205. [DOI] [PubMed] [Google Scholar]
- Hughes G. Youden's index and the weight of evidence. Methods Inf Med 2015;54:198–199. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002;143:2119–2138. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003;144:2870–2881. [DOI] [PubMed] [Google Scholar]
- Kumar S, Brudney A, Cheon YP, Fazleabas AT, Bagchi IC. Progesterone induces calcitonin expression in the baboon endometrium within the window of uterine receptivity. Biol Reprod 2003;68:1318–1323. [DOI] [PubMed] [Google Scholar]
- Lai TH, King JA, Shih Ie M, Vlahos NF, Zhao Y. Immunological localization of syndecan-1 in human endometrium throughout the menstrual cycle. Fertil Steril 2007;87:121–126. [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod 1997;12:569–574. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab 1999;84:3355–3363. [DOI] [PubMed] [Google Scholar]
- Leach RE, Jessmon P, Coutifaris C, Kruger M, Myers ER, Ali-Fehmi R, Carson SA, Legro RS, Schlaff WD, Carr BR et al. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod 2012;27:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA. Adhesion molecules and implantation. J Reprod Immunol 2002;55:101–112. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Fine tuning of endometrial function by estrogen and progesterone through microRNAs. Biol Reprod 2010;82:653–655. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 2013;154:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 2012;39:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 2006;21:3036–3043. [DOI] [PubMed] [Google Scholar]
- Martel D, Psychoyos A. Estrogen receptors in the nidatory sites of the rat endometrium. Science 1981;211:1454–1455. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Vaurs-Barriere C, Pouly JL, Boespflug-Tanguy O, Penault-Llorca F, Dechelotte P, Dastugue B, Okamura K, Mage G. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol Hum Reprod 2004;10:719–728. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Pouly JL, Dechelotte P, Okamura K, Mage G. The macrophage stimulating protein/RON system: a potential novel target for prevention and treatment of endometriosis. Mol Hum Reprod 2005;11:345–349. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab 2010;95:3437–3445. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Wirstlein P, Skrzypczak J. The impact of leukemia inhibitory factor in uterine flushing on the reproductive potential of infertile women–a prospective study. Am J Reprod Immunol 2007;58:65–74. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotrophin-stimulated cycles. J Clin Endocrinol Metab 2004;89:5742–5752. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 2005;20:2104–2117. [DOI] [PubMed] [Google Scholar]
- Nallasamy S, Li Q, Bagchi MK, Bagchi IC. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet 2012;8:e1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, Psychoyos A. Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod 1995;10:1208–1213. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Dassen H, Klomp J, Dunselman G, Kamps R, Dijcks F, Ederveen A, de Goeij A, Groothuis P. Oestrogen-modulated gene expression in the human endometrium. Cell Mol Life Sci 2005;62:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH + 2 versus LH + 7 by microarray technology. Mol Hum Reprod 2003;9:253–264. [DOI] [PubMed] [Google Scholar]
- Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet 2012;29:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, Murphy C, Adams S, Riesewijk A, Mannaerts B et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod 2005;20:3318–3327. [DOI] [PubMed] [Google Scholar]
- Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta 2010;31:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature (London) 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- Sun X, Park CB, Deng W, Potter SS, Dey SK. Uterine inactivation of muscle segment homeobox (Msx) genes alters epithelial cell junction proteins during embryo implantation. FASEB J 2015;30:1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 2003;88:3860–3866. [DOI] [PubMed] [Google Scholar]
- Wang J, Armant DR. Integrin-mediated adhesion and signaling during blastocyst implantation. Cells Tissues Organs 2002;172:190–201. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int J Dev Biol 2014;58:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–1799. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, Wang Y, Wang X, Ghosh S, Guo SW. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology 2006;147:232–246. [DOI] [PubMed] [Google Scholar]
- Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T, Okai T. Differences in gene expression in the proliferative human endometrium. Fertil Steril 2005;83 (Suppl 1):1206–1215. [DOI] [PubMed] [Google Scholar]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013;34:939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]